Multivariate Analysis of Structural and Functional Neuroimaging Can Inform Psychiatric Differential Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Image Acquisition
2.3. fMRI Task
2.4. MRI Data Analysis
2.4.1. Voxel-Based Morphometry
2.4.2. Task-Related Functional Data Processing
2.4.3. Resting State Data Processing—Whole Brain Residual Partial Activations
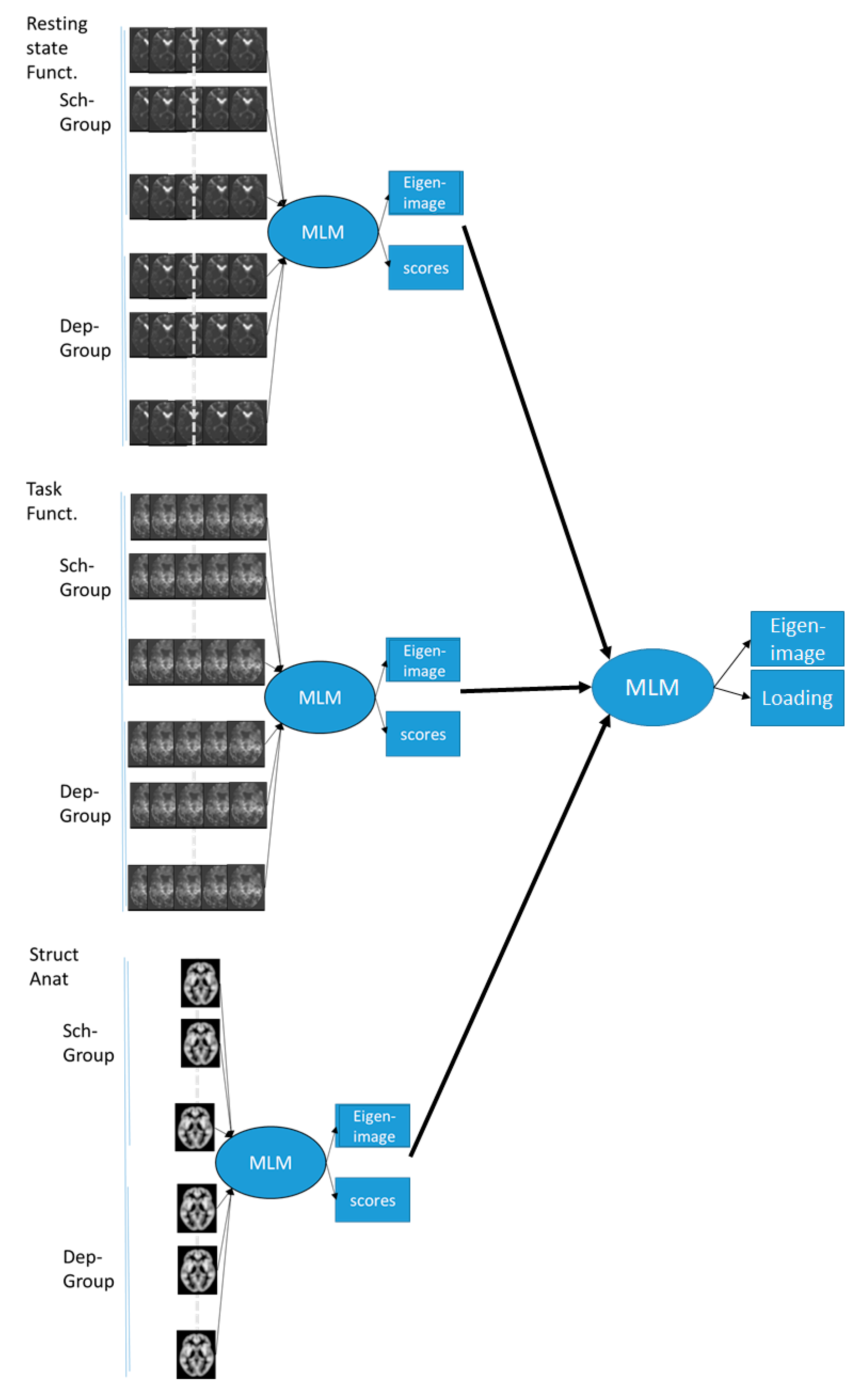
2.4.4. MLM Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
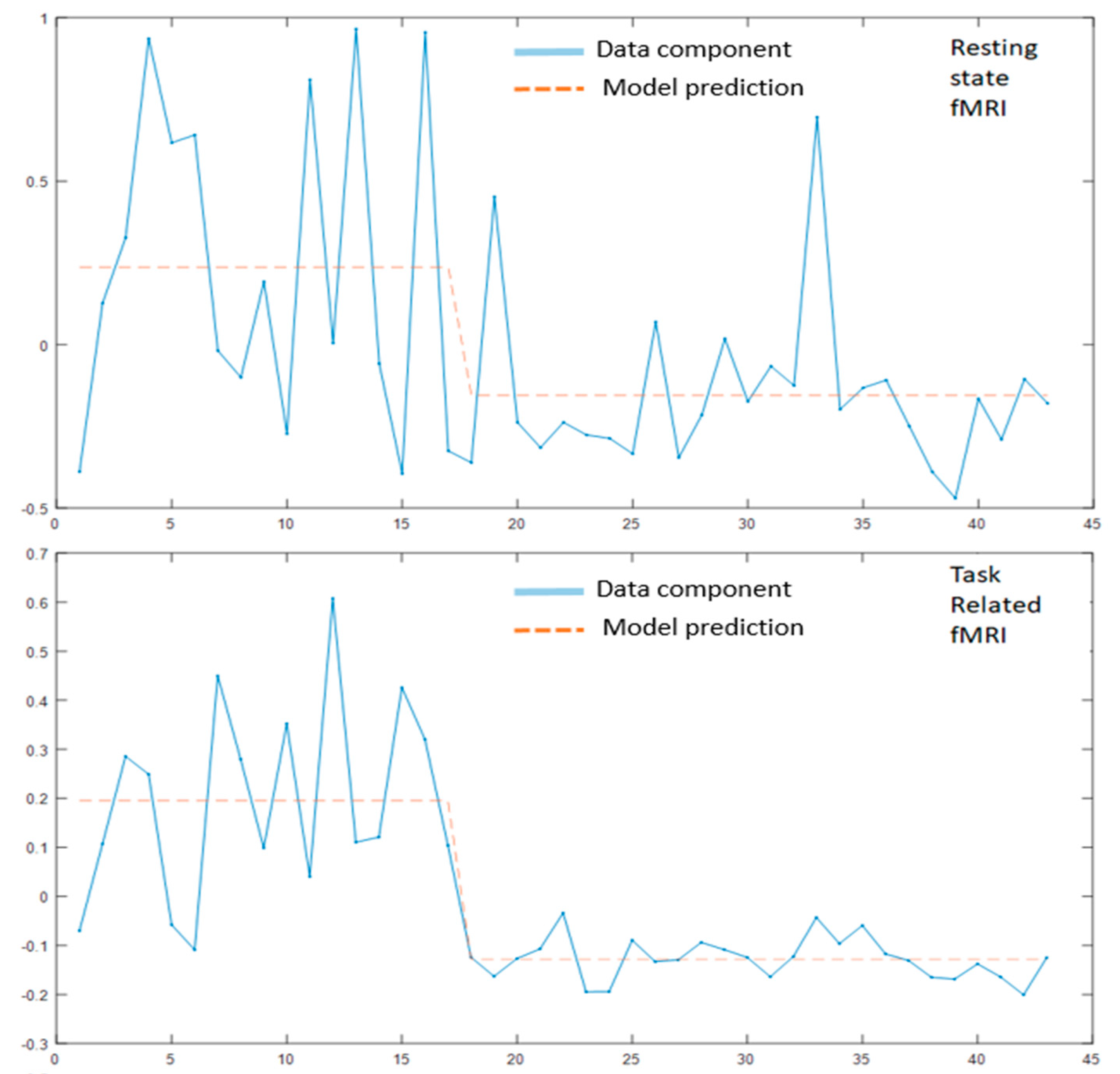
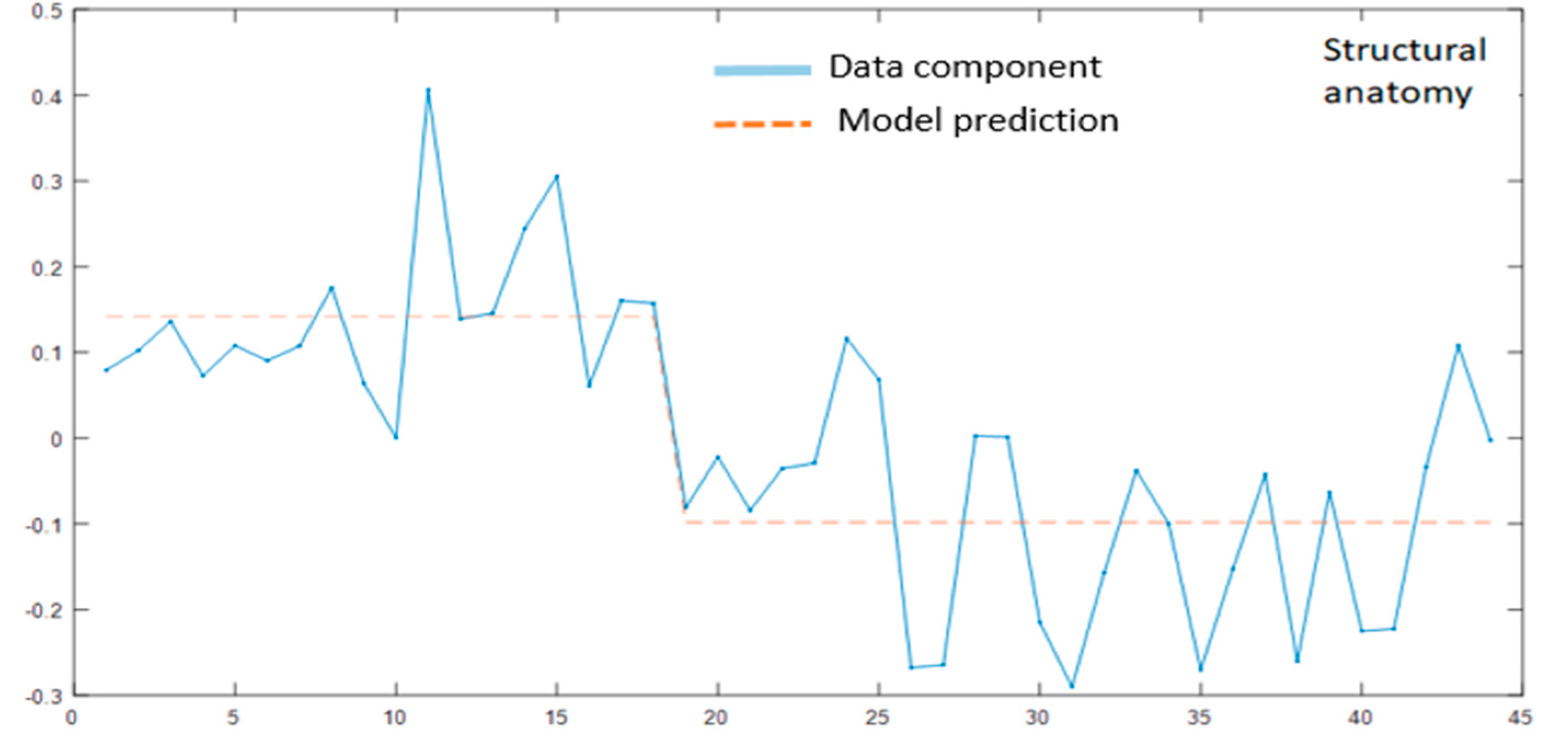
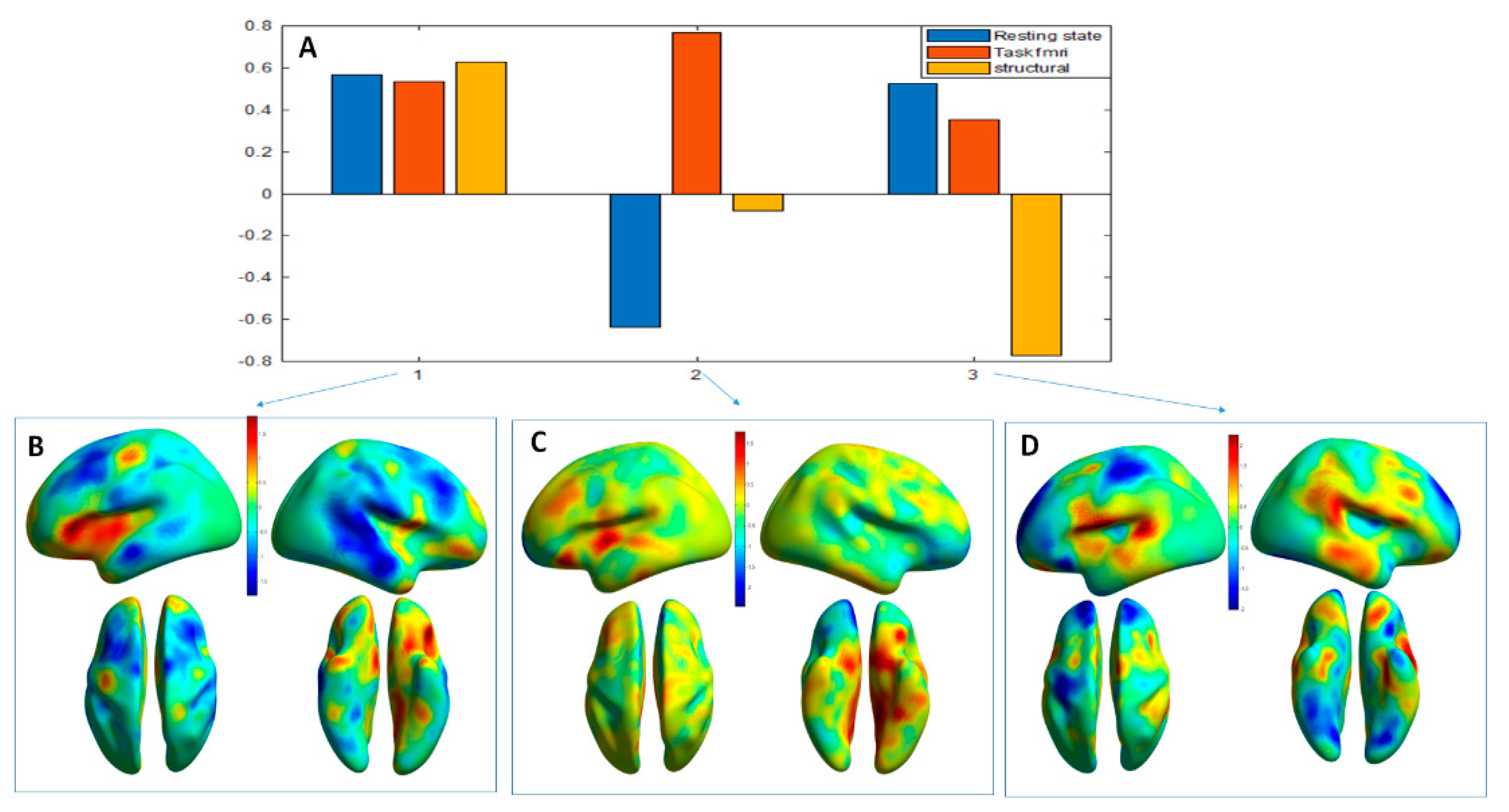
3.2. MLM Analysis
3.2.1. Modality Specific MLM
3.2.2. MLM Analyses across the Modalities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vigo, D.; Thornicroft, G.; Antun, R. Estimating the true global burden of mental illness. Lancet Psychiatry 2016, 3, 171–178. [Google Scholar] [CrossRef]
- World Health Organization. Management of Physical Health Conditions in Adults with Severe Mental Disorders: WHO Guidelines; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Hany, M.; Rehman, B.; Azhar, Y.; Chapman, J. Schizophrenia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- McIntyre, R.S.; O’Donovan, C. The human cost of not achieving full remission in depression. Can. J. Psychiatry 2004, 49 (Suppl. S1), 10S–16S. [Google Scholar] [PubMed]
- Aryutova, K.; Kandilarova, S.; Todeva-Radneva, A.; Stoyanov, D. Clinical Use of Neurophysiological Biomarkers and Self-Assessment Scales to Predict and Monitor Treatment Response for Psychotic and Affective disorders. Curr. Pharm. Des. in press.
- Cuthbert, B.N. Research Domain Criteria: Toward future psychiatric nosologies. Dialogues Clin. Neurosci. 2015, 17, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Cassano, G.B.; Rucci, P.; Stat, D.; Frank, E.; Fagiolini, A.; Dell’Osso, L.; Shear, M.K.; Kupfer, D.J. The Mood Spectrum in Unipolar and Bipolar Disorder: Arguments for a Unitary Approach. Am. J. Psychiatry 2004, 161, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Moessnang, C.; Schweiger, J.I.; Baumeister, S.; Plichta, M.M.; Brandeis, D.; Banaschewski, T.; Wackerhagen, C.; Erk, S.; Walter, H.; et al. Transdiagnostic Prediction of Affective, Cognitive, and Social Function Through Brain Reward Anticipation in Schizophrenia, Bipolar Disorder, Major Depression, and Autism Spectrum Diagnoses. Schizophr. Bull. 2020, 46, 592–602. [Google Scholar] [CrossRef]
- Cardoso de Almeida, J.R.; Phillips, M.L. Distinguishing between unipolar depression and bipolar depression: Current and future clinical and neuroimaging perspectives. Biol. Psychiatry 2013, 73, 111–118. [Google Scholar] [CrossRef]
- Zachar, P.; Stoyanov, S.D.; Aragona, M.; Jablensky, A. (Eds.) Alternative Perspectives on Psychiatric Validation: DSM, IDC, RDoC, and Beyond; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Stoyanov, D.; Kandilarova, S.; Borgwardt, S.; Stieglitz, R.-D.; Hugdahl, K.; Kostianev, S. Psychopathology Assessment Methods Revisited: On Translational Cross-Validation of Clinical Self-Evaluation Scale and fMRI. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef]
- Stoyanov, D.; Kandilarova, S.; Arabadzhiev, Z.; Paunova, R.; Schmidt, A.; Borgwardt, S. Cross-Validation of Paranoid-Depressive Scale and Functional MRI: New Paradigm for Neuroscience Informed Clinical Psychopathology. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef]
- Stoyanov, D.; Kandilarova, S.; Paunova, R.; Barranco Garcia, J.; Latypova, A.; Kherif, F. Cross-Validation of Functional MRI and Paranoid-Depressive Scale: Results From Multivariate Analysis. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef]
- Stoyanov, D.S. An Essay on the Mind-Brain Problem and Legal Proof. Balk. J. Philos. 2018, 10, 27–36. [Google Scholar] [CrossRef]
- Specht, K. Current Challenges in Translational and Clinical fMRI and Future Directions. Front. Psychiatry 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, J.; Sui, J.; Pearlson, G.; Calhoun, V.D. A Hybrid Machine Learning Method for Fusing fMRI and Genetic Data: Combining both Improves Classification of Schizophrenia. Front. Hum. Neurosci. 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Marquand, A.F.; Ehlis, A.-C.; Dresler, T.; Kittel-Schneider, S.; Jarczok, T.A.; Lesch, K.-P.; Jakob, P.M.; Mourao-Miranda, J.; Brammer, M.J.; et al. Integrating Neurobiological Markers of Depression. Arch. Gen. Psychiatry 2010, 68, 361. [Google Scholar] [CrossRef]
- Castro, E.; Martínez-Ramón, M.; Pearlson, G.; Sui, J.; Calhoun, V.D. Characterization of groups using composite kernels and multi-source fMRI analysis data: Application to schizophrenia. NeuroImage 2011, 58, 526–536. [Google Scholar] [CrossRef]
- Sundermann, B.; Herr, D.; Schwindt, W.; Pfleiderer, B. Multivariate Classification of Blood Oxygen Level-Dependent fMRI Data with Diagnostic Intention: A Clinical Perspective. Am. J. Neuroradiol. 2013. [Google Scholar] [CrossRef]
- Sui, J.; He, H.; Yu, Q.; Chen, J.; Rogers, J.; Pearlson, G.D.; Mayer, A.; Bustillo, J.; Canive, J.; Calhoun, V.D. Combination of Resting State fMRI, DTI, and sMRI Data to Discriminate Schizophrenia by N-way MCCA + jICA. Front. Hum. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Kalcher, K.; Boubela, R.N.; Huf, W.; Biswal, B.B.; Baldinger, P.; Sailer, U.; Filzmoser, P.; Kasper, S.; Lamm, C.; Lanzenberger, R.; et al. RESCALE: Voxel-specific task-fMRI scaling using resting state fluctuation amplitude. NeuroImage 2013, 70, 80–88. [Google Scholar] [CrossRef]
- Gao, S.; Calhoun, V.D.; Sui, J. Machine learning in major depression: From classification to treatment outcome prediction. CNS Neurosci. Ther. 2018, 24, 1037–1052. [Google Scholar] [CrossRef]
- Worsley, K.J.; Poline, J.-B.; Friston, K.J.; Evans, A.C. Characterizing the Response of PET and fMRI Data Using Multivariate Linear Models. NeuroImage 1997, 6, 305–319. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Suzuki, M.; Kherif, F.; Takahashi, T.; Zhou, S.-Y.; Nakamura, K.; Matsui, M.; Sumiyoshi, T.; Seto, H.; Kurachi, M. Multivariate voxel-based morphometry successfully differentiates schizophrenia patients from healthy controls. NeuroImage 2007, 34, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kherif, F.; Poline, J.-B.; Flandin, G.; Benali, H.; Simon, O.; Dehaene, S.; Worsley, K.J. Multivariate Model Specification for fMRI Data. NeuroImage 2002, 16, 1068–1083. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheenan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33. [Google Scholar] [PubMed]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Radua, J.; Phillips, M.L.; Russell, T.; Lawrence, N.; Marshall, N.; Kalidindi, S.; El-Hage, W.; Mcdonald, C.; Giampietro, V.; Brammer, M.; et al. Neural response to specific components of fearful faces in healthy and schizophrenic adults. NeuroImage 2010, 49, 939–946. [Google Scholar] [CrossRef]
- Adolphs, R. Is the human amygdala specialized for processing social information? Ann. New York Acad. Sci. 2003, 985, 326–340. [Google Scholar] [CrossRef]
- Kasai, K.; Shenton, M.E.; Salisbury, D.F.; Hirayasu, Y.; Lee, C.-U.; Ciszewski, A.A.; Yurgelun-Todd, D.; Kikinis, R.; Jolesz, F.A.; McCarley, R.W. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am. J. Psychiatry 2003, 160, 156–164. [Google Scholar] [CrossRef]
- Rajarethinam, R.P.; DeQuardo, J.R.; Nalepa, R.; Tandon, R. Superior temporal gyrus in schizophrenia: A volumetric magnetic resonance imaging study. Schizophr. Res. 2000, 41, 303–312. [Google Scholar] [CrossRef]
- Kühn, S.; Gallinat, J. Quantitative Meta-Analysis on State and Trait Aspects of Auditory Verbal Hallucinations in Schizophrenia. Schizophr. Bull. 2012, 38, 779–786. [Google Scholar] [CrossRef]
- Modinos, G.; Costafreda, S.G.; van Tol, M.-J.; McGuire, P.K.; Aleman, A.; Allen, P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: A quantitative meta-analysis of voxel-based morphometry studies. Cortex 2013, 49, 1046–1055. [Google Scholar] [CrossRef]
- Allen, P.; Larøi, F.; McGuire, P.K.; Aleman, A. The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev. 2008, 32, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Schremm, A.; Novén, M.; Horne, M.; Söderström, P.; van Westen, D.; Roll, M. Cortical thickness of planum temporale and pars opercularis in native language tone processing. Brain Lang. 2018, 176, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Tops, M.; Boksem, M.A.S. A Potential Role of the Inferior Frontal Gyrus and Anterior Insula in Cognitive Control, Brain Rhythms, and Event-Related Potentials. Front. Psychol. 2011, 2. [Google Scholar] [CrossRef]
- Tops, M.; Boksem, M.A.S. Cortisol involvement in mechanisms of behavioral inhibition. Psychophysiology 2011, 48, 723–732. [Google Scholar] [CrossRef]
- Wu, J.C. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J. Affect. Disord. 2008, 107, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Britt, J.P.; Benaliouad, F.; McDevitt, R.A.; Stuber, G.D.; Wise, R.A.; Bonci, A. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron 2012, 76, 790–803. [Google Scholar] [CrossRef]
- Rowley, H.L.; Kulkarni, R.S.; Gosden, J.; Brammer, R.J.; Hackett, D.; Heal, D.J. Differences in the neurochemical and behavioural profiles of lisdexamfetamine methylphenidate and modafinil revealed by simultaneous dual-probe microdialysis and locomotor activity measurements in freely-moving rats. J. Psychopharmacol. 2013. [Google Scholar] [CrossRef]
- Schrantee, A.; Ferguson, B.; Stoffers, D.; Booij, J.; Rombouts, S.; Reneman, L. Effects of dexamphetamine-induced dopamine release on resting-state network connectivity in recreational amphetamine users and healthy controls. Brain Imaging Behav. 2016, 10, 548–558. [Google Scholar] [CrossRef]
- Dang, L.C.; O’Neil, J.P.; Jagust, W.J. Dopamine Supports Coupling of Attention-Related Networks. J. Neurosci. 2012, 32, 9582–9587. [Google Scholar] [CrossRef]
- Duncan, N.W.; Wiebking, C.; Northoff, G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—A review of multimodal imaging studies. Neurosci. Biobehav. Rev. 2014, 47, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, K.; Raichle, M.E.; Mitra, A.; Specht, K. On the existence of a generalized non-specific task-dependent network. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The restless brain: How intrinsic activity organizes brain function. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140172. [Google Scholar] [CrossRef]
- Eichele, T.; Debener, S.; Calhoun, V.D.; Specht, K.; Engel, K.A.; Hugdahl, K.; von Cramon, Y.; Ullsperger, M. Prediction of human errors by maladaptive changes in event-related brain networks. Proc. Natl. Acad. Sci. USA 2008, 105, 6173–6178. [Google Scholar] [CrossRef] [PubMed]
- Løberg, E.-M.; Nygård, M.; Øystein Berle, J.; Johnsen, E.; Kroken, R.A.; Jørgensen, H.A.; Hugdahl, K. An fMRI Study of Neuronal Activation in Schizophrenia Patients with and without Previous Cannabis Use. Front. Psychiatry 2012, 3. [Google Scholar] [CrossRef]
- Nygård, M.; Eichele, T.; Løberg, E.-M.; Jørgensen, H.A.; Johnsen, E.; Kroken, R.A.; Øystein Berle, J.; Hugdahl, K. Patients with Schizophrenia Fail to Up-Regulate Task-Positive and Down-Regulate Task-Negative Brain Networks: An fMRI Study Using an ICA Analysis Approach. Front. Hum. Neurosci. 2012, 6. [Google Scholar] [CrossRef]
- van Lutterveld, R.; Diederen, K.M.J.; Otte, W.M.; Sommer, I.E. Network analysis of auditory hallucinations in nonpsychotic individuals. Human Brain Mapp. 2014, 35, 1436–1445. [Google Scholar] [CrossRef]
- Hugdahl, K.; Løberg, E.-M.; Nygård, M. Left temporal lobe structural and functional abnormality underlying auditory hallucinations. Front. Neurosci. 2009, 3. [Google Scholar] [CrossRef]
- Phillips, L.K.; Seidman, L.J. Emotion Processing in Persons at Risk for Schizophrenia. Schizophr. Bull. 2008, 34, 888–903. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Donaldson7.pdf. Available online: https://dspace.stir.ac.uk/bitstream/1893/2389/1/Donaldson7.pdf (accessed on 27 November 2020).
- Modinos, G.; Tseng, H.-H.; Falkenberg, I.; Samson, C.; McGuire, P.; Allen, P. Neural correlates of aberrant emotional salience predict psychotic symptoms and global functioning in high-risk and first-episode psychosis. Soc. Cogn. Affect. Neurosci. 2015, 10, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, E.C.; Bellaire, M.; Ewing, S.G.; Grace, A.A. Abnormal Stress Responsivity in a Rodent Developmental Disruption Model of Schizophrenia. Neuropsychopharmacology 2013, 38. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.J.; Grace, A.A. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol. Sci. 2011, 32, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.W.; Szczepanik, J.; Brutsché, N.; Park, L.T.; Nugent, A.C.; Zarate, C.A. Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biol. Psychiatry 2018, 84, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Marsman, A.; van den Heuvel, M.P.; Klomp, D.W.J.; Kahn, R.S.; Luijten, P.R.; Hulshoff Pol, H.E. Glutamate in Schizophrenia: A Focused Review and Meta-Analysis of 1H-MRS Studies. Schizophr. Bull. 2013, 39, 120–129. [Google Scholar] [CrossRef]
- Kucyi, A.; Moayedi, M.; Weissman-Fogel, I.; Hodaie, M.; Davis, K.D. Hemispheric asymmetry in white matter connectivity of the temporoparietal junction with the insula and prefrontal cortex. PLoS ONE 2012, 7, e35589. [Google Scholar] [CrossRef]
- de Schotten, M.T.; Dell’Acqua, F.; Forkel, S.J.; Simmons, A.; Vergani, F.; Murphy, D.G.M.; Catani, M. A lateralized brain network for visuospatial attention. Nat. Neurosci. 2011, 14, 1245–1246. [Google Scholar] [CrossRef]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.; Zarate, C.A., Jr. Glutamate and GABA Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol. Psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef]
- Graziano, M.S.A. The temporoparietal junction and awareness. Neurosci. Conscious. 2018, 2018. [Google Scholar] [CrossRef]
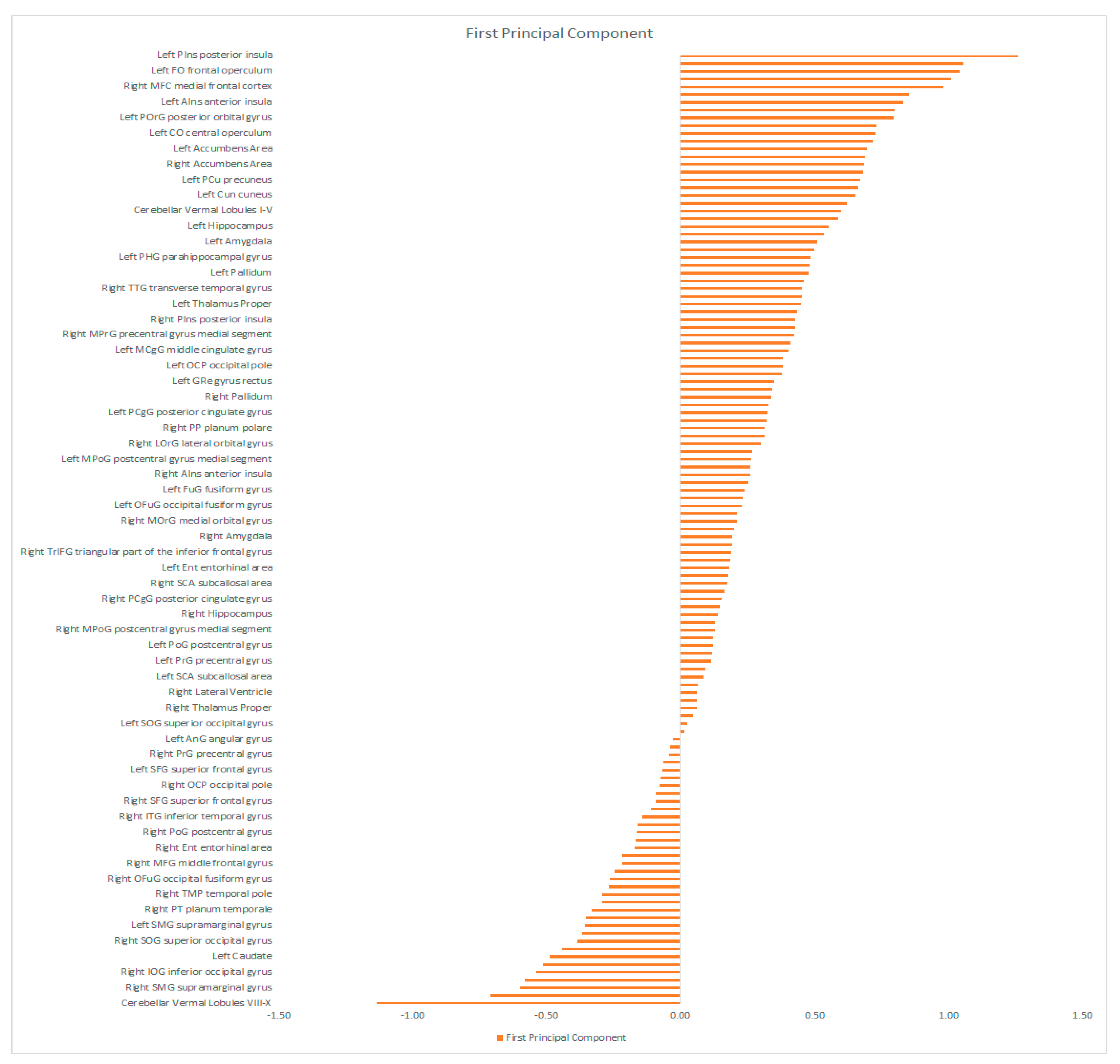
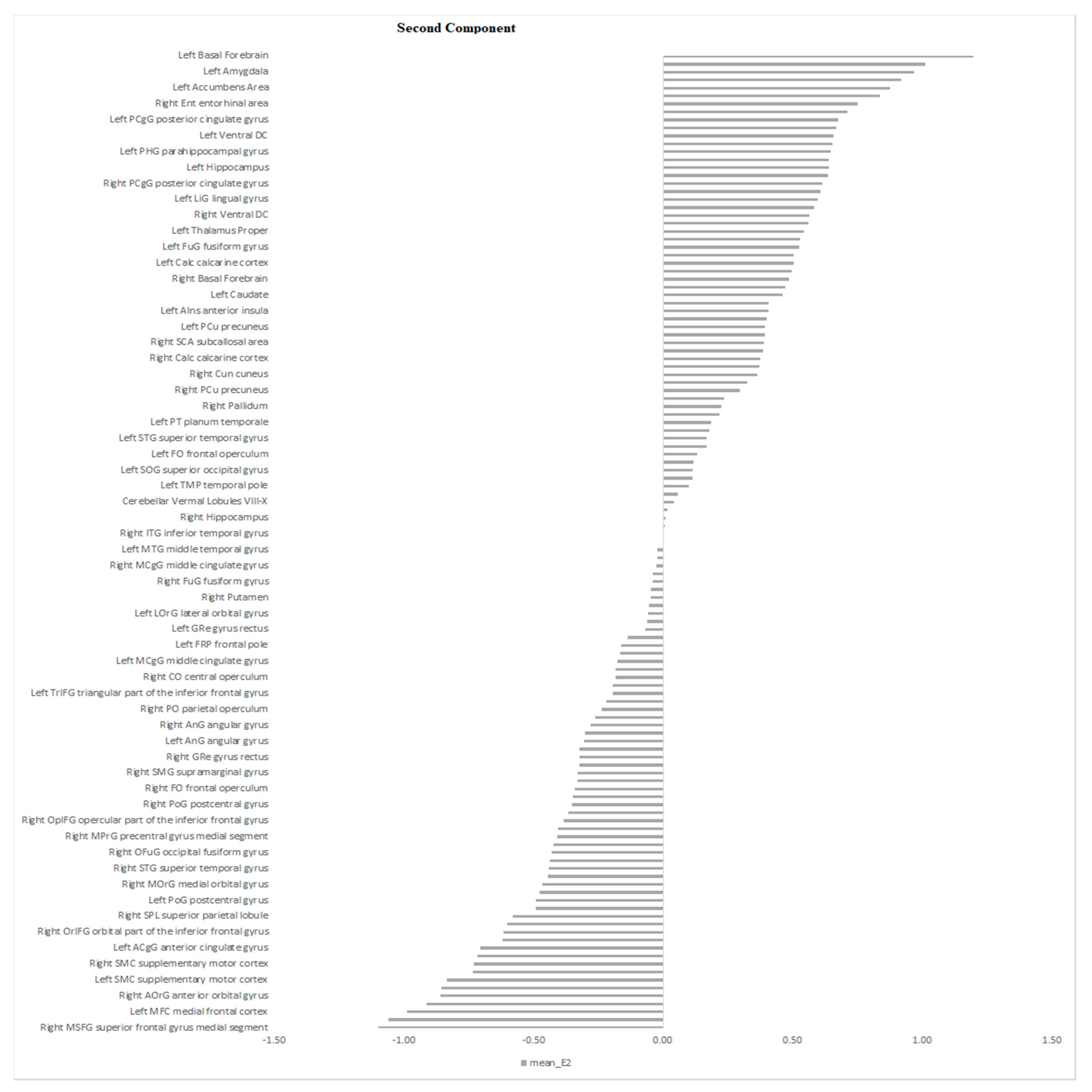
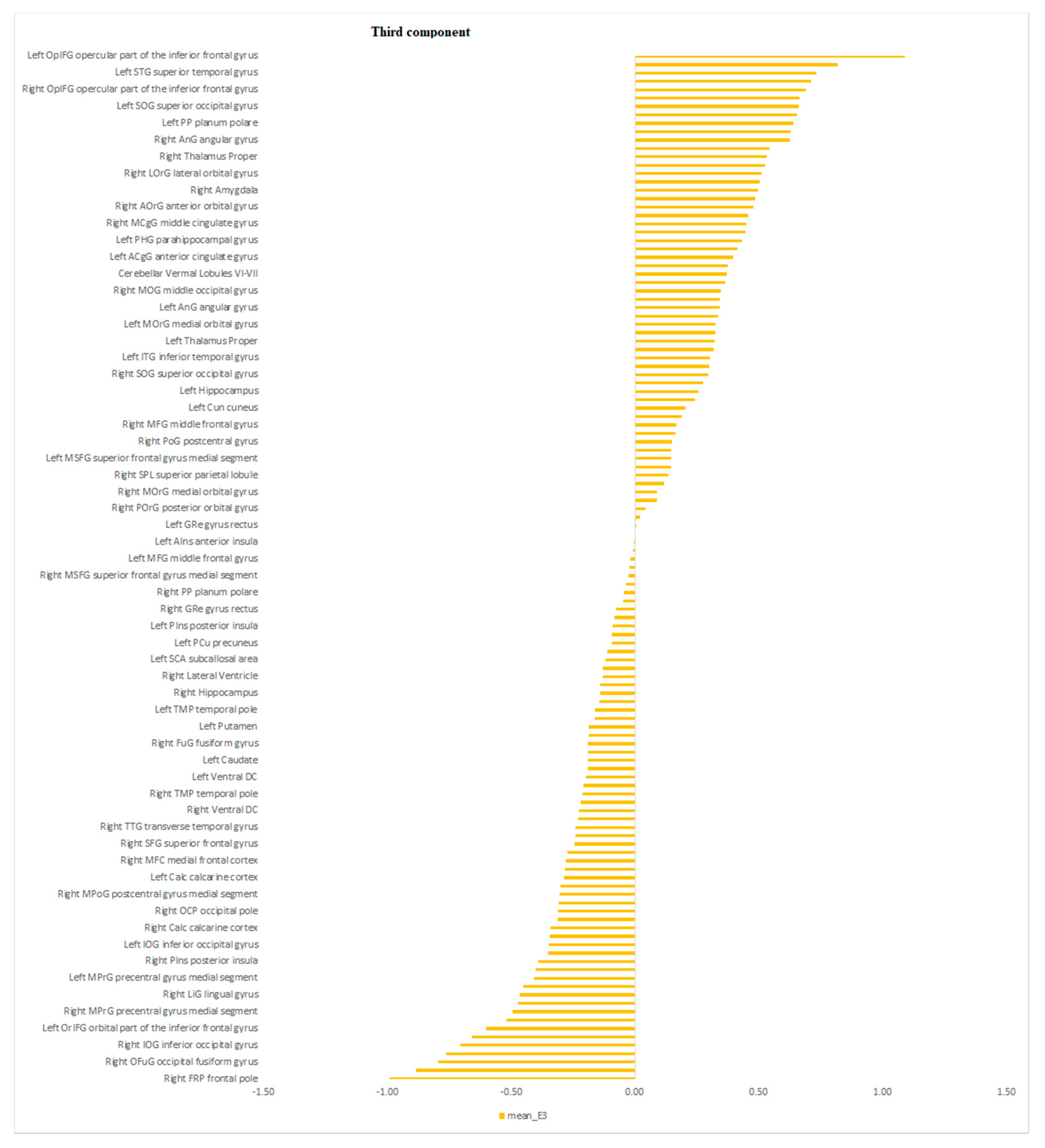
| Characteristics | Schizophrenia Patients (n = 19) | Depressed Patients (n = 25) | Statistical Significance |
|---|---|---|---|
| Age (mean ± SD) | 39.3 ± 14.8 | 44.2 ± 12.1 | 0.231 a |
| Sex (M/F) | 9/10 | 9/16 | 0.542 b |
| Education (years) | 13.5 ± 2.8 | 14.1 ± 3.5 | 0.548 a |
| Age at onset (years) | 27.1 ± 9.1 | 33.8 ± 12.4 | 0.139 a |
| Illness duration (months) | 142.8 ± 121.6 | 121.8 ± 84.5 | 0.505 a |
| Episode duration (weeks) | 15.4 ± 14.1 | 11.9 ± 10.4 | 0.403 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanov, D.; Kandilarova, S.; Aryutova, K.; Paunova, R.; Todeva-Radneva, A.; Latypova, A.; Kherif, F. Multivariate Analysis of Structural and Functional Neuroimaging Can Inform Psychiatric Differential Diagnosis. Diagnostics 2021, 11, 19. https://doi.org/10.3390/diagnostics11010019
Stoyanov D, Kandilarova S, Aryutova K, Paunova R, Todeva-Radneva A, Latypova A, Kherif F. Multivariate Analysis of Structural and Functional Neuroimaging Can Inform Psychiatric Differential Diagnosis. Diagnostics. 2021; 11(1):19. https://doi.org/10.3390/diagnostics11010019
Chicago/Turabian StyleStoyanov, Drozdstoy, Sevdalina Kandilarova, Katrin Aryutova, Rositsa Paunova, Anna Todeva-Radneva, Adeliya Latypova, and Ferath Kherif. 2021. "Multivariate Analysis of Structural and Functional Neuroimaging Can Inform Psychiatric Differential Diagnosis" Diagnostics 11, no. 1: 19. https://doi.org/10.3390/diagnostics11010019
APA StyleStoyanov, D., Kandilarova, S., Aryutova, K., Paunova, R., Todeva-Radneva, A., Latypova, A., & Kherif, F. (2021). Multivariate Analysis of Structural and Functional Neuroimaging Can Inform Psychiatric Differential Diagnosis. Diagnostics, 11(1), 19. https://doi.org/10.3390/diagnostics11010019









