Viscoelastic Hemostatic Assays and Platelet Function Testing in Patients with Atherosclerotic Vascular Diseases
Abstract
1. Introduction
2. Viscoelastic Hemostatic Assays and Platelet Function Testing
2.1. Platelet Dysfunction and Adverse Vascular Events
2.2. Platelet Function Testing in Cardiovascular Medicine
2.3. Thromboelastography and Thromboelastometry: Assays Principle, Advantages and Disadvantages
2.4. Platelet Function Testing with Thromboelastography
2.5. Platelet Function Testing with Thromboelastometry
2.6. Clinical Experiences with Viscoelastic Hemostatic Assays (VHA) for Platelet Function Testing and Gaps in Evidence
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndromes |
| ADP | adenosinediphosphate |
| ADPRB | ADP receptor blockers |
| ASA | acetylsalicylic acid |
| CABG | coronary artery bypass graft |
| CI | confidence interval |
| GRAVITAS | Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety |
| HPR | high on-treatment platelet reactivity |
| HR | hazard ratio |
| LTA | light transmission aggregometry |
| MPR | maximal platelet reactivity |
| PCI | percutaneous coronary interventions |
| PFA | Platelet Function Analyzer |
| PFT | platelet function testing |
| POC | point-of-care |
| PRI | platelet reactivity index |
| PRP | platelet rich plasma |
| PRU | platelet response units |
| ROTEM® | thromboelastometry |
| SCAD | stable coronary artery disease |
| STEMI | ST elevation myocardial infarction (MI) |
| TEG® | thromboelastography |
| TRAP | thrombin receptor activating peptide |
| VASP-P | vasodilator-stimulated phosphoprotein (VASP) phosphorylation |
| VHA | viscoelastic hemostatic assays |
References
- Sibbing, D.; Byrne, R.A.; Kastrati, A. Role of platelet function testing in clinical practice: Current concepts and future perspec-tives. Curr. Drug Targets 2011, 12, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Aradi, D.; Sibbing, D.; Gross, L. Platelet Function Testing in Patients on Antiplatelet Medications. Semin. Thromb. Hemost. 2016, 42, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.W.A.; Berg, J.M.T. Platelet Function Testing and Tailored Antiplatelet Therapy. J. Cardiovasc. Transl. Res. 2013, 6, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Korpallová, B.; Samoš, M.; Bolek, T.; Škorňová, I.; Kovář, F.; Kubisz, P.; Staško, J.; Mokáň, M. Role of Thromboelastography and Rotational Thromboelastometry in the Management of Cardiovascular Diseases. Clin. Appl. Thromb. 2018, 24, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.B.; Hvas, A.M. Predictive Value of Whole Blood and Plasma Coagulation Tests for Intra- and Postoperative Bleed-ing Risk: A Systematic Review. Semin. Thromb. Hemost. 2017, 43, 772–805. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Angiolillo, D.; Bates, E.; Berger, P.B.; Bhatt, D.; Cannon, C.P.; Furman, M.I.; Gurbel, P.; Michelson, A.D.; Peterson, E.; et al. The problem of persistent platelet activation in acute coronary syndromes and following percutaneous coro-nary intervention. Clin. Cardiol. 2008, 31, I17–I20. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Peacock, W.F.; Kottke-Marchant, K. Importance of platelets and platelet response in acute coronary syndromes. Clevel. Clin. J. Med. 2009, 76, S2–S7. [Google Scholar] [CrossRef]
- Marcucci, R.; Gori, A.M.; Paniccia, R.; Giusti, B.; Valente, S.; Giglioli, C.; Buonamici, P.; Antoniucci, D.; Abbate, R.; Gensini, G.F. Car-diovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: A 12-month follow-up. Circulation 2009, 119, 237–242. [Google Scholar] [CrossRef]
- Marcucci, R.; Giusti, B.; Paniccia, R.; Gori, A.M.; Saracini, C.; Valente, S.; Giglioli, C.; Parodi, G.; Antoniucci, D.; Gensini, G.F.; et al. High on-treatment platelet reactivity by ADP and increased risk of MACE in good clopidogrel metabolizers. Platelets 2012, 23, 586–593. [Google Scholar] [CrossRef][Green Version]
- Siller-Matula, J.M.; Delle-Karth, G.; Christ, G.; Neunteufl, T.; Maurer, G.; Huber, K.; Tolios, A.; Drucker, C.; Jilma, B. Dual non-responsiveness to antiplatelet treatment is a stronger predictor of cardiac adverse events than isolated non-responsiveness to clopidogrel or aspirin. Int. J. Cardiol. 2013, 167, 430–435. [Google Scholar] [CrossRef]
- Droppa, M.; Tschernow, D.; Müller, K.A.L.; Tavlaki, E.; Karathanos, A.; Stimpfle, F.; Schaeffeler, E.; Schwab, M.; Tolios, A.; Siller-Matula, J.M.; et al. Evaluation of Clinical Risk Factors to Predict High On-Treatment Platelet Reactivity and Outcome in Patients with Stable Coronary Artery Disease (PREDICT-STABLE). PLoS ONE 2015, 10, e0121620. [Google Scholar] [CrossRef] [PubMed]
- Viviani Anselmi, C.; Briguori, C.; Roncarati, R.; Papa, L.; Visconti, G.; Focaccio, A.; De Micco, F.; Latronico, M.V.; Pagnotta, P.; Condorelli, G. Routine assessment of on-clopidogrel platelet reactivity and gene polymorphisms in predicting clinical out-come following drug-eluting stent implantation in patients with stable coronary artery disease. JACC Cardiovasc. Interv. 2013, 6, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Oikonomou, E.; Zaromitidou, M.; Kioufis, S.; Vavuranakis, M.; Maniatis, K.; Kokkou, E.; Papageorgiou, N.; Papaioannou, S.; Tourikis, P.; et al. High platelet reactivity is associated with vascular function in patients after percutaneous coronary intervention receiving clopidogrel. Int. J. Cardiol. 2014, 177, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Gianetti, J.; Parri, M.S.; Sbrana, S.; Paoli, F.; Maffei, S.; Paradossi, U.; Berti, S.; Clerico, A.; Biagini, A. Platelet activation predicts re-current ischemic events after percutaneous coronary angioplasty: A 6 months prospective study. Thromb. Res. 2006, 118, 487–493. [Google Scholar] [CrossRef]
- Wasilewski, J.; Desperak, P.; Hawranek, M.; Ciślak, A.; Osadnik, T.; Pyka, Ł.; Gawlita, M.; Bujak, K.; Niedziela, J.; Krawczyk, M.; et al. Prognostic implications of mean platelet volume on short- and long-term outcomes among patients with non-ST-segment elevation myocardial infarction treated with percutaneous coronary intervention: A single-center large observational study. Platelets 2016, 27, 452–458. [Google Scholar] [CrossRef]
- Bladowski, M.; Gawryś, J.; Gajecki, D.; Szahidewicz-Krupska, E.; Sawicz-Bladowska, A.; Doroszko, A. Role of the Platelets and Nitric Oxide Biotransformation in Ischemic Stroke: A Translative Review from Bench to Bedside. Oxidative Med. Cell. Longev. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Denorme, F.; De Meyer, S.F. The VWF-GPIb axis in is chaemic stroke: Lessons from animal models. Thromb. Haemost. 2016, 116, 597–604. [Google Scholar] [CrossRef]
- VanRooy, M.J.; Pretorius, E. Metabolic syndrome, platelet activation and the development of transient ischemic attack or thromboembolic stroke. Thromb Res. 2015, 135, 434–442. [Google Scholar] [CrossRef]
- Rayt, H.S.; Merker, L.; Davies, R.S. Coagulation, Fibrinolysis, and Platelet Activation Following Open Surgical or Percutane-ous Angioplasty Revascularization for Symptomatic Lower Limb Chronic Ischemia. Vasc. Endovasc. Surg. 2016, 50, 193–201. [Google Scholar] [CrossRef]
- Maiocchi, S.; Alwis, I.; Wu, M.C.L.; Yuan, Y.; Jackson, S.P. Thrombo inflammatory Functions of Platelets in Ischemia–Reperfusion Injury and Its Dysregulation in Diabetes. Semin. Thromb. Hemost. 2018, 44, 102–113. [Google Scholar] [CrossRef]
- Crafa, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Barbagallo, F.; Aversa, A.; Izzo, G.; Perri, A.; Calogero, A.E.; La Vignera, S. Mean Platelet Volume as a Marker of Vasculogenic Erectile Dysfunction and Future Cardiovascular Risk. J. Clin. Med. 2020, 9, 2513. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.J.; Gohs, F.X.; Kirtane, A.J.; Brodie, B.R.; Stuckey, T.D.; Redfors, B.; McAndrew, T.; Witzenbichler, B.; Weisz, G.; Neumann, F.-J.; et al. Impact of Point-of-Care Platelet Function Testing Among Patients with and Without Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention With Drug-Eluting Stents (from the ADAPT-DES Study). Am. J. Cardiol. 2019, 123, 549–557. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, R.; Palmerini, T.; De Servi, S.; Belmonte, M.; Crimi, G.; Cornara, S.; Calabrò, P.; Cattaneo, M.; Maffeo, D.; Toso, A.; et al. High on-treatment platelet reactivity and outcome in elderly with non ST-segment elevation acute coronary syndrome–Insight from the GEPRESS study. Int. J. Cardiol. 2018, 259, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Berger, P.B.; Angiolillo, D.J.; Teirstein, P.S.; Tanguay, J.F.; Kandzari, D.E.; Cannon, C.P.; Topol, E.J. Evaluation of individu-alizedclopidogrel therapy after drug-eluting stent implantation in patients with high residual platelet reactivity: Design and rationale of the GRAVITAS trial. Am. Heart J. 2009, 157, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Wang, Q.; Xu, Q.; Lv, Q. Clopidogrel-associated genetic variants on inhibition of platelet activity and clinical outcome for acute coronary syndrome patients. Basic Clin. Pharmacol. Toxicol. 2019, 124, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Spectre, G.; Mosseri, M.; Abdelrahman, N.M.; Briskin, E.; Bulut, A.; Loncar, S.; Varon, D.; Alcalai, R. Clinical and prognostic im-plications of the initial response to aspirin in patients with acute coronary syndrome. Am. J. Cardiol. 2011, 108, 1112–1118. [Google Scholar] [CrossRef]
- Beigel, R.; Hod, H.; Fefer, P.; Asher, E.; Novikov, I.; Shenkman, B.; Savion, N.; Varon, D.; Matetzky, S. Relation of Aspirin Failure to Clinical Outcome and to Platelet Response to Aspirin in Patients with Acute Myocardial Infarction. Am. J. Cardiol. 2011, 107, 339–342. [Google Scholar] [CrossRef]
- Aradi, D.; Gross, L.; Trenk, D.; Geisler, T.; Merkely, B.; Kiss, R.G.; Komócsi, A.; Dézsi, C.A.; Ruzsa, Z.; Ungi, I.; et al. Platelet reactivity and clinical outcomes in acute coronary syndrome patients treated with prasugrel and clopidogrel: A pre-specified exploratory analysis from the TROPICAL-ACS trial. Eur. Hear. J. 2019, 40, 1942–1951. [Google Scholar] [CrossRef]
- Sato, T.; Namba, Y.; Kashihara, Y.; Tanaka, M.; Fuke, S.; Yumoto, A.; Saito, H. Clinical significance of platelet reactivity during prasugrel therapy in patients with acute myocardial infarction. J. Cardiol. 2017, 70, 35–40. [Google Scholar] [CrossRef]
- Jariwala, P.; Bhatia, H.; Kumar, E.A.P. Sub-acute stent thrombosis secondary to ticagrelor resistance—Myth or reality!! Indian Hear. J. 2017, 69, 804–806. [Google Scholar] [CrossRef]
- Mahla, E.; Tantry, U.S.; Schoerghuber, M.; Gurbel, P.A. Platelet Function Testing in Patients on Antiplatelet Therapy before Cardiac Surgery. Anesthesiology 2020, 133, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, D.; Lancé, M.D.; Siegemund, M. Point-of-Care Platelet Function Monitoring: Implications for Patients with Platelet Inhibitors in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2020, 21, 1053. [Google Scholar] [CrossRef]
- Petricevic, M.; Knezevic, J.; Biocina, B.; Mikus, M.; Konosic, L.; Rasic, M.; Milosevic, M.; Rotim, C.; Madzar, T.; Rotim, A.; et al. Association among Clopidogrel Cessation, Platelet Function, and Bleeding in Coronary Bypass Surgery: An Observational Trial. Thorac. Cardiovasc. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Björklund, E.; Hansson, E.C.; Romlin, B.S.; Jeppsson, A.; Malm, C.J. Postoperative platelet function is associated with severe bleeding in ticagrelor-treated patients. Interact. Cardiovasc. Thorac. Surg. 2018, 28, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Vlot, E.; Willemsen, L.M.; Van Dongen, E.P.; Janssen, P.W.; Hackeng, C.M.; Kloppenburg, G.T.; Kelder, J.C.; Berg, J.M.T.; Noordzij, P.G. Perioperative point of care platelet function testing and postoperative blood loss in high-risk cardiac surgery patients. Platelets 2018, 30, 982–988. [Google Scholar] [CrossRef]
- Mahla, E.; Metzler, H.; Bornemann-Cimenti, H.; Prueller, F.; Raggam, R.; Pregartner, G.; Berghold, A.; Baumann, A.; Goeroeg, C.; Gurbel, P.A. Platelet Inhibition and Bleeding in Patients Undergoing Non-Cardiac Surgery—The BIANCA Observational Study. Thromb. Haemost. 2018, 118, 864–872. [Google Scholar] [CrossRef]
- Raggam, R.; Toller, W.; Mahla, E. Platelet function testing to time surgery in patients on dual antiplatelet therapy? Hämostaseologie 2014, 34, 40–45. [Google Scholar] [CrossRef]
- James, T.W.; Thomson, B.J.; Naumann, D.N.; Stevenson, D.S. Platelet function testing in patients with post-operative tonsillec-tomy bleeding may be a useful early identifier of inherited platelet function disorders. J. Laryngol. Otol. 2020, 5, 1–5. [Google Scholar]
- Dovlatova, N. Current status and future prospects for platelet function testing in the diagnosis of inherited bleeding dis-orders. Br. J. Haematol. 2015, 170, 150–161. [Google Scholar] [CrossRef]
- Koltai, K.; Kesmarky, G.; Feher, G.; Tibold, A.; Toth, K. Platelet Aggregometry Testing: Molecular Mechanisms, Techniques and Clinical Implications. Int. J. Mol. Sci. 2017, 18, 1803. [Google Scholar] [CrossRef]
- Dyszkiewicz-Korpanty, A.M.; Kim, A.; Burner, J.D.; Frenkel, E.P.; Sarode, R. Comparison of a rapid platelet function as-say--Verify Now Aspirin--with whole blood impedance aggregometry for the detection of aspirin resistance. Thromb. Res. 2007, 120, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Cuisset, T.; Hamilos, M.; Sarma, J.; Sarno, G.; Wyffels, E.; Vanderheyden, M.; Barbato, E.; Bartunek, J.; De Bruyne, B.; Wijns, W. Relation of low response to clopidogrel assessed with point-of-care assay to periproceduralmyonecrosis in patients under-going elective coronary stenting for stable angina pectoris. Am. J. Cardiol. 2008, 101, 1700–1703. [Google Scholar] [CrossRef] [PubMed]
- Fedor, M.; Samoš, M.; Šimonová, R.; Fedorová, J.; Škorňová, I.; Duraj, L.; Staško, J.; Kovář, F.; Mokáň, M.; Kubisz, P. Monitoring the efficacy of ADP inhibitor treatment in patients with acute STEMI post-PCI by VASP-P flow cytometry assay. Clin. Appl. Thromb. Hemost. 2015, 21, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Lemesle, G.; Landel, J.B.; Bauters, A.; Delhaye, C.; Bonello, L.; Sudre, A.; Susen, S.; Bauters, C.; Lablanche, J.M. Poor agreement between light transmission aggregometry, Verify Now P2Y12 and vasodilatator-stimulated phosphoprotein for clopidogrel low-response assessment: A potential explanation of negative results of recent randomized trials. Platelets 2014, 25, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, D.; Seeberger, M.D.; Tanaka, K.A. Principles and practice of thromboelastography in clinical coagulationmanage-ment and transfusion practice. Transfus Med. Rev. 2012, 26, 1–13. [Google Scholar] [CrossRef]
- Zaky, A. Thromboelastometry Versus Rotational Thromboelastography in Cardiac Surgery. Semin. Cardiothorac. Vasc. Anesth. 2017, 21, 206–211. [Google Scholar] [CrossRef]
- Whiting, D.; Dinardo, J.A. TEG and ROTEM: Technology and clinical applications. Am. J. Hematol. 2014, 89, 228–232. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bliden, K.P.; Gurbel, P.A. Overestimation of Platelet Aspirin Resistance Detection by Thrombelastograph Platelet Mapping and Validation by Conventional Aggregometry Using Arachidonic Acid Stimulation. J. Am. Coll. Cardiol. 2005, 46, 1705–1709. [Google Scholar] [CrossRef]
- Alstrom, U.; Tydén, H.; Oldgren, J.; Siegbahn, A.; Ståhle, E. The platelet inhibiting effect of a clopidogrel bolus dose in patients on long-term acetylsalicylic acid treatment. Thromb. Res. 2007, 120, 353–359. [Google Scholar] [CrossRef]
- Bochsen, L.; Wiinberg, B.; Kjelgaard-Hansen, M.; Steinbrüchel, D.A.; Johansson, P.I. Evaluation of the TEG platelet mapping assay in blood donors. Thromb. J. 2007, 5, 3. [Google Scholar] [CrossRef]
- Bochsen, L.; Nielsen, A.B.; Steinbrüchel, D.A.; Johansson, P.I. Higher Thrombelastograph platelet reactivity in cardiac surgery patients than in blood donors. Scand. Cardiovasc. J. 2007, 41, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Collyer, T.C.; Gray, D.J.; Sandhu, R.; Berridge, J.; Lyons, G. Assessment of platelet inhibition secondary to clopidogrel and aspi-rin therapy in preoperative acute surgical patients measured by Thrombelastography Platelet Mapping. Br. J. Anaesth. 2009, 102, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Preisman, S.; Kogan, A.; Itzkovsky, K.; Leikin, G.; Raanani, E. Modified thromboelastography evaluation of platelet dysfunc-tion in patients undergoing coronary artery surgery. Eur. J. Cardiothorac. Surg. 2010, 37, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, N.S.; Weitzel, L.B.; Epperson, L.E.; Karimpour-Ford, A.; Tran, Z.V.; Seres, T. Platelet mapping as part of modified throm-boelastography (TEG) in patients undergoing cardiac surgery and cardiopulmonary bypass. Anaesthesia 2012, 67, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Johnson, R.I.; Kirmani, B.H. Pre- and Post-Bypass Platelet Function Testing with Multiple Electrode Aggregome-try and TEG Platelet Mapping in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1272–1276. [Google Scholar] [CrossRef]
- Ellis, J.; Valencia, O.; Crerar-Gilbert, A.; Phillips, S.; Meeran, H.; Sharma, V. Point-of-care platelet function testing to predict blood loss after coronary artery bypass grafting surgery: A prospective observational pilot study. Perfusion 2016, 31, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Sivapalan, P.; Bäck, A.C.; Ostrowski, S.R.; Ravn, H.B.; Johansson, P.I. Transfusion requirements in elective cardiopulmonary by-pass surgery patients: Predictive value of Multiplate and Thromboelastography (TEG) Platelet Mapping Assay. Scand. J. Clin. Lab Investig. 2017, 77, 345–351. [Google Scholar] [CrossRef]
- Barker, E.E.; Saini, A.; Gazit, A.Z.; Shea, S.M.; Baltagi, S.; Gage, B.F.; Spinella, P.C. TEG Platelet Mapping and Impedance Ag-gregometry to Predict Platelet Transfusion During Cardiopulmonary Bypass in Pediatric Patients. Front Pediatr. 2019, 7, 509. [Google Scholar] [CrossRef]
- Tian, L.; Gao, X.; Yang, J.; Yao, Y.; Ji, H. Association of Adenosine Diphosphate–Induced Platelet Maximum Amplitude with Postoperative Bleeding and Blood Transfusions in Patients Undergoing Coronary Artery Bypass Grafting. J. Cardiothorac. Vasc. Anesth. 2021, 35, 421–428. [Google Scholar] [CrossRef]
- Walsh, M.; Thomas, S.G.; Howard, J.C.; Evans, E.; Guyer, K.; Medvecz, A.; Swearingen, A.; Navari, R.M.; Ploplis, V.; Castellino, F.J. Blood Component Therapy in Trauma Guided with the Utilization of the Perfusionist and Thromboelastography. J. Extra-Corporeal Technol. 2011, 43, 162–167. [Google Scholar]
- Cattano, D.; Altamirano, A.V.; Kaynak, H.E.; Seitan, C.; Paniccia, R.; Chen, Z.; Huang, H.; Prisco, D.; Hagberg, C.A.; Pivalizza, E.G. Perioperative assessment of platelet function by Thromboelastograph Platelet Mapping in cardiovascular patients under-going non-cardiac surgery. J. Thromb. Thrombol. 2013, 35, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Callender, R.; Altamirano, A.; Tezino, T.; Pivalizza, E.G.; Cattano, D. Is it possible? Predicting complications and morbidity in surgical patients on clopidogrel therapy with Thrombelastography Platelet Mapping. J. Orthop. Traumatol. 2013, 15, 69–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kasivisvanathan, R.; Abbassi-Ghadi, N.; Kumar, S.; MacKenzie, H.; Thompson, K.; James, K.; Mallett, S.V. Risk of bleeding and adverse outcomes predicted by thromboelastography platelet mapping in patients taking clopidogrel within 7 days of non-cardiac surgery. BJS 2014, 101, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Mamczak, C.N.; Maloney, M.; Fritz, B.; Boyer, B.; Thomas, S.; Evans, E.; Ploplis, V.A.; Castellino, F.J.; McCollester, J.; Walsh, M. Thromboelastography in Orthopaedic Trauma Acute Pelvic Fracture Resuscitation: A Descriptive Pilot Study. J. Orthop. Trauma 2016, 30, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Cao, J.; Fan, L.; Liu, L.; Li, J.; Hu, G.L.; Hu, Y.X.; Li, X.L. Prevalence of and risk factors for aspirin resistance in elderly pa-tients with coronary artery disease. J. Geriatr. Cardiol. 2013, 10, 21–27. [Google Scholar] [PubMed]
- Mir, A.; Frank, S.G.; Journeycake, J.; Wolovits, J.; Guleserian, K.; Heistein, L.; Lemler, M.; Wolovitis, J. Aspirin Resistance in Single-Ventricle Physiology: Aspirin Prophylaxis Is Not Adequate to Inhibit Platelets in the Immediate Postoperative Period. Ann. Thorac. Surg. 2015, 99, 2158–2164. [Google Scholar] [CrossRef]
- Berganza, F.M.; Gonzalez de Alba, C.; Egbe, A.C.; Bartakian, S.; Brownlee, J. Prevalence of aspirin resistance by thromboelas-tography plus platelet mapping in children with CHD: A single-centre experience. Cardiol. Young 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Morton, J.; Nelluri, S. Use of a thrombelastograph platelet mapping assay for diagnosis of clopidogrel resistance: A case report. J. Extra Corpor. Technol. 2009, 41, 32–36. [Google Scholar]
- Jeong, Y.-H.; Bliden, K.P.; Antonino, M.J.; Tantry, U.; Gurbel, P.A. Usefulness of thrombelastography platelet mapping assay to measure the antiplatelet effect of P2Y12receptor inhibitors and high on-treatment platelet reactivity. Platelets 2012, 24, 166–169. [Google Scholar] [CrossRef]
- Sun, B.; Li, J.; Dong, M.; Yang, L.; Wu, C.; Zhu, L.; Cong, Y. Diversity of platelet function and genetic polymorphism in clopidogrel-treated Chinese patients. Genet. Mol. Res. 2015, 14, 1434–1442. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, S.; Hao, Y. Net platelet clot strength of thromboelastography platelet mapping assay for the identification of high on-treatment platelet reactivity in post-PCI patients. Biosci. Rep. 2020, 40, 20201346. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, W.; Pan, Y.; Yan, H.; Meng, X.; Liu, L.; Wang, Y.; Wang, Y. Effect of ticagrelor versus clopidogrel on platelet reactivity measured by thrombelastography in patients with minor stroke or TIA. Aging 2020, 12, 20085–20094. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Jahngir, M.U.; Qualls, K.; Akinci, Y.; Lobanova, I.; Liaqat, J.; Gao, X.; Akhtar, I.N.; Kraus, J.; Uzun, G.; et al. The Effect of Ticagrelor on Platelet Reactivity in Patients with Clopidogrel Resistance Undergoing Neuroendovascular Procedures. J. Neuroimaging 2020, 30, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ghamraoui, A.K.; Ricotta, J.J. Outcomes and strategy of tailored antiplatelet therapy with ticagrelor in patients undergoing transcarotid artery revascularization. J. Vasc. Surg. 2021, 73, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Tang, X.F.; Zhang, Y.; Wang, J.; Yao, Y.; Ma, Y.L.; Xu, B.; Gao, R.L.; Gao, Z.; Chen, J.; et al. Relationship between ABCB1 polymorphisms, thromboelastography and risk of bleeding events in clopidogrel-treated patients with ST-elevation myocardial infarction. Thromb. Res. 2014, 134, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Samoš, M.; Stančiaková, L.; Duraj, L.; Kovář, F.; Fedor, M.; Šimonová, R.; Bolek, T.; Galajda, P.; Staško, J.; Kubisz, P.; et al. Monitoring the hemostasis with rotation thromboelastometry in patients with acute STEMI on dual antiplatelet therapy: First experiences. Medicine (Baltimore) 2017, 96, e6045. [Google Scholar] [CrossRef]
- Scharbert, G.; Auer, A.; Kozek-Langenecker, S.A. Evaluation of the Platelet Mapping™ Assay on rotational thromboelastometry ROTEM®. Platelets 2009, 20, 125–130. [Google Scholar] [CrossRef]
- Braun, D.; Knipper, A.; Orban, M.; Sibbing, D.; Petzold, T.; Braun, S.; Schulz, S.; Hausleiter, J.; Kastrati, A.; Mehilli, J.; et al. Platelet function and coagulation in patients with STEMI and peri-interventional clopidogrel plus heparin vs. prasugrel plus bivalirudin therapy (BRAVE 4 substudy). Thromb. Res. 2016, 137, 72–78. [Google Scholar] [CrossRef]
- Nissen, P.H.; Skipper, M.T.; Hvas, A.M. Whole blood platelet aggregation determined by the ROTEM platelet equipment; ref-erence intervals and stability. Platelets 2020, 31, 215–220. [Google Scholar] [CrossRef]
- Kapoor, P.M.; Gorlinger, K.; Bhardwaj, V. Simulation in coagulation testing using rotational thromboelastometry: A fast emerging, reliable point of care technique. Ann. Card. Anaesth. 2016, 19, 516–520. [Google Scholar] [CrossRef]
- Ogawa, S.; Szlam, F.; Chen, E.P.; Nishimura, T.; Kim, H.; Roback, J.D.; Levy, J.H.; Tanaka, K.A. A comparative evaluation of rota-tionthromboelastometry and standard coagulation tests in hemodilution-induced coagulation changes after cardiac surgery. Transfusion 2012, 52, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Tarzia, V.; Bortolussi, G.; Buratto, E.; Paolini, C.; Lin, C.D.; Rizzoli, G.; Bottio, T.; Gerosa, G. Single vs double antiplatelet therapy in acute coronary syndrome: Predictors of bleeding after coronary artery bypass grafting. World J. Cardiol. 2015, 7, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Azarfarin, R.; Noohi, F.; Kiavar, M.; Totonchi, Z.; Heidarpour, A.; Hendiani, A.; Koleini, Z.S.; Rahimi, S. Relationship between maximum clot firmness in ROTEM() and postoperative bleeding after coronary artery bypass graft surgery in patients using clopidogrel. Ann. Card. Anaesth. 2018, 21, 175–180. [Google Scholar] [PubMed]
- Corliss, B.M.; Freedman, R.; Brennan, M.M.; Smith, J.; Nerva, J.D.; Harris, N.S.; Polifka, A.J.; Hoh, B.L.; Fox, W.C. Laboratory assessments of therapeutic platelet inhibition in endovascular neurosurgery: Complication prediction using the VerifyNow P2Y12 assay and thromboelastography with platelet mapping. J. Neurosurg. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.; Katyal, N.; Parker, C.; Natteru, P.; Nattanamai, P.; Newey, C.R.; Kraus, C.K. Thromboelastography with Platelet Map-ping is Not an Effective Measure of Platelet Inhibition in Patients with Spontaneous Intracerebral Hemorrhage on Antiplatelet Therapy. Cureus 2018, 10, e2515. [Google Scholar]
- Daley, M.J.; Trust, M.D.; Peterson, E.J.; Luftman, K.; Miller, A.H.; Ali, S.; Clark, A.; Aydelotte, J.D.; Coopwood, T.B.; Brown, C.V. Thromboelastography Does Not Detect Preinjury Antiplatelet Therapy in Acute Trauma Patients. Am. Surg. 2016, 82, 175–180. [Google Scholar] [CrossRef]
- Lee, J.-K.; Wu, C.; Juang, J.-M.J.; Tsai, C.-T.; Hwang, J.-J.; Lin, J.-L.; Chiang, F.-T. Non-Carriers of Reduced-Function CYP2C19 Alleles are Most Susceptible to Impairment of the Anti-Platelet Effect of Clopidogrel by Proton-Pump Inhibitors: A Pilot Study. Acta Cardiol. Sin. 2016, 32, 215–222. [Google Scholar]
- Connelly, C.; Yonge, J.D.; McCully, S.P.; Hart, K.D.; Hilliard, T.C.; Lape, D.E.; Watson, J.J.; Rick, B.; Houser, B.; Deloughery, T.G.; et al. Assessment of three point-of-care platelet function assays in adult trauma patients. J. Surg. Res. 2017, 212, 260–269. [Google Scholar] [CrossRef]
- Karon, B.S.; Tolan, N.V.; Koch, C.D.; Wockenfus, A.M.; Miller, R.S.; Lingineni, R.K.; Pruthi, R.K.; Chen, D.; Jaffe, A.S. Precision and re-liability of 5 platelet function tests in healthy volunteers and donors on daily antiplatelet agent therapy. Clin. Chem. 2014, 60, 1524–1531. [Google Scholar] [CrossRef]
- Schött, U.; Johansson, P.I., II. Bringing flow into haemostasis diagnostics. Br. J. Anaesth. 2013, 111, 864–867. [Google Scholar] [CrossRef][Green Version]
- Israels, S.J.; Rand, M.L. What we have learned from inherited platelet disorders. Pediatr. Blood Cancer 2013, 60, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, M.A.; de Maat, M.P.; Leebeek, F.W. Von Willebrand factor and ADAMTS13 in arterial thrombosis: A systematic review and meta-analysis. Blood Rev. 2014, 28, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, W.J.; van der Merwe, P. Comparison of Commercially Available Blood Collection Tubes Containing Sodium Citrate and Hirudin in Platelet Aggregation Testing. Med. Sci. Monit. Basic Res. 2017, 23, 264–269. [Google Scholar] [CrossRef] [PubMed]
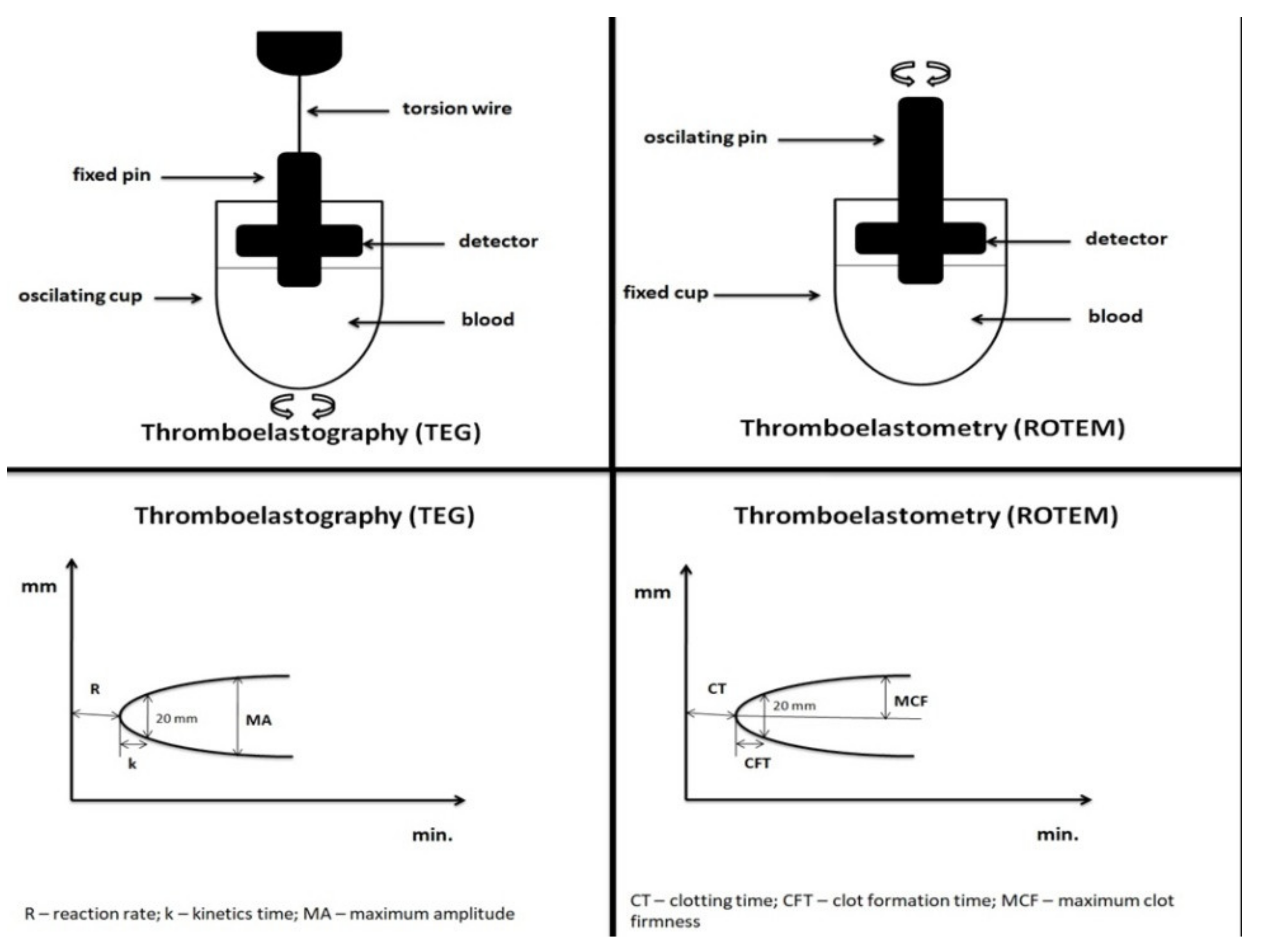
| Assay Principle | Viscoelastic hemostatic assay, uses 340 µL of whole blood in a rotating cylindrical cup while a fixed pin on a torsion wire is suspended in the blood, coagulation is activated by selected activators in a pre-defined assay | |
| Manufacturer | Haemoscope, Haemonetics, Niles, IL, USA. | |
| Platelet Function Testing | Yes (Platelet mapping assay specifically designed for thromboelastography is commercially available) | |
| Parameter | Calculation | Information Provided |
| R (Reaction rate) | the time elapsed from the coagulation trigger until the formation of a clot of 2 mm | Time from initiation of clotting process until clot starts to form |
| K (Kinetics time) | the time elapsed from 2 to 20 mm | Flags the speed of formation of a solid clot |
| MA (Maximum amplitude) | the maximum amplitude of the signal | Reflects the maximum clot strength |
| LY30 (Lysis 30) | percentage of remaining clot stability in relation to the MA value at 30 min after R | Reflects loss of clot stability |
| Assay | Description | |
| Kaolin | Kaolin acts as contact activators. | |
| Rapid TEG | Reagent contains tissue factor and kaolin as inducers. | |
| HTEG | Reagent with lipophilized heparinase to neutralize unfractionated heparin. Used in conjunction with kaolin to measure heparin effect. | |
| Functional fibrinogen | Reagent with tissue factor and abciximab (glycoprotein IIb/IIIa platelet receptor blocker), inhibiting platelet contribution to clot formation. Allows qualitative measurement of the fibrinogen contribution to clot strength independent of platelets. | |
| Native | Native whole blood sample analyzed following only recalcification. Impractical for clinical use given long R time. | |
| Platelet mapping | Assay uses heparinized blood mixed with ActivatorF (reptilase and activated factor XIII). Sufficient heparin is present to entirely suppress the generation of thrombin while fibrinogen is converted to fibrin and cross- linked due to the presence of reptilase and activated factor XIII. Subsequent addition of either arachadonic acid (AA) or adenosine diphosphate (ADP) allows measurement of the platelet activation response to these inducers in the absence of thrombin. These results are compared to kaolin analysis to determine platelet response to AA and ADP. | |
| Assay Principle | Viscoelastic hemostatic assay, uses 340 µL of whole blood in a fixed cylindrical cup while a pin suspended on a ball-bearing mechanism oscillates with the application of a constant force, coagulation is activated by selected activators in a pre-defined assays | |
| Manufacturer | Instrumentation Laboratory, Bedford, MA, USA | |
| Platelet Function Testing | No (FIBTEM indirectly assesses platelet function, platelet mapping assay could be adapted for ROTEM®) | |
| Parameter | Calculation | Information Provided |
| CT (Clotting time) | the time elapsed from the coagulation trigger until the formation of a clot of 2 mm | Time from initiation of clotting process until clot starts to form |
| CFT (Clot forming time) | the time elapsed from 2 to 20 mm | Flags the speed of formation of a solid clot |
| MCF (Maximum clot firmness) | the maximum amplitude of the signal | Reflects the maximum clot strength |
| LI 30 (Lysis index after 30 min) | percentage of remaining clot stability in relation to the MCF value at 30 min after CT | Reflects loss of clot stability |
| Assay | Activator/Inhibitor | Information Provided |
| INTEM | Contact activation | Fast assessment of clot forming, fibrin polymerization, and fibrinolysis through the intrinsic pathway |
| HEPTEM | Contact activation + heparinase | ROTEM measurement without heparin contribution: specific detection of heparin (compared to INTEM), measurement of clotting in heparinized ones |
| EXTEM | Tissue factor activation | Fast assessment of clot forming, fibrin polymerization, and fibrinolysis through the extrinsic pathway |
| FIBTEM | Tissue factor activation + platelet inhibition | ROTEM measurement without platelets: qualitative measurement of fibrinogen status |
| APTEM | Tissue factor activation + aprotinin | In vitro fibrinolysis inhibition: fast detection of lysis when compared with EXTEM |
| NATEM | Recalcification only = classical TEM (thromboelastometry) | Sensitive measurement of the equilibrium of coagulation activation or inhibition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoš, M.; Škorňová, I.; Bolek, T.; Stančiaková, L.; Korpallová, B.; Galajda, P.; Staško, J.; Kubisz, P.; Mokáň, M. Viscoelastic Hemostatic Assays and Platelet Function Testing in Patients with Atherosclerotic Vascular Diseases. Diagnostics 2021, 11, 143. https://doi.org/10.3390/diagnostics11010143
Samoš M, Škorňová I, Bolek T, Stančiaková L, Korpallová B, Galajda P, Staško J, Kubisz P, Mokáň M. Viscoelastic Hemostatic Assays and Platelet Function Testing in Patients with Atherosclerotic Vascular Diseases. Diagnostics. 2021; 11(1):143. https://doi.org/10.3390/diagnostics11010143
Chicago/Turabian StyleSamoš, Matej, Ingrid Škorňová, Tomáš Bolek, Lucia Stančiaková, Barbora Korpallová, Peter Galajda, Ján Staško, Peter Kubisz, and Marián Mokáň. 2021. "Viscoelastic Hemostatic Assays and Platelet Function Testing in Patients with Atherosclerotic Vascular Diseases" Diagnostics 11, no. 1: 143. https://doi.org/10.3390/diagnostics11010143
APA StyleSamoš, M., Škorňová, I., Bolek, T., Stančiaková, L., Korpallová, B., Galajda, P., Staško, J., Kubisz, P., & Mokáň, M. (2021). Viscoelastic Hemostatic Assays and Platelet Function Testing in Patients with Atherosclerotic Vascular Diseases. Diagnostics, 11(1), 143. https://doi.org/10.3390/diagnostics11010143





