The Role of Breast Cancer Stem Cell-Related Biomarkers as Prognostic Factors
Abstract
1. Introduction
2. Types of Stem Cells
2.1. Adult and Embryonic Stem Cells
2.2. Cancer Stem Cells (CSCs)
2.3. Breast Cancer Stem Cells (BCSCs)
3. Prognostic Value of BCSC-Related Biomarkers
3.1. Oct4
3.2. Nanog
3.3. CD133
3.4. CD44
3.5. ALDH-1
3.6. Autophagy-Related Genes (Autophagy Protein Light Chain 3 (LC3) and Beclin-1)
3.7. Zinc Finger E-box-Binding Homeobox 1 (ZEB1)
3.8. Other Potential Prognostic Markers
4. miRNAs in Stem Cells and Cancer Stem Cells
5. Prognostic Value of BCSC-Related MiRNAs
5.1. miR-1
5.2. Let-7 miRNA
5.3. miR-9 and miR-221
5.4. miR-24
5.5. miR-27a
5.6. miR-125a
5.7. miR-128
5.8. miR-142
5.9. miR-200
5.10. miR-205
5.11. miR-210
5.12. miR-495
5.13. miR-590-5p
5.14. miR-628
5.15. miR-638
5.16. miR-760
5.17. Other miRNAs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| AKT | Protein kinase B |
| ALDH1 | Aldehyde Dehydrogenase 1 |
| BMI-1 | B-lymphoma Mo-MLV insertion region 1 homolog |
| BIML | BCL21-interacting Protein BIM |
| BCSCs | Breast cancer stem cells |
| CSCs | Cancer stem cells |
| CA12 | Carbonic Anhydrase 12 |
| Dnmt1 | DNA (cytosine-5)-methyltransferase 1 |
| E2F2 | E2F Transcription Factor 2 |
| EMT | Epithelial–mesenchymal transition |
| ER | Oestrogen receptor |
| ESA | Epithelial specific antigen |
| FOXP2 | Forkhead box protein P2 |
| H-RAS | Harvey rat sarcoma viral oncogene homolog |
| HMGA2 | High-mobility group AT-hook 2 |
| HER2 | Human epidermal growth factor receptor 2 |
| MICA and MICB | MHC class I chain-related protein A and B |
| miRNAs | MicroRNAs |
| mRNA | Messenger RNA |
| NEK2 | Serine/threonine-protein kinase Nek2 |
| NK cells | Natural killer cells |
| Oct-4 | Octamer binding transcription factor 4 |
| PARP1 | Poly (ADP-ribose) polymerase 1 |
| PI3K | Phosphoinositide 3-kinase |
| POU5F1 | POU Class 5 Homeobox 1 |
| PR | Progesterone receptor |
| PTEN | Phosphatase and tensin homolog |
| REDD1 | Regulated in development and DNA damage responses 1 |
| RT-PCR | Real time polymerase chain reaction |
| RUNX2 | Runt-related transcription factor 2 |
| SALL4 | Sal-like protein 4 |
| SOS1 | Son of sevenless homolog 1 |
| SOX2 | SRY (sex determining region Y)-box 2 |
| Src | SRC Proto-Oncogene, Non-Receptor Tyrosine Kinase |
| SUZ12 | SUZ12 Polycomb Repressive Complex 2 Subunit |
| TNBC | Triple negative breast cancer |
| TNM stage | tumour (T), nodes (N), and metastases (M) stage |
| Twist1 | Twist-related protein 1 |
| uPAR | Urokinase receptor |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless-related integration site |
| ZEB1 | Zinc Finger E-Box Binding Homeobox 1 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Azizah, A.M.; Nor Saleha, I.T.; Noor Hashimah, A.; Asmah, Z.A.; Mastulu, W. Malaysian National Cancer Registry Report 2007–2011; National Cancer Institute, Ministry of Health: Putrajaya, Malaysia, 2016. Available online: https://www.crc.gov.my/wp-content/uploads/documents/report/MNCRRrepor2007-2011.pdf (accessed on 19 September 2020).
- Azizah, A.M.; Hashimah, B.; Nirmal, K.; Siti Zubaidah, A.R.; Puteri, N.A.; Nabihah, A.; Sukumaran, R.; Balqis, B.; Nadia, S.M.R.; Sharifah, S.S.S.; et al. National Cancer Registry Report 2012–2016; National Cancer Registry Department, National Cancer Institute: Putrajaya, Malaysia, 2019; Available online: https://drive.google.com/file/d/1BuPWrb05N2Jez6sEP8VM5r6JtJtlPN5W/view (accessed on 19 September 2020).
- Donovan, P.J.; Gearhart, J. The end of the beginning for pluripotent stem cells. Nature 2001, 414, 92–97. [Google Scholar] [CrossRef]
- Hall, P.A.; Watt, F.M. Stem cells: The generation and maintenance of cellular diversity. Development 1989, 106, 619–633. [Google Scholar] [PubMed]
- Spillane, J.B.; Henderson, M.A. Cancer stem cells: A review. ANZ. J. Surg. 2007, 77, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Gentile, P.; Fabbri, G.; Cervelli, V.; Orlandi, A. The role of breast cancer stem cells as a prognostic marker and a target to improve the efficacy of breast cancer therapy. Cancers 2019, 11, 1021. [Google Scholar] [CrossRef]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef]
- Desai, A.; Yan, Y.; Gerson, S.L. Concise reviews: Cancer stem cell targeted therapies: Toward clinical success. Stem Cells Transl. Med. 2019, 8, 75–81. [Google Scholar] [CrossRef]
- Dragu, D.L.; Necula, L.G.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Therapies targeting cancer stem cells: Current trends and future challenges. World J. Stem Cells 2015, 7, 1185–1201. [Google Scholar]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Toledo-Guzmán, M.E.; Bigoni-Ordóñez, G.D.; Ibáñez Hernández, M.; Ortiz-Sánchez, E. Cancer stem cell impact on clinical oncology. World J. Stem Cells. 2018, 10, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Ricardo, S.; Vieira, A.F.; Gerhard, R.; Leitão, D.; Pinto, R.; Cameselle-Teijeiro, J.F.; Milanezi, F.; Schmitt, F.; Paredes, J. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J Clin. Pathol. 2011, 64, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Gwak, J.M.; Kim, M.; Kim, H.J.; Jang, M.H.; Park, S.Y. Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance. Oncotarget 2017, 8, 36305–36318. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Stender, J.D.; Joshi, S.; Wu, G.; Katzenellenbogen, B.S. OCT-4: A novel estrogen receptor-α collaborator that promotes tamoxifen resistance in breast cancer cells. Oncogene 2016, 35, 5722–5734. [Google Scholar] [CrossRef]
- Rasti, A.; Mehrazma, M.; Madjd, Z.; Abolhasani, M.; Zanjani, L.S.; Asgari, M. Co-expression of cancer stem cell markers OCT4 and NANOG predicts poor prognosis in renal cell carcinomas. Sci. Rep. 2018, 8, 11739. [Google Scholar] [CrossRef]
- Kosaka, T.; Mikami, S.; Yoshimine, S.; Miyazaki, Y.; Daimon, T.; Kikuchi, E.; Miyajima, A.; Oya, M. The prognostic significance of OCT4 expression in patients with prostate cancer. Hum. Pathol. 2016, 51, 1–8. [Google Scholar] [CrossRef]
- Huang, P.; Chen, J.; Wang, L.; Na, Y.; Kaku, H.; Ueki, H.; Sasaki, K.; Yamaguchi, K.; Zhang, K.; Saika, T.; et al. Implications of transcriptional factor, OCT-4, in human bladder malignancy and tumor recurrence. Med. Oncol. 2012, 29, 829–834. [Google Scholar] [CrossRef]
- Kim, B.W.; Cho, H.; Choi, C.H.; Ylaya, K.; Chung, J.Y.; Kim, J.H.; Hewitt, S.M. Clinical significance of OCT4 and SOX2 protein expression in cervical cancer. BMC Cancer 2015, 15, 1015. [Google Scholar] [CrossRef]
- Zhai, Y.; Han, Y.; Han, Z. Aberrant expression of WWOX and its association with cancer stem cell biomarker expression. Int. J. Clin. Exp. Pathol. 2020, 13, 1176–1184. [Google Scholar] [PubMed]
- Croker, A.K.; Goodale, D.; Chu, J.; Postenka, C.; Hedley, B.D.; Hess, D.A.; Allan, A.L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell Mol. Med. 2009, 13(8B), 2236–2252. [Google Scholar] [CrossRef] [PubMed]
- Borgna, S.; Armellin, M.; di Gennaro, A.; Maestro, R.; Santarosa, M. Mesenchymal traits are selected along with stem features in breast cancer cells grown as mammospheres. Cell Cycle 2012, 11, 4242–4251. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, F.; Grassilli, S.; Al-Qassab, Y.; Capitani, S.; Bertagnolo, V. CD133 in breast cancer cells: More than a stem cell marker. J. Oncol. 2019, 2019, 7512632. [Google Scholar] [CrossRef] [PubMed]
- Currie, M.J.; Beardsley, B.E.; Harris, G.C.; Gunningham, S.P.; Dachs, G.U.; Dijkstra, B.; Morrin, H.R.; Wells, J.E.; Robinson, B.A. Immunohistochemical analysis of cancer stem cell markers in invasive breast carcinoma and associated ductal carcinoma in situ: Relationships with markers of tumor hypoxia and microvascularity. Hum. Pathol. 2013, 44, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Xia, P. CD133 mRNA may be a suitable prognostic marker for human breast cancer. Stem Cell Investig. 2017, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Arshad, M.; Kurozomi, S.; Althobiti, M.; Miligy, I.M.; Al-Izzi, S.; Toss, M.S.; Goh, F.Q.; Johnston, S.J.; Martin, S.G.; et al. Overexpression of the cancer stem cell marker CD133 confers a poor prognosis in invasive breast cancer. Breast Cancer Res. Treat 2019, 174, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, F.; Grassilli, S.; Piazzi, M.; Palomba, M.; Nika, E.; Bavelloni, A.; Capitani, S.; Bertagnolo, V. In triple negative breast tumor cells, PLC-β2 promotes the conversion of CD133high to CD133low phenotype and reduces the CD133-related invasiveness. Mol. Cancer 2013, 12, 165. [Google Scholar] [CrossRef]
- Zhao, P.; Lu, Y.; Jiang, X.; Li, X. Clinicopathological significance and prognostic value of CD133 expression in triple-negative breast carcinoma. Cancer Sci. 2011, 102, 1107–1111. [Google Scholar] [CrossRef]
- Cantile, M.; Collina, F.; D’Aiuto, M.; Rinaldo, M.; Pirozzi, G.; Borsellino, C.; Franco, R.; Botti, G.; Di Bonito, M. Nuclear localization of cancer stem cell marker CD133 in triple-negative breast cancer: A case report. Tumori 2013, 99, e245–e250. [Google Scholar] [CrossRef]
- Collina, F.; Di Bonito, M.; Li Bergolis, V.; De Laurentiis, M.; Vitagliano, C.; Cerrone, M.; Nuzzo, F.; Cantile, M.; Botti, G. Prognostic value of cancer stem cells markers in triple-negative breast cancer. Biomed Res. Int. 2015, 2015, 158682. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cong, Y.; Wang, D.; Sun, Y.; Deng, L.; Liu, Y.; Martin-Trevino, R.; Shang, L.; McDermott, S.P.; Landis, M.D.; et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2013, 2, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Charafe-Jauffret, E.; Ginestier, C.; Bertucci, F.; Cabaud, O.; Wicinski, J.; Finetti, P.; Josselin, E.; Adelaide, J.; Nguyen, T.T.; Monville, F.; et al. ALDH1-positive cancer stem cells predict engraftment of primary breast tumors and are governed by a common stem cell program. Cancer Res. 2013, 73, 7290–7300. [Google Scholar] [CrossRef]
- Xu, H.; Tian, Y.; Yuan, X.; Liu, Y.; Wu, H.; Liu, Q.; Wu, G.S.; Wu, K. Enrichment of CD44 in basal-type breast cancer correlates with EMT, cancer stem cell gene profile, and prognosis. Onco. Targets Ther. 2016, 9, 431–444. [Google Scholar]
- Watanabe, O.; Kinoshita, J.; Shimizu, T.; Imamura, H.; Hirano, A.; Okabe, T.; Aiba, M.; Ogawa, K. Expression of a CD44 variant and VEGF-C and the implications for lymphatic metastasis and long-term prognosis of human breast cancer. J. Exp. Clin Cancer Res. 2005, 24, 75–82. [Google Scholar]
- Niitsu, N.; Iijima, K. High serum soluble CD44 is correlated with a poor outcome of aggressive non-Hodgkin’s lymphoma. Leuk. Res. 2002, 26, 241–248. [Google Scholar] [CrossRef]
- Lee, S.C.; Harn, H.J.; Lin, T.S.; Yeh, K.T.; Liu, Y.C.; Tsai, C.S.; Cheng, Y.L. Prognostic significance of CD44v5 expression in human thymic epithelial neoplasms. Ann. Thorac. Surg. 2003, 76, 213–218. [Google Scholar] [CrossRef]
- Sun, X.; Gong, Y.; Talamonti, M.S.; Rao, M.S. Expression of cell adhesion molecules, CD44s and E-cadherin, and microvessel density in carcinoid tumors. Mod. Pathol. 2002, 15, 1333–1338. [Google Scholar] [CrossRef][Green Version]
- Montgomery, E.; Abraham, S.C.; Fisher, C.; Deasel, M.R.; Amr, S.S.; Sheikh, S.S.; House, M.; Lilliemoe, K.; Choti, M.; Brock, M.; et al. CD44 loss in gastric stromal tumors as a prognostic marker. Am. J. Surg. Pathol. 2004, 28, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Esteban, F.; Bravo, J.J.; González-Moles, M.A.; Bravo, M.; Ruiz-Avila, I.; Gil-Montoya, J.A. Adhesion molecule CD44 as a prognostic factor in laryngeal cancer. Anticancer Res. 2005, 25, 1115–1121. [Google Scholar]
- Pirinen, R.; Hirvikoski, P.; Böhm, J.; Moisio, K.; Viren, M.; Johansson, R.; Hollmen, S.; Kosma, V.M. Reduced expression of CD44v3 variant isoform is associated with unfavorable outcome in non-small cell lung carcinoma. Hum. Pathol. 2000, 31, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Combaret, V.; Gross, N.; Lasset, C.; Frappaz, D.; Peruisseau, G.; Philip, T.; Beck, D.; Favrot, M.C. Clinical relevance of CD44 cell-surface expression and N-myc gene amplification in a multicentric analysis of 121 pediatric neuroblastomas. J. Clin. Oncol. 1996, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, S.; Anttila, M.A.; Voutilainen, K.; Tammi, R.H.; Tammi, M.I.; Saarikoski, S.V.; Kosma, V.M. CD44 expression indicates favorable prognosis in epithelial ovarian cancer. Clin. Cancer Res. 2003, 9, 5318–5324. [Google Scholar] [PubMed]
- Balicki, D. Moving forward in human mammary stem cell biology and breast cancer prognostication using ALDH1. Cell Stem Cell 2007, 1, 485–487. [Google Scholar] [CrossRef]
- Shima, H.; Yamada, A.; Ishikawa, T.; Endo, I. Are breast cancer stem cells the key to resolving clinical issues in breast cancer therapy? Gland Surg. 2017, 6, 82–88. [Google Scholar] [CrossRef]
- Ma, F.; Li, H.; Li, Y.; Ding, X.; Wang, H.; Fan, Y.; Lin, C.; Qian, H.; Xu, B. Aldehyde dehydrogenase 1 (ALDH1) expression is an independent prognostic factor in triple negative breast cancer (TNBC). Med. 2017, 96, e6561. [Google Scholar] [CrossRef]
- Panigoro, S.S.; Kurnia, D.; Kurnia, A.; Haryono, S.J.; Albar, Z.A. ALDH1 cancer stem cell marker as a prognostic factor in triple-negative breast cancer. Int. J. Surg. Oncol. 2020, 2020, 7863243. [Google Scholar] [CrossRef]
- Chang, S.J.; Ou-Yang, F.; Tu, H.P.; Lin, C.H.; Huang, S.H.; Kostoro, J.; Hou, M.F.; Chai, C.Y.; Kwan, A.L. Decreased expression of autophagy protein LC3 and stemness (CD44+/CD24-/low) indicate poor prognosis in triple-negative breast cancer. Hum. Pathol. 2016, 48, 48–55. [Google Scholar] [CrossRef]
- Hamurcu, Z.; Delibaşı, N.; Geçene, S.; Sener, E.F.; Dönmez-Altuntaş, H.; Özkul, Y.; Canatan, H.; Ozpolat, B. Targeting LC3 and Beclin-1 autophagy genes suppresses proliferation, survival, migration and invasion by inhibition of Cyclin-D1 and uPAR/Integrin β1/ Src signaling in triple negative breast cancer cells. J. Cancer Res. Clin. Oncol. 2018, 144, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Kim, H.J.; Kim, E.J.; Chung, Y.R.; Park, S.Y. Expression of epithelial-mesenchymal transition-related markers in triple-negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome. Hum. Pathol. 2015, 46, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Eger, A.; Aigner, K.; Sonderegger, S.; Dampier, B.; Oehler, S.; Schreiber, M.; Berx, G.; Cano, A.; Beug, H.; Foisner, R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 2005, 24, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- El Abbass, K.A.; Abdellateif, M.S.; Gawish, A.M.; Zekri, A.N.; Malash, I.; Bahnassy, A.A. The role of breast cancer stem cells and some related molecular biomarkers in metastatic and nonmetastatic breast cancer. Clin. Breast Cancer 2020, S1526-820930756-6. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Zhang, X.Y.; Zhou, J.P.; Mu, G.N.; Li, Y.W.; Zhang, Y.X.; Pang, D. Transcriptome sequencing of HER2-positive breast cancer stem cells identifies potential prognostic marker. Tumour Biol. 2016, 37, 14757–14764. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.C.; Chan, E.; Molnar, A.; Sarkar, R.; Alexieva, D.; Isa, I.M.; Robinson, S.; Zhang, S.; Ellis, P.; Langford, C.F.; et al. 5’ isomiR variation is of functional and evolutionary importance. Nucleic Acids Res. 2014, 42, 9424–9435. [Google Scholar] [CrossRef]
- Tan, G.C.; Dibb, N. IsomiRs have functional importance. Malays. J. Pathol. 2015, 37, 73–81. [Google Scholar]
- Tan, G.C.; Wong, Y.P.; Cui, W.; Dibb, N. Construction of a doxycycline inducible lentivirus that expresses stem cell-specific miR-302 cluster. Malays. J. Pathol. 2020, 42, 91–97. [Google Scholar]
- Shimono, Y.; Mukohyama, J.; Nakamura, S.; Minami, H. MicroRNA regulation of human breast cancer stem cells. J. Clin. Med. 2015, 5, 2. [Google Scholar] [CrossRef]
- Xu, Y.F.; Hannafon, B.N.; Ding, W.Q. microRNA regulation of human pancreatic cancer stem cells. Stem Cell Investig. 2017, 4, 5. [Google Scholar] [CrossRef]
- Mukohyama, J.; Isobe, T.; Hu, Q.; Hayashi, T.; Watanabe, T.; Maeda, M.; Yanagi, H.; Qian, X.; Yamashita, K.; Minami, H.; et al. miR-221 targets QKI to enhance the tumorigenic capacity of human colorectal cancer stem cells. Cancer Res. 2019, 79, 5151–5158. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Yue, F.; Xian, X.; Ren, Q.; Cui, H.; Wang, Y. Inhibiting effect of MicroRNA-3619-5p/PSMD10 axis on liver cancer cell growth in vivo and in vitro. Life Sci. 2020, 254, 117632. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.O.N.; Deng, W.; Ye, T.M.; Ngan, H.Y.S.; Tsao, S.W.; Cheung, A.N.Y.; Ziru, N.; Yuen, D.C.K.; Pang, R.T.K.; Yeung, W.S.B. MicroRNA-135a-induced formation of CD133+ subpopulation with cancer stem cell properties in cervical cancer. Carcinogenesis 2020, bgaa025. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbacher, D.; Balic, M.; Pichler, M. The role of microRNAs in breast cancer stem cells. Int. J. Mol. Sci. 2013, 14, 14712–14723. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Yu, J.C.; Hsieh, Y.H.; Liao, W.L.; Shieh, J.C.; Yao, C.C.; Lee, H.J.; Chen, P.M.; Wu, P.E.; Shen, C.Y. Increased cellular levels of microRNA-9 and microRNA-221 correlate with cancer stemness and predict poor outcome in human breast cancer. Cell Physiol. Biochem. 2018, 48, 2205–2218. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Q.; Wang, Z.; Jiang, J.; Yu, S.C.; Ping, Y.F.; Yang, J.; Xu, S.L.; Ye, X.Z.; Xu, C.; et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014, 74, 5746–5757. [Google Scholar] [CrossRef]
- Roscigno, G.; Puoti, I.; Giordano, I.; Donnarumma, E.; Russo, V.; Affinito, A.; Adamo, A.; Quintavalle, C.; Todaro, M.; Vivanco, M.D.; et al. MiR-24 induces chemotherapy resistance and hypoxic advantage in breast cancer. Oncotarget 2017, 8, 19507–19521. [Google Scholar] [CrossRef]
- Tang, W.; Yu, F.; Yao, H.; Cui, X.; Jiao, Y.; Lin, L.; Chen, J.; Yin, D.; Song, E.; Liu, Q. miR-27a regulates endothelial differentiation of breast cancer stem like cells. Oncogene 2014, 33, 2629–2638. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, J.; Su, S.; Wu, W.; Liu, Q.; Su, F.; Yu, F. MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS ONE 2012, 7, e51702. [Google Scholar] [CrossRef]
- Nandy, S.B.; Arumugam, A.; Subramani, R.; Pedroza, D.; Hernandez, K.; Saltzstein, E.; Lakshmanaswamy, R. MicroRNA-125a influences breast cancer stem cells by targeting leukemia inhibitory factor receptor which regulates the Hippo signaling pathway. Oncotarget 2015, 6, 17366–17378. [Google Scholar] [CrossRef]
- Isobe, T.; Hisamori, S.; Hogan, D.J.; Zabala, M.; Hendrickson, D.G.; Dalerba, P.; Cai, S.; Scheeren, F.; Kuo, A.H.; Sikandar, S.S.; et al. miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. eLife 2014, 3, e01977. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, A.Y.; Fan, C.; Zheng, H.; Li, Y.; Zhang, C.; Wu, S.; Yu, D.; Huang, Z.; Liu, F.; et al. MicroRNA-33b Inhibits Breast Cancer Metastasis by Targeting HMGA2, SALL4 and Twist1. Sci Rep. 2015, 5, 9995. [Google Scholar] [CrossRef] [PubMed]
- Shimono, Y.; Ugalde, M.Z.; Cho, R.W.; Lobo, N.; Dalerba, P.; Qian, D.; Diehn, M.; Liu, H.; Panula, S.P.; Chiao, E.; et al. Down-regulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009, 138, 592–603. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodal, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Lindahl-Allen, M.; Polytarchou, C.; Hirsch, H.A.; Tsichlis, P.N.; Struhl, K. Loss of miR-200 inhibition of SUZ12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol. Cell 2010, 39, 761–772. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Pardal, R.; Iwashita, T.; Park, I.K.; Clarke, M.F.; Morrison, S.J. BMI-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003, 425, 962–967. [Google Scholar] [CrossRef]
- Tang, T.; Yang, Z.; Zhu, Q.; Wu, Y.; Sun, K.; Alahdal, M.; Zhang, Y.; Xing, Y.; Shen, Y.; Xia, T.; et al. Up-regulation of miR-210 induced by a hypoxic microenvironment promotes breast cancer stem cells metastasis, proliferation, and self-renewal by targeting E-cadherin. FASEB J. 2018, fj201801013R. [Google Scholar] [CrossRef]
- Hwang-Verslues, W.W.; Chang, P.H.; Wei, P.C.; Yang, C.Y.; Huang, C.K.; Kuo, W.H.; Shew, J.Y.; Chang, K.J.; Lee, Y.H.P.; Lee, W.H. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene 2011, 30, 2463–2474. [Google Scholar] [CrossRef]
- Liu, T.; Hu, K.; Zhao, Z.; Chen, G.; Ou, X.; Zhang, H.; Zhang, X.; Wei, X.; Wang, D.; Cui, M.; et al. MicroRNA-1 down-regulates proliferation and migration of breast cancer stem cells by inhibiting the Wnt/β-catenin pathway. Oncotarget 2015, 6, 41638–41649. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, C.; Zhang, X. Mitochondrial Damage Mediated by miR-1 Overexpression in Cancer Stem Cells. Mol. Ther. Nucleic Acids 2019, 18, 938–953. [Google Scholar] [CrossRef]
- Yu, F.; Yao, H.; Zhu, P.; Zhang, X.; Pan, Q.; Gong, C.; Huang, Y.; Hu, X.; Su, F.; Lieberman, J.; et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007, 131, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Pode-Shakked, N.; Shukrun, R.; Mark-Danieli, M.; Tsvetkov, P.; Bahar, S.; Pri-Chen, S.; Goldstein, R.S.; Rom-Gross, E.; Mor, Y.; Fridman, E.; et al. The isolation and characterization of renal cancer initiating cells from human Wilms’ tumour xenografts unveils new therapeutic targets. EMBO Mol. Med. 2013, 5, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.E. Let-7 and miR-200 microRNAs: Guardians against pluripotency and cancer progression. Cell Cycle. 2009, 8, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, N.; Liu, L.; Dong, H.; Liu, X. microRNA-128-3p overexpression inhibits breast cancer stem cell characteristics through suppression of Wnt signalling pathway by down-regulating NEK2. J. Cell Mol. Med. 2020, 24, 7353–7369. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Xu, H.; Wang, G.; Fan, C.; Zheng, Q.; Li, F. miR-205/RunX2 axis negatively regulates CD44+/CD24− breast cancer stem cell activity. Am. J. Cancer Res. 2020, 10, 1871–1887. [Google Scholar]
- Zhou, L.; Zhao, L.C.; Jiang, N.; Wang, X.L.; Zhou, X.N.; Luo, X.L.; Ren, J. MicroRNA miR-590-5p inhibits breast cancer cell stemness and metastasis by targeting SOX2SOX2. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 87–94. [Google Scholar]
- Lin, C.; Gao, B.; Yan, X.; Lei, Z.; Chen, K.; Li, Y.; Zeng, Q.; Chen, Z.; Li, H. MicroRNA 628 suppresses migration and invasion of breast cancer stem cells through targeting SOS1. Onco. Targets Ther. 2018, 11, 5419–5428. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Wang, J.Q.; Wu, L.L.; Zheng, W.E.; Chen, P.R. miR-638 represses the stem cell characteristics of breast cancer cells by targeting E2F2. Breast Cancer. 2020, 27, 147–158. [Google Scholar] [CrossRef]
- Han, M.L.; Wang, F.; Gu, Y.T.; Pei, X.H.; Guo, G.C.; Li, L.; Duan, X.; Zhu, M.Z.; Wang, Y.M. MicroR-760 suppresses cancer stem cell subpopulation and breast cancer cell proliferation and metastasis: By down-regulating NANOG. Biomed. Pharmacother. 2016, 80, 304–310. [Google Scholar] [CrossRef]
- Das, P.K.; Siddika, M.A.; Asha, S.Y.; Aktar, S.; Rakib, M.A.; Khanam, J.A.; Pillai, S.; Islam, F. MicroRNAs, a promising target for breast cancer stem cells. Mol. Diagn. Ther. 2020, 24, 69–83. [Google Scholar] [CrossRef]
- Cuiffo, B.G.; Campagne, A.; Bell, G.W.; Lembo, A.; Orso, F.; Lien, C.E.; Bhasin, M.K.; Raimo, M.; Hanson, S.E.; Marusyk, A.; et al. MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell. 2014, 15, 762–774. [Google Scholar] [CrossRef] [PubMed]
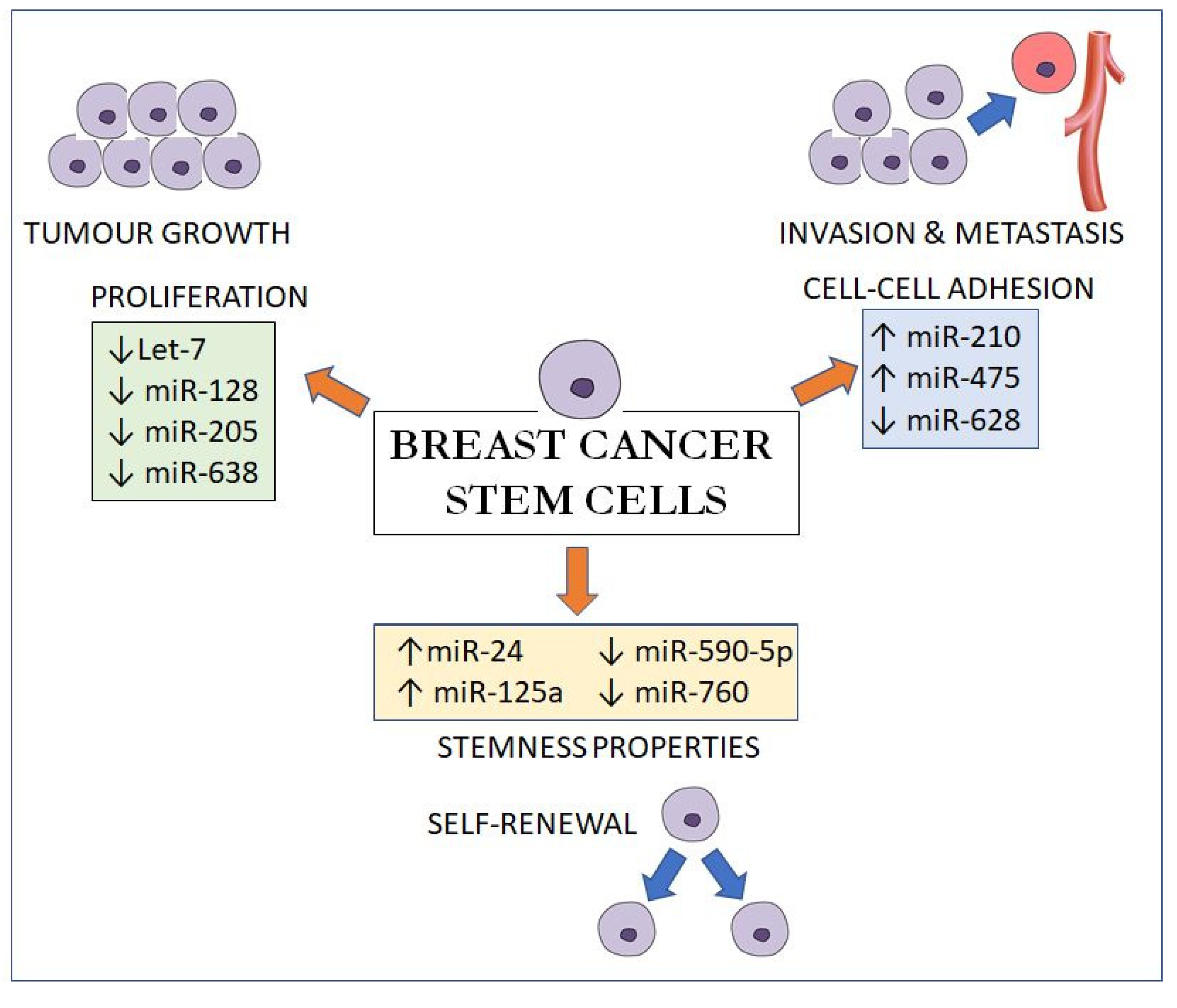
| Upregulated miRNAs | Remarks | References | |
|---|---|---|---|
| 1 | miR-9/221 | Increases tumour size, poor differentiation, lymph node metastasis and lower overall survival | [67] |
| 2 | miR-20a | Downregulates MICA/MICB to promote the resistance of BCSCs to NK cell cytotoxicity | [68] |
| 3 | miR-24 | Induces the upregulation of Oct3-4 and Nanog, as well as the expression of antiapoptotic BIML | [69] |
| 4 | miR-27a | VEGF increases miR-27a and, together, promote angiogenesis and tumour metastasis | [70,71] |
| 5 | miR-125a | Promotes SOX2 expression | [72] |
| 6 | miR-142 | Regulates BCSCs properties by in part activating the WNT signalling pathway | [73] |
| 7 | miR-199a | Suppresses FOXP2 | [74] |
| 8 | miR-200 | Modulates CSC self-renewal via BMI-1 and SUZ12 | [75,76,77,78] |
| 9 | miR-210 | miR-210 is reduced in the hypoxic environment and, in turn, promotes BCSC proliferation, self-renewal and metastasis by targeting E-cadherin | [79] |
| 10 | miR-495 | Promotes cell invasion by suppressing E-cadherin | [80] |
| Downregulated miRNAs | |||
| 1 | miR-1 | Increases in basal-like subtype Triggered mitophagy of cancer stem cells by targeting MINOS1 and GPD2 mRNAs | [81,82] |
| 2 | let-7 | Suppresses self-renewal and differentiation by targeting HRAS and HMGA2 | [83,84,85] |
| 3 | miR-33b | Targets HMGA2, SALL4 and TWIST1 | [74] |
| 4 | miR-128 | Inhibits proliferation, migration, invasion, self-renewal and tumorigenesis by downregulating NEK2 | [86] |
| 5 | miR-205 | A tumour suppressor that inhibits breast cancer malignancy by regulating RUNX2 | [87] |
| 6 | miR-590-5p | Inhibits breast cancer cell stemness by downregulating SOX2 | [88] |
| 7 | miR-628 | Suppresses BCSCs invasiveness by targeting SOS1 and enhancing E-cadherin | [89] |
| 8 | miR-638 | Reciprocal effect with E2F2 in BCSCs, inhibiting self-renewal, proliferation and invasion | [90] |
| 9 | miR-760 | Inhibits cell proliferation and migration by downregulating Nanog | [91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, C.C.H.; Chia, W.K.; Selvarajah, G.T.; Cheah, Y.K.; Wong, Y.P.; Tan, G.C. The Role of Breast Cancer Stem Cell-Related Biomarkers as Prognostic Factors. Diagnostics 2020, 10, 721. https://doi.org/10.3390/diagnostics10090721
Ko CCH, Chia WK, Selvarajah GT, Cheah YK, Wong YP, Tan GC. The Role of Breast Cancer Stem Cell-Related Biomarkers as Prognostic Factors. Diagnostics. 2020; 10(9):721. https://doi.org/10.3390/diagnostics10090721
Chicago/Turabian StyleKo, Clarence Ching Huat, Wai Kit Chia, Gayathri Thevi Selvarajah, Yoke Kqueen Cheah, Yin Ping Wong, and Geok Chin Tan. 2020. "The Role of Breast Cancer Stem Cell-Related Biomarkers as Prognostic Factors" Diagnostics 10, no. 9: 721. https://doi.org/10.3390/diagnostics10090721
APA StyleKo, C. C. H., Chia, W. K., Selvarajah, G. T., Cheah, Y. K., Wong, Y. P., & Tan, G. C. (2020). The Role of Breast Cancer Stem Cell-Related Biomarkers as Prognostic Factors. Diagnostics, 10(9), 721. https://doi.org/10.3390/diagnostics10090721





