The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and κ and μ Opioid Receptors Are Associated with Interleukin-6
Abstract
1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Measurements
2.2.1. Clinical Evaluations
2.2.2. Assays
2.3. Statistical Analysis
3. Results
3.1. Socio–Demographic Data
3.2. Differences in Biomarkers between the Study Groups
3.3. Effects of Background Variables
3.4. Prediction of Symptom Domains by Biomarkers
3.5. Prediction of Cognitive Impairments by Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SCZ | schizophrenia |
| IRS | immune-inflammatory response system |
| IL | Interleukin |
| TNF | tumor necrosis factor |
| Th | T helper |
| CIRS | compensatory immune regulatory system |
| TRS | treatment resistant schizophrenia |
| NRTT | non-responders to treatment |
| EO | endogenous opioids |
| MOR | mu-opioid receptor |
| KOR | kappa-opioid receptor |
| EOS | endogenous opioid system |
| PRTT | partial responders to treatment |
| CGI | Clinical Global Impression |
| CRP | C reactive protein |
| MINI | Mini-International Neuropsychiatric Interview |
| CGI-I | CGI Improvement |
| CGI-S | CGI Severity |
| SANS | the Scale for the Assessments of Negative Symptoms |
| FTD | formal thought disorders |
| PMR | psychomotor retardation |
| BPRS | Brief Psychiatric Rating Scale |
| PANNS | Positive and Negative Syndrome Scale |
| BACS | Brief Assessment of Cognition in Schizophrenia |
| TUD | tobacco use disorder |
| BMI | body mass index |
| GLM | generalized linear model |
References
- Smith, R.S.; Maes, M. The macrophage-T-lymphocyte theory of schizophrenia: Additional evidence. Med. Hypotheses 1995, 45, 135–141. [Google Scholar] [CrossRef]
- Noto, C.; Maes, M.; Ota, V.K.; Teixeira, A.L.; Bressan, R.A.; Gadelha, A.; Brietzke, E. High predictive value of immune-inflammatory biomarkers for schizophrenia diagnosis and association with treatment resistance. World J. Biol. Psychiatry 2015, 16, 422–429. [Google Scholar] [CrossRef]
- Rubesa, G.; Gudelj, L.; Makovac, D. J Immunological characteristics of schizophrenia. Psychiatr. Danub. 2018, 30, 180–187. [Google Scholar] [PubMed]
- Al-Hakeim, H.K.; Almulla, A.F.; Maes, M. The neuroimmune and neurotoxic fingerprint of major neurocognitive psychosis or deficit schizophrenia: A supervised machine learning study. Neurotox. Res. 2020, 37, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Almulla, A.F.; Al-Hakeim, H.K.; Abed, M.S.; Carvalho, A.F.; Maes, M. Chronic fatigue and fibromyalgia symptoms are key components of deficit schizophrenia and are strongly associated with activated immune-inflammatory pathways. Schizophr. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Sirivichayakul, S.; Matsumoto, A.K.; Maes, A.; Michelin, A.P.; de Oliveira Semeão, L.; de Lima Pedrão, J.V.; Moreira, E.G.; Barbosa, D.S.; Geffard, M. Increased Levels of Plasma Tumor Necrosis Factor-α Mediate Schizophrenia Symptom Dimensions and Neurocognitive Impairments and Are Inversely Associated with Natural IgM Directed to Malondialdehyde and Paraoxonase 1 Activity. Mol. Neurobiol. 2020, 57, 2333–2345. [Google Scholar] [CrossRef]
- Noto, M.N.; Maes, M.; Nunes, S.O.V.; Ota, V.K.; Rossaneis, A.C.; Verri, W.A., Jr.; Cordeiro, Q.; Belangero, S.I.; Gadelha, A.; Bressan, R.A. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur. Neuropsychopharmacol. 2019, 29, 416–431. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Noto, C.; Kanchanatawan, B.; Anderson, G.; Kubera, M.; Carvalho, A.F.; Maes, M. The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: The IRS-CIRS theory of schizophrenia. Mol. Neurobiol. 2020, 57, 778–797. [Google Scholar] [CrossRef] [PubMed]
- Kanchanatawan, B.; Hemrungrojn, S.; Thika, S.; Sirivichayakul, S.; Ruxrungtham, K.; Carvalho, A.F.; Geffard, M.; Anderson, G.; Maes, M. Changes in tryptophan catabolite (TRYCAT) pathway patterning are associated with mild impairments in declarative memory in schizophrenia and deficits in semantic and episodic memory coupled with increased false-memory creation in deficit schizophrenia. Mol. Neurobiol. 2018, 55, 5184–5201. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Sirivichayakul, S.; Kanchanatawan, B.; Carvalho, A.F. In schizophrenia, psychomotor retardation is associated with executive and memory impairments, negative and psychotic symptoms, neurotoxic immune products and lower natural IgM to malondialdehyde. World J. Biol. Psychiatry 2020, 21, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rammahi, D.A.; Al-Dujaili, A.H. IL-6, IL-18, sIL-2R, and TNFα proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J. Affect. Disord. 2015, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Vojdani, A.; Geffard, M.; Moreira, E.G.; Barbosa, D.S.; Michelin, A.P.; de Oliveira Semeão, L.; Sirivichayakul, S.; Kanchanatawan, B. Schizophrenia phenomenology comprises a bifactorial general severity and a single-group factor, which are differently associated with neurotoxic immune and immune-regulatory pathways. Biomol. Concepts 2019, 10, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Sirivichayakul, S.; Kanchanatawan, B.; Thika, S.; Carvalho, A.F.; Maes, M. A New Schizophrenia Model: Immune Activation is Associated with the Induction of Different Neurotoxic Products which Together Determine Memory Impairments and Schizophrenia Symptom Dimensions. CNS Neurol. Disord. Drug Targets 2019, 18, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Sirivichayakul, S.; Kanchanatawan, B.; Thika, S.; Carvalho, A.F.; Maes, M. Eotaxin, an endogenous cognitive deteriorating chemokine (ECDC), is a major contributor to cognitive decline in normal people and to executive, memory, and sustained attention deficits, formal thought disorders, and psychopathology in schizophrenia patients. Neurotox. Res. 2019, 35, 122–138. [Google Scholar]
- Maes, M.; Chiavetto, L.B.; Bignotti, S.; Tura, G.-J.B.; Pioli, R.; Boin, F.; Kenis, G.; Bosmans, E.; De Jongh, R.; Lin, A. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur. Neuropsychopharmacol. 2000, 10, 119–124. [Google Scholar] [CrossRef]
- Charles, S.J.; Farias, M.; Dunbar, R.I. The aetiology of social deficits within mental health disorders: The role of the immune system and endogenous opioids. Brain Behav. Immun. Health 2020, 1, 100003. [Google Scholar] [CrossRef]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef]
- Plein, L.M.; Rittner, H.L. Opioids and the immune system–friend or foe. Br. J. Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef]
- Li, Z.-H.; Chu, N.; Shan, L.-D.; Gong, S.; Yin, Q.-Z.; Jiang, X.H. Inducible expression of functional mu opioid receptors in murine dendritic cells. J. Neuroimmune Pharmacol. 2009, 4, 359–367. [Google Scholar] [CrossRef]
- Hu, S.; Peterson, P.K.; Chao, C.C. Kappa-opioid modulation of human microglial cell superoxide anion generation. Biochem. Pharmacol. 1998, 56, 285–288. [Google Scholar] [CrossRef]
- Sacerdote, P. Opioids and the immune system. Palliat. Med. 2006, 20, 9–15. [Google Scholar] [CrossRef]
- Finley, M.J.; Chen, X.; Bardi, G.; Davey, P.; Geller, E.B.; Zhang, L.; Adler, M.W.; Rogers, T. Bi-directional heterologous desensitization between the major HIV-1 co-receptor CXCR4 and the κ-opioid receptor. J. Neuroimmunol. 2008, 197, 114–123. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.J.; McHugh, D.P.; Magister, M.J.; Zagon, I.S. Endogenous opioid inhibition of proliferation of T and B cell subpopulations in response to immunization for experimental autoimmune encephalomyelitis. BMC Immunol. 2015, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, C. 30 years of dynorphins—New insights on their functions in neuropsychiatric diseases. Pharmacol. Ther. 2009, 123, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.H.; Myers, J.; Marques, T.R.; Rabiner, E.A.; Howes, O.D. Reduced mu opioid receptor availability in schizophrenia revealed with [11 C]-carfentanil positron emission tomographic imaging. Nat. Commun 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Trezza, V.; Damsteegt, R.; Achterberg, E.M.; Vanderschuren, L. Nucleus accumbens μ-opioid receptors mediate social reward. J. Neurosci. 2011, 31, 6362–6370. [Google Scholar] [CrossRef]
- Urban-Kowalczyk, M.; Śmigielski, J.; Strzelecki, D. Comparison of beta-endorphin and CGRP levels before and after treatment for severe schizophrenia. Neuropsychiatr. Dis. Treat. 2016, 12, 863–868. [Google Scholar] [CrossRef]
- Szűcs, E.; Büki, A.; Kékesi, G.; Horváth, G.; Benyhe, S. Mu-Opioid (MOP) receptor mediated G-protein signaling is impaired in specific brain regions in a rat model of schizophrenia. Neurosci. Lett. 2016, 619, 29–33. [Google Scholar] [CrossRef]
- Fichna, J.; Janecka, A.; Costentin, J.; Do Rego, J.C. The endomorphin system and its evolving neurophysiological role. Pharmacol. Rev. 2007, 59, 88–123. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Fadhel, S.Z.; Al-Dujaili, A.H.; Maes, M. In major depression, increased kappa and mu opioid receptor levels are associated with immune activation. Acta Neuropsychiatr. 2020, 32, 99–108. [Google Scholar] [CrossRef]
- Jenab, S.; Morris, P.L. Interleukin-6 regulation of kappa opioid receptor gene expression in primary sertoli cells. Endocrine 2000, 13, 11–15. [Google Scholar] [CrossRef]
- Börner, C.; Kraus, J.; Schröder, H.; Ammer, H.; Höllt, V. Transcriptional regulation of the human μ-opioid receptor gene by interleukin-6. Mol. Pharmacol. 2004, 66, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Mao, X.F.; Tang, X.Q.; Ali, U.; Apryani, E.; Liu, H.; Li, X.Y.; Wang, Y.X. Spinal interleukin-10 produces antinociception in neuropathy through microglial β-endorphin expression, separated from antineuroinflammation. Brain Behav. Immun. 2018, 73, 504–519. [Google Scholar] [CrossRef]
- Guy, W. Clinical Global Impressions Scale (CGI). In Handbook of Psychiatric Measures; Rush, A.J., Ed.; American Psychiatric Association: Washington, DC, USA, 2000; pp. 100–102. [Google Scholar]
- Andreasen, N.C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and theoretical foundations. Br. J. Psychiatry Suppl. 1989, 155, 49–52. [Google Scholar] [CrossRef]
- Overall, J.E.; Gorham, D.R. The brief psychiatric rating scale. Psychol. Rep. 1962, 10, 799–812. [Google Scholar] [CrossRef]
- Hamilton, M.J. A rating scale for depression. J. Neurol. Neurosurg. Psychiat. 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Keefe, R.S.; Goldberg, T.E.; Harvey, P.D.; Gold, J.M.; Poe, M.P.; Coughenour, L.J. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004, 68, 283–297. [Google Scholar] [CrossRef]
- Almulla, A.F.; Al-Hakeim, H.K.; Maes, M. Schizophrenia phenomenology revisited: Positive and negative symptoms are strongly related reflective manifestations of an underlying single trait indicating overall severity of schizophrenia. CNS Spectr. 2020, 1–10. [Google Scholar] [CrossRef]
- Volk, D.W.; Radchenkova, P.V.; Walker, E.M.; Sengupta, E.J.; Lewis, D.A. Cortical opioid markers in schizophrenia and across postnatal development. Cereb Cortex 2012, 22, 1215–1223. [Google Scholar] [CrossRef]
- Ding, S.; Chen, B.; Zheng, Y.; Lu, Q.; Liu, L.; Zhuge, Q.C. Association study of OPRM1 polymorphisms with Schizophrenia in Han Chinese population. BMC Psychiat. 2013, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Šerý, O.; Přikryl, R.; Častulík, L.; Šťastný, F. A118G polymorphism of OPRM1 gene is associated with schizophrenia. J. Mol. Neurosci. 2010, 41, 219–222. [Google Scholar] [CrossRef]
- Scarr, E.; Money, T.T.; Pavey, G.; Neo, J.; Dean, B. Mu opioid receptor availability in people with psychiatric disorders who died by suicide: A case control study. BMC Psychiat. 2012, 12, 126. [Google Scholar] [CrossRef]
- Maes, M.; Chiavetto, L.B.; Bignotti, S.; Tura, G.-J.B.; Pioli, R.; Boin, F.; Kenis, G.; Bosmans, E.; De Jongh, R.; Altamura, C.A. Increased serum interleukin-8 and interleukin-10 in schizophrenic patients resistant to treatment with neuroleptics and the stimulatory effects of clozapine on serum leukemia inhibitory factor receptor. Schizophr. Res. 2002, 54, 281–291. [Google Scholar] [CrossRef]
- Jimenez, N.; Puig, M.M.; Pol, O. Antiexudative effects of opioids and expression of κ-and δ-opioid receptors during intestinal inflammation in mice: Involvement of nitric oxide. J. Pharmacol. Exp. Ther. 2006, 316, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Hipólito, L.; Wilson-Poe, A.; Campos-Jurado, Y.; Zhong, E.; Gonzalez-Romero, J.; Virag, L.; Whittington, R.; Comer, S.D.; Carlton, S.M.; Walker, B.M. Inflammatory pain promotes increased opioid self-administration: Role of dysregulated ventral tegmental area μ opioid receptors. J. Neurosci. 2015, 35, 12217–12231. [Google Scholar] [CrossRef]
- Przewlocki, R.; Hassan, A.; Lason, W.; Epplen, C.; Herz, A.; Stein, C. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: Functional role in antinociception. Neuroscience 1992, 48, 491–500. [Google Scholar] [CrossRef]
- Panerai, A.E.; Sacerdote, P. β-endorphin in the immune system: A role at last? Immunol. Today 1997, 18, 317–319. [Google Scholar] [CrossRef]
- Menzebach, A.; Hirsch, J.; Hempelmann, G.; Welters, I. Effects of endogenous and synthetic opioid peptides on neutrophil function in vitro. Br. J. Anaesth. 2003, 91, 546–550. [Google Scholar] [CrossRef]
- Bidlack, J.M. Detection and function of opioid receptors on cells from the immune system. Clin. Diagnos Lab. Immunol. 2000, 7, 719–723. [Google Scholar] [CrossRef]
- Suzuki, S.; Chuang, T.K.; Chuang, L.F.; Doi, R.H.; Chuang, R.Y. Morphine upregulates kappa-opioid receptors of human lymphocytes. Adv. Exp. Med. Biol. 2001, 493, 81–87. [Google Scholar] [PubMed]
- Machelska, H.; Celik, M.Ö. Opioid Receptors in Immune and Glial Cells—Implications for Pain Control. Front. Immunol. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Maher, D.P.; Walia, D.; Heller, N.M. Morphine decreases the function of primary human natural killer cells by both TLR4 and opioid receptor signaling. Brain Behav. Immun. 2020, 83, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Sharp, B.M. Multiple opioid receptors on immune cells modulate intracellular signaling. Brain Behav. Immun. 2006, 20, 9–14. [Google Scholar] [CrossRef]
- Suzuki, S.; Miyagi, T.; Chuang, T.K.; Chuang, L.F.; Doi, R.H.; Chuang, R.Y. Morphine upregulates mu opioid receptors of human and monkey lymphocytes. Biochem. Biophys Res. Commun. 2000, 279, 621–628. [Google Scholar] [CrossRef]
- Kraus, J. Regulation of mu-opioid receptors by cytokines. Front. Biosci 2009, 1, 164. [Google Scholar] [CrossRef]
- Maes, M.; Kanchanatawan, B.; Sirivichayakul, S.; Carvalho, A.F. In schizophrenia, increased plasma IgM/IgA responses to gut commensal bacteria are associated with negative symptoms, neurocognitive impairments, and the deficit phenotype. Neurotox. Res. 2019, 35, 684–698. [Google Scholar] [CrossRef]
- Langsdorf, E.F.; Mao, X.; Chang, S.L. A role for reactive oxygen species in endotoxin-induced elevation of MOR expression in the nervous and immune systems. J. Neuroimmunol. 2011, 236, 57–64. [Google Scholar] [CrossRef]
- Basso, L.; Garnier, L.; Bessac, A.; Boué, J.; Blanpied, C.; Cenac, N.; Laffont, S.; Dietrich, G. T-lymphocyte-derived enkephalins reduce T h 1/T h 17 colitis and associated pain in mice. J. Gastroenterol. 2018, 53, 215–226. [Google Scholar] [CrossRef]
- Laumet, G.; Ma, J.; Robison, A.J.; Susmita, K.; Heijnen, C.; Kavelaars, A. T cells as an emerging target for chronic pain therapy. Front. Mol. Neurosci. 2019, 12, 216. [Google Scholar] [CrossRef]
- Mousa, S.A.; Machelska, H.; Schäfer, M.; Stein, C. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J. Neuroimmunol. 2002, 126, 5–15. [Google Scholar] [CrossRef]
- Ninković, J.; Roy, S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, E.; Dubynin, V.; Sarucheva, N.; Kalikhevich, V.; Ardemasova, Z.; Kamensky, A. Opioid peptides endomorphin-2 and soymorphin-5-amide are able to cross blood-brain barrier after intraperitoneal administration. J. Neurochem. 2013, 125, 229. [Google Scholar]
- Ting, P.; Cushenberry, P.; Friedman, T.; Loh, Y. Enhanced Brain Opioid Receptor Activity Precedes Blood-Brain Barrier Disruption. In Brain Edema X; Springer: Vienna, Austria, 1997; pp. 250–253. [Google Scholar]
- Maes, M.; Sirivichayakul, S.; Kanchanatawan, B.; Vodjani, A. Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox. Res. 2019, 36, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, R.; Chen, C.; Ji, F.; Li, T. Opioid system modulates the immune function: A review. Transl. Perioper. Pain Med. 2016, 1, 5–13. [Google Scholar] [PubMed]
- Al-Fadhel, S.Z.; Al-Hakeim, H.K.; Al-Dujaili, A.H.; Maes, M. IL-10 is associated with increased mu-opioid receptor levels in major depressive disorder. Eur. Psychiat. 2019, 57, 46–51. [Google Scholar] [CrossRef]
- Grimm, M.; Ben-Baruch, A.; Taub, D.; Howard, O.; Wang, J.; Oppenheim, J. Opiate inhibition of chemokine-induced chemotaxis. Ann. N. Y. Acad. Sci. 1998, 840, 9–20. [Google Scholar] [CrossRef]
- Martin, J.L.; Koodie, L.; Krishnan, A.G.; Charboneau, R.; Barke, R.A.; Roy, S. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am. J. Pathol. 2010, 176, 786–799. [Google Scholar] [CrossRef]
- Long, X.; Li, Y.; Qiu, S.; Liu, J.; He, L.; Peng, Y. MiR-582-5p/miR-590-5p targeted CREB1/CREB5–NF-κB signaling and caused opioid-induced immunosuppression in human monocytes. Transl. Psychiatry 2016, 6, e757. [Google Scholar] [CrossRef]
- Azuma, Y.; Ohura, K. Immunomodulation by Endomorphins 1 and 2 in Neutrophils, Macrophages and Microglia. Antiinflamm. Antiallergy Agents Med. Chem. 2003, 2, 1–8. [Google Scholar] [CrossRef]
- Yang, X.; Xia, H.; Chen, Y.; Liu, X.; Zhou, C.; Gao, Q.; Li, Z. Inducible expression of endomorphins in murine dendritic cells. Neural Regen. Res. 2012, 7, 2811–2817. [Google Scholar] [PubMed]
- Azuma, Y.; Ohura, K.; Wang, P.-L.; Shinohara, M. Endomorphins delay constitutive apoptosis and alter the innate host defense functions of neutrophils. Immunol. Lett. 2002, 81, 31–40. [Google Scholar] [CrossRef]
- Jessop, D.S. Endomorphins as agents for the treatment of chronic inflammatory disease. Bio. Drugs 2006, 20, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Wolff, C.; Fassold, A.; Hofbauer, R.; Chover-Gonzalez, A.; Richards, L.J.; Jessop, D.S. Antiinflammatory role of endomorphins in osteoarthritis, rheumatoid arthritis, and adjuvant-induced polyarthritis. Arthritis Rheum 2008, 58, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Anderson, G.; Kubera, M.; Berk, M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin. Ther. Tar. 2014, 18, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Borovcanin, M.M.; Jovanovic, I.; Radosavljevic, G.; Pantic, J.; Janicijevic, S.M.; Arsenijevic, N.; Lukic, M.L. Interleukin-6 in Schizophrenia—Is There a Therapeutic Relevance? Front. Psychiat. 2017, 8, 221. [Google Scholar] [CrossRef]
- Fineberg, A.M.; Ellman, L.M. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol. Psychiat. 2013, 73, 951–966. [Google Scholar] [CrossRef]
- Clark, S.D.; Abi-Dargham, A. The role of dynorphin and the kappa opioid receptor in the symptomatology of schizophrenia: A review of the evidence. Biol. Psychiat. 2019, 86, 502–511. [Google Scholar] [CrossRef]
- Land, B.B.; Bruchas, M.R.; Lemos, J.C.; Xu, M.; Melief, E.J.; Chavkin, C. The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. J. Neurosci. 2008, 28, 407–414. [Google Scholar] [CrossRef]
- Nemeth, C.L.; Paine, T.A.; Rittiner, J.E.; Béguin, C.; Carroll, F.I.; Roth, B.L.; Cohen, B.M.; Carlezon, W.A. Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology 2010, 210, 263–274. [Google Scholar] [CrossRef]
- Shekhar, A. Role of Kappa Opioid Receptors in Symptoms of Schizophrenia: What Is the Neurobiology? Biol. Psychiat. 2019, 86, 494–496. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Hareyama, N.; Ikeda, K.; Kurokawa, T.; Nakajima, M.; Nakao, K.; Mochizuki, H.; Ichinose, H. Effects of TRK-820, a selective kappa opioid receptor agonist, on rat schizophrenia models. Eur. J. Pharmacol. 2009, 606, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.P.; González, M.P.; Meza, R.C.; Noches, V.; Henny, P.; Gysling, K.; España, R.A.; Fuentealba, J.A.; Andrés, M.E. Mechanisms of kappa opioid receptor potentiation of dopamine D2 receptor function in quinpirole-induced locomotor sensitization in rats. Int. J. Neuropsychopharmacol. 2017, 20, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Heinke, B.; Gingl, E.; Sandkühler, J. Multiple targets of μ-opioid receptor-mediated presynaptic inhibition at primary afferent Aδ-and C-fibers. J. Neurosci. 2011, 31, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-B.; Huang, F.-S.; Fen, B.; Yin, J.-B.; Wang, W.; Li, Y.-Q. Inhibitory effects of endomorphin-2 on excitatory synaptic transmission and the neuronal excitability of sacral parasympathetic preganglionic neurons in young rats. Front. Cell Neurosci. 2015, 9, 206. [Google Scholar] [CrossRef]
- Gelman, P.L.; Herrera, N.E.G.; Ortega, M.E.M.; Villanueva, E.B.; Santillán, C.T.; Juárez, A.S.; Palma, B.A. Endomorphin peptides: Pharmacological and functional implications of these opioid peptides in the brain of mammals. Part two. Salud Ment. 2010, 33, 257–272. [Google Scholar]
- Sakurada, S.; Sawai, T.; Mizoguchi, H.; Watanabe, H.; Watanabe, C.; Yonezawa, A.; Morimoto, M.; Sato, T.; Komatsu, T.; Sakurada, T. Possible involvement of dynorphin A release via μ1-opioid receptor on supraspinal antinociception of endomorphin-2. Peptides 2008, 29, 1554–1560. [Google Scholar] [CrossRef]
- Willis, W.D., Jr.; Coggeshall, R.E. Chemical Anatomy of Dorsal Root Ganglion Cell. In Sensory Mechanisms of the Spinal Cord: Volume 1 Primary Afferent Neurons and the Spinal Dorsal Horn; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Chapter 4; pp. 103–154. [Google Scholar]
- Gein, S.V. Dynorphins in regulation of immune system functions. Biochem (Mosc) 2014, 79, 397–405. [Google Scholar] [CrossRef]
- Offermanns, S.; Rosenthal, W. Encyclopedia of molecular pharmacology; Springer Science & Business Media: Berlin, Germany, 2008; pp. 904–908. [Google Scholar]
- Terskiy, A.; Wannemacher, K.M.; Yadav, P.N.; Tsai, M.; Tian, B.; Howells, R.D. Search of the human proteome for endomorphin-1 and endomorphin-2 precursor proteins. Life Sci. 2007, 81, 1593–1601. [Google Scholar] [CrossRef][Green Version]
- Kou, Z.-Z.; Wan, F.-P.; Bai, Y.; Li, C.-Y.; Hu, J.-C.; Zhang, G.-T.; Zhang, T.; Chen, T.; Wang, Y.-Y.; Li, H. Decreased endomorphin-2 and μ-opioid receptor in the spinal cord are associated with painful diabetic neuropathy. Front. Mol. Neurosci. 2016, 9, 80. [Google Scholar] [CrossRef]
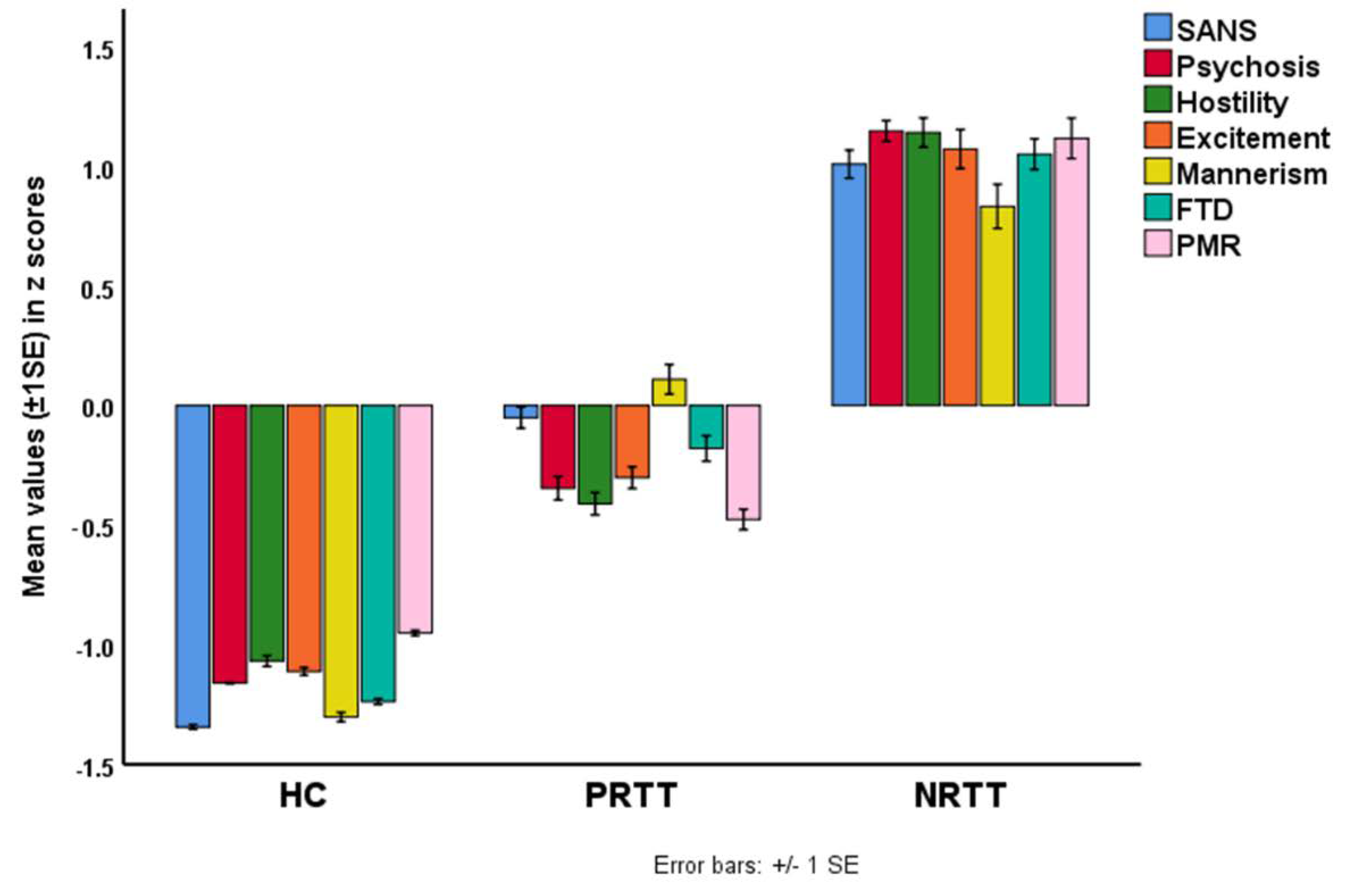
| Variables | HC A (n = 43) | PRTT B (n = 55) | NRTT C (n = 60) | F/φ/χ2 | df | p |
|---|---|---|---|---|---|---|
| Age (years) | 33.2 (11.1) | 36.5 (9.5) | 36.2 (12.3) | F = 1.29 | 2/155 | 0.280 |
| Sex (Female/Male) | 19/24 | 15/40 | 22/38 | χ2 = 3.08 | 2 | 0.214 |
| Married (No/Yes) | 12/31 C | 35/30 | 32/28 A | χ2 = 6.69 | 2 | 0.035 |
| BMI (kg/m2) | 27.9 (4.1) | 29.6 (4.3) | 28.4 (4.9) | F = 1.90 | 2/155 | 0.153 |
| TUD (No/Yes) | 30/13 | 44/11 | 40/20 | χ2 = 2.71 | 2 | 0.258 |
| Employment (No/Yes) | 17/26 B,C | 36/19 A | 43/17 A | χ2 = 11.63 | 2 | 0.003 |
| Education (years) | 11.1 (3.6) C | 10.8 (4.5) C | 8.9 (4.7) A,B | F = 4.21 | 2/155 | 0.017 |
| Age at onset (years) | 27.5 (7.5) | 29.3 (10.2) | F = 1.14 | 1/113 | 0.287 | |
| List learning * | 0.797 (0.596) | 0.437 (0.580) | −0.959 (0.688) | KW | <0.001 | |
| Digit sequencing task * | 1.369 (0.530) | −0.221 (0.604) | −0.755 (0.322) | KW | <0.001 | |
| Category instances * | 0.821 (0.398) | 0.136 (0.641) | −0.693 (1.064) | KW | <0.001 | |
| COWA * | 1.386 (0.373) | −0.149 (0.515) | −0.833 (0.399) | KW | <0.001 | |
| Symbol coding | 1.559 (0.403) B,C | −0.518 (0.216) A | −0.617 (0.267) A | KW | <0.001 | |
| Tower of London * | 1.195 (0.463) | 0.131 (0.709) | −0.848 (0.486) | KW | <0.001 | |
| SANS total score * | 11.2 (5.0) | 52.6 (12.2) | 91.9 (17.0) | KW | <0.001 | |
| CGI-I | 2.73 (0.45) | 4.20 (0.40) | F = 342.92 | 1/113 | <0.001 | |
| CGI-S | 4.38 (0.49) | 5.95 (0.70) | F = 190.63 | 1/113 | <0.001 | |
| Clozapine (No/Yes) | 55/0 | 46/14 | φ = 0.356 | <0.001 | ||
| Quietiapin (No/Yes) | 55/0 | 54/6 | φ = 0.225 | 0.016 | ||
| Haloperidol (No/Yes) | 43/12 | 60/0 | φ = 0.357 | <0.001 | ||
| Olanzapine (No/Yes) | 2/53 | 25/35 | φ = 0.448 | <0.001 | ||
| Risperidone | 53/2 | 48/12 | φ = 0.250 | 0.007 |
| Variables | IL-6 | IL-10 | β-Endorphin | Endomorphin 2 | KOR | MOR |
|---|---|---|---|---|---|---|
| IL-6 | ||||||
| IL-10 | 0.152 | |||||
| β-endorphin | 0.173 * | 0.090 | ||||
| Endomorphin 2 | 0.253 ** | 0.005 | 0.254 ** | |||
| KOR | 0.491 ** | 0.084 | 0.113 | 0.385 ** | ||
| MOR | 0.333 ** | 0.289 ** | 0.090 | 0.015 | 0.449 ** |
| Type | Dependent Variables | Explanatory Variables | F | df | p | Partial η2 |
|---|---|---|---|---|---|---|
| Multivariate | β-Endorphin, Endomorphin 2, KOR, MOR, IL-6, IL-10 | Diagnosis | 6.68 | 12/296 | <0.001 | 0.213 |
| Sex | 1.23 | 6/147 | 0.296 | 0.048 | ||
| Age | 1.14 | 6/147 | 0.341 | 0.045 | ||
| BMI | 0.68 | 6/147 | 0.667 | 0.027 | ||
| Tests for between-subject effects | β-Endorphin | Diagnosis | 4.25 | 2/152 | 0.016 | 0.053 |
| Endomorphin 2 | Diagnosis | 13.44 | 2/152 | <0.001 | 0.150 | |
| KOR | Diagnosis | 13.38 | 2/152 | <0.001 | 0.150 | |
| MOR | Diagnosis | 14.71 | 2/152 | <0.001 | 0.162 | |
| IL-6 | Diagnosis | 15.22 | 2/152 | <0.001 | 0.167 | |
| IL-10 | Diagnosis | 3.56 | 2/152 | 0.031 | 0.045 |
| Biomarkers | HC A | PRTT B | NRTT C |
|---|---|---|---|
| β-Endorphin (pg/mL) | 20.37 (2.52) | 16.57 (2.32) C | 24.62 (2.14) B |
| Endomorphin 2 (pg/mL) | 256.84 (39.69) B,C | 315.77 (36.61) A,C | 478.08 (33.71) A,B |
| KOR (ng/mL) | 4.24 (1.07) B,C | 7.70 (0.98) A | 7.32 (0.91) A |
| MOR (pg/mL) | 3.03 (0.36) C | 3.59 (0.34) C | 4.85 (0.31) A,B |
| IL-6 (pg/mL) | 4.82 (0.86) C | 5.73 (0.80) C | 7.79 (0.73) A,B |
| IL-10 (pg/mL) | 10.83 (0.87) C | 12.59 (0.80) | 14.12 (0.74) A |
| Dichotomies | Explanatory Variables | B | SE | Wald | df | p | OR | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Schizophrenia/Controls | Endomorphin 2 | 0.496 | 0.237 | 4.386 | 1 | 0.036 | 1.642 | 1.032–2.61 |
| KOR | 0.979 | 0.280 | 12.231 | 1 | <0.001 | 2.663 | 1.538–4.61 | |
| IL-10 | 0.591 | 0.225 | 6.902 | 1 | 0.009 | 1.806 | 1.162–2.81 | |
| NRTT/PRTT | Endomorphin 2 | 0.711 | 0.261 | 7.434 | 1 | 0.006 | 2.037 | 1.22–3.40 |
| MOR | 0.673 | 0.260 | 6.705 | 1 | 0.010 | 1.960 | 1.18–3.26 | |
| IL-6 | 0.757 | 0.258 | 8.600 | 1 | 0.003 | 2.132 | 1.29–3.54 |
| Dependent Variables | Explanatory Variables | β | t | p | F model | df | p | R2 |
|---|---|---|---|---|---|---|---|---|
| #1. SANS | Model | 19.94 | 3/154 | <0.001 | 0.280 | |||
| Endomorphin 2 | 0.302 | 4.290 | <0.001 | |||||
| MOR | 0.268 | 3.724 | <0.001 | |||||
| IL-6 | 0.191 | 2.600 | 0.010 | |||||
| #2. Psychosis | Model | 15.13 | 4/153 | <0.001 | 0.283 | |||
| Endomorphin 2 | 0.259 | 3.674 | <0.001 | |||||
| MOR | 0.263 | 3.644 | <0.001 | |||||
| IL-6 | 0.188 | 2.511 | 0.013 | |||||
| Education | –0.157 | –2.248 | 0.026 | |||||
| #3. Hostility | Model | 18.91 | 3/154 | <0.001 | 0.269 | |||
| Endomorphin 2 | 0.246 | 3.460 | 0.001 | |||||
| MOR | 0.270 | 3.724 | <0.001 | |||||
| IL-6 | 0.231 | 3.128 | 0.002 | |||||
| #4. Excitation | Model | 14.82 | 3/154 | <0.001 | 0.224 | |||
| Endomorphin 2 | 0.218 | 2.984 | 0.003 | |||||
| MOR | 0.248 | 3.314 | 0.001 | |||||
| IL-6 | 0.215 | 2.822 | 0.005 | |||||
| #5. PMR | Model | 20.39 | 3/154 | <0.001 | 0.284 | |||
| Endomorphin 2 | 0.310 | 4.412 | <0.001 | |||||
| MOR | 0.223 | 3.104 | 0.002 | |||||
| IL-6 | 0.233 | 3.189 | 0.002 | |||||
| #6. Mannerism | Model | 11.91 | 4/153 | <0.001 | 0.237 | |||
| Endomorphin 2 | 0.170 | 2.252 | 0.026 | |||||
| MOR | 0.183 | 2.310 | 0.022 | |||||
| KOR | 0.211 | 2.613 | 0.010 | |||||
| IL-10 | 0.199 | 2.710 | 0.008 | |||||
| #7. FTD | Model | 11.71 | 5/152 | <0.001 | 0.278 | |||
| Endomorphin 2 | 0.203 | 2.850 | 0.005 | |||||
| MOR | 0.246 | 3.285 | 0.001 | |||||
| IL-6 | 0.168 | 2.223 | 0.028 | |||||
| Education | –0.159 | –2.254 | 0.026 | |||||
| IL-10 | 0.147 | 2.047 | 0.042 |
| Dependent Variables | Explanatory Variables | β | t | p | F model | df | p | R2 |
|---|---|---|---|---|---|---|---|---|
| #1. List learning | Model | 15.08 | 2/155 | <0.001 | 0.163 | |||
| MOR | 0.304 | 3.945 | <0.001 | |||||
| IL-6 | 0.188 | 2.441 | 0.016 | |||||
| #2. Digit sequencing task | Model | 10.43 | 4/153 | <0.001 | 0.214 | |||
| MOR | 0.224 | 2.965 | 0.004 | |||||
| IL-6 | 0.190 | 2.431 | 0.016 | |||||
| Endomorphin 2 | 0.158 | 2.143 | 0.034 | |||||
| Education | 0.188 | 2.575 | 0.011 | |||||
| #3. Category instances | Model | 13.03 | 3/154 | <0.001 | 0.202 | |||
| MOR | 0.283 | 3.891 | <0.001 | |||||
| Endomorphin 2 | 0.246 | 3.379 | 0.001 | |||||
| Education | 0.192 | 2.672 | 0.008 | |||||
| #4. COWA | Model | 15.12 | 4/153 | <0.001 | 0.283 | |||
| MOR | 0.293 | 4.070 | <0.001 | |||||
| IL-6 | 0.183 | 2.497 | 0.014 | |||||
| Endomorphin 2 | 0.259 | 3.669 | <0.001 | |||||
| Age | 0.161 | 2.376 | 0.019 | |||||
| #5. Symbol coding | Model | 15.15 | 3/153 | <0.001 | 0.229 | |||
| KOR | 0.306 | 4.013 | <0.001 | |||||
| IL-10 | 0.201 | 2.826 | 0.005 | |||||
| Endomorphin 2 | 0.201 | 2.647 | 0.009 | |||||
| #6. Tower of London | Model | 12.61 | 5/152 | <0.001 | 0.293 | |||
| MOR | 0.273 | 3.783 | <0.001 | |||||
| IL-6 | 0.150 | 2.007 | 0.046 | |||||
| Endomorphin 2 | 0.146 | 2.070 | 0.040 | |||||
| Sex | 0.183 | 2.656 | 0.009 | |||||
| Education | 0.290 | 4.178 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustafa, S.R.; Al-Rawi, K.F.; Stoyanov, D.; Al-Dujaili, A.H.; Supasitthumrong, T.; Al-Hakeim, H.K.; Maes, M. The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and κ and μ Opioid Receptors Are Associated with Interleukin-6. Diagnostics 2020, 10, 633. https://doi.org/10.3390/diagnostics10090633
Moustafa SR, Al-Rawi KF, Stoyanov D, Al-Dujaili AH, Supasitthumrong T, Al-Hakeim HK, Maes M. The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and κ and μ Opioid Receptors Are Associated with Interleukin-6. Diagnostics. 2020; 10(9):633. https://doi.org/10.3390/diagnostics10090633
Chicago/Turabian StyleMoustafa, Shatha Rouf, Khalid F. Al-Rawi, Drozdstoi Stoyanov, Arafat Hussein Al-Dujaili, Thitiporn Supasitthumrong, Hussein Kadhem Al-Hakeim, and Michael Maes. 2020. "The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and κ and μ Opioid Receptors Are Associated with Interleukin-6" Diagnostics 10, no. 9: 633. https://doi.org/10.3390/diagnostics10090633
APA StyleMoustafa, S. R., Al-Rawi, K. F., Stoyanov, D., Al-Dujaili, A. H., Supasitthumrong, T., Al-Hakeim, H. K., & Maes, M. (2020). The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and κ and μ Opioid Receptors Are Associated with Interleukin-6. Diagnostics, 10(9), 633. https://doi.org/10.3390/diagnostics10090633







