Abstract
Fecal calprotectin (FC) is a quick, cost-effective, and noninvasive test, which is used to diagnose patients with active inflammatory bowel diseases (IBD). Recent studies suggest the possible predictive role of FC in the diagnosis of small intestinal bacterial overgrowth (SIBO) in patients with systemic sclerosis (SSc). This study aimed to assess the predictive value of FC in SSc patients and its’ possible use as a SIBO marker. A total of 40 SSc patients and 39 healthy volunteers were enrolled in the study. All subjects completed questionnaires evaluating gastrointestinal symptoms, FC measurements, and lactulose hydrogen breath test (LHBT) assessing SIBO presence. After rifaximin treatment, patients with SIBO underwent the same diagnostic procedures. Significantly higher FC values were observed in the study group compared to controls (97 vs. 20 μg/g; p < 0.0001) and in SSc patients diagnosed with SIBO compared to SSc patients without SIBO (206 vs. 24 μg/g; p = 0.0010). FC turned out to be a sensitive (94.12%) and specific (73.68%) marker in the detection of SIBO in patients with SSc (AUC = 0.82, 95% CI = 0.66–0.93; p < 0.0001). Our study suggests the potential value of FC in SSc in detecting gastrointestinal impairment and its promising role as an additional diagnostic tool for SIBO.
1. Introduction
Calprotectin is a heterodimer of 36.5 kDa belonging to the S100 family and characterized by calcium and zinc binding, a central hinge, N- and C-terminal domains, and solubility in 100% ammonium sulphate at neutral pH [1,2,3]. Its antimicrobial role is based on binding transition metals, thereby preventing their utilization by invading microbes. During infection and inflammation, increased calprotectin release is observed, mainly within neutrophils but also within monocytes and macrophages in lower quantities [4,5,6]. The antimicrobial role of calprotectin has been demonstrated against Escherichia coli, Klebsiella sp., Staphylococcus aureus, Listeria monocytogenes, and Salmonella enterica as [7]. Additionally, as a damage-associated molecular pattern molecule (DAMP), it has the ability to activate toll-like receptors, which is part of the innate immune response [8].
Apart from other body fluids, calprotectin can be found in stool samples. Fecal calprotectin (FC) levels are dependent on the inflammatory state of the intestinal mucosa and the accumulation of neutrophils responsible for its production. This correlation has paved the way for FC in gastrointestinal symptom diagnostics. Elevated FC levels has been demonstrated in patients with inflammatory bowel disease (IBD) [9]. FC distinguishes IBD from functional bowel disorders at 63–100% sensitivity and 80–100% specificity rates [10,11,12,13,14,15,16,17]. Furthermore, recent studies have indicated FC utility in IBD, irritable bowel syndrome (IBS), and nonorganic bowel disease differentiation [18]. Additionally, FC can serve as therapeutic follow-up marker in disease monitoring, and its increased levels may allow early endoscopic intervention [18]. Furthermore, FC measurement is an easy, cost-effective, quick, noninvasive, and reproductible diagnostic procedure [7]. However, false-positive results of FC may be observed during treatment with anti-inflammatory agents (NSAIDS) and proton pump inhibitors (PPI), and therefore their administration should be ceased before conducting FC measurement [18]. The FC values in healthy individuals should not exceed 50 μg/g of feces. However, the normal FC values depend on the patient’s age and are much higher in pediatric population, particularly in children aged under five years [7,19].
Systemic sclerosis (SSc) is a chronic, connective tissue disease of autoimmune origin and characterized by multiorgan malfunction [20]. Approximately, 90% of SSc patients are at risk of developing gastrointestinal complications [21]. The progressive fibrosis within gastrointestinal tract causes hypomotility as well as the stasis of intestinal content, which promote extensive bacterial colonization. This quite frequently results in small intestinal bacterial overgrowth (SIBO) syndrome development, which is described as overgrowth of bacteria to over 105 CFU/mL (colony forming units per mL of jejunal juice) or the presence of atypical flora [22,23,24,25,26]. One of the first-choice diagnostic procedures for SIBO is lactulose hydrogen breath test (LHBT) [27]. Due to progressive skin fibrosis and the development of microstomia, SSc patients find it difficult to exhale through a mouthpiece and sometimes even through a facemask. In these cases, LHBT becomes a more challenging test. Therefore, the search for other possible diagnostic markers of SIBO remains crucial. Some recent studies have suggested possible value of fecal calprotectin in detecting SIBO in SSc patient population [28,29,30].
The aims of the present prospective study were as follows: (i) determine the FC levels in patients with SSc and healthy controls; (ii) establish the correlation between SSc clinical characteristics and levels of FC; and (iii) evaluate the correlation between FC levels and digestive symptoms as well as gastrointestinal involvement, including the presence of SIBO.
2. Patients and Methods
A total of 40 patients with SSc diagnosis based on the 2013 classification criteria for systemic sclerosis by the American College of Rheumatology/European League against Rheumatism collaborative initiative and 39 healthy controls were enrolled in the study in the Department of Dermatology, Venerology, and Pediatric Dermatology, Medical University of Lublin, Poland [31]. All participants joined the study in the period from December 2018 to February 2020. Written informed consent was obtained from all study subjects before enrolment to the study. The study was approved by the Local Ethics Committee: KE-0254/238/2018 (25/10/2018)), and data was analyzed from a prospectively maintained database.
All study subjects enrolled in the study underwent the same diagnostic procedures, including gastrointestinal symptom questionnaires, lactulose hydrogen breath test (LHBT), and fecal calprotectin measurement. Initially, all study participants completed a gastrointestinal symptom questionnaire, which classified the complaints—diarrhea, constipation, abdominal pain/discomfort, flatulence, abdominal tenderness, nausea, vomiting, dysuria, tenesmus, fever, general illness, reflux, dysphagia, and early satiety—in the range from 0 (no symptoms) to 3 (very often). Moreover, the subjects completed the UCLA Scleroderma Clinical Trial Consortium GIT 2.0 (UCLA SCTC GIT 2.0) questionnaire, which consists of seven items—reflux, distention/bloating, diarrhea, fecal soilage, constipation, emotional well-being, and social functioning—scored from 0.00 to 3.00, except diarrhea and constipation, which are scored from 0.00 to 2.00 and from 0.00 to 2.50, respectively. The total score of gastrointestinal symptom severity is the summation of all scales (except constipation) [32].
All study participants underwent LHBT after preparation (restricted diet, avoidance of certain drugs and endoscopic procedures, fasting period of at least 12 h). Exhaled air sample was analyzed using Gastrolyzer (Bedfont, Maidstone, Kent, UK). Basal air sample was assessed at fasting and then after oral administration of lactulose (20 g in 200 mL of sterile water) at intervals of 20 min during the period of 3 h. An increase of 20 parts per million (ppm) of expired hydrogen between the maximum reading and the basal value equaled a positive result.
Fecal calprotectin was measured using CALEX® Cap Stool Extraction Device and Cobas® 6000 analyzer series (Roche, Switzerland) according to the manufacturer’s instructions in the study and control group. As PPI and NSAID use appears to be associated with significantly elevated fecal calprotectin, the patients enrolled in the study were instructed to discontinue PPIs and NSAIDs at least one week prior to FC measurement. The cut-off level for abnormal FC values was estimated above 50 μg/g. We evaluated the biochemical, demographic, and clinical characteristics of SSc patients, including measurements of erythrocyte sedimentation rate (ESR; mm/h), C-reactive protein (CRP; mg/L), total protein (g/dL), albumin (% of total protein), C3 complement (g/L), C4 complement (g/L), creatine kinase (CK, IU/L), rheumatoid factor (IU/mL), NT-proBNP (pg/mL), leukocytes (K/uL), alanine transaminase (ALT, U/L), aspartate transaminase (AST, IU/L), neutrophils (K/µL), antibody status (anticentromere (ACA), antitopoisomerase I (anti-Scl70), and anti-RNA polymerase III antibodies), median age, weight, body mass index (BMI), median SSc duration, SSc subsets, and the presence of gastrointestinal comorbidities.
All study subjects with SIBO diagnosis underwent rifaximin therapy (400 mg 3 times daily for 10 days). One month after the therapy, the patients underwent LHBT to determine the eradication of SIBO, filled the gastrointestinal symptom questionnaires, and had FC values measured. Out of the 19 SSc patients with SIBO diagnosis, 15 patients attended the check-up; four patients were lost from the follow-up due to illness or withdrawal of willingness to participate in the study.
We compared the values of FC between SSc patients and healthy controls, FC levels between SIBO-positive and SIBO-negative SSc patients, and FC before and after eradication treatment with rifaximin in SSc patients. We tried to establish the connection between FC levels and certain demographic, clinical, and biochemical characteristics in both SIBO-positive and SIBO-negative SSc patients.
The statistical analyses were conducted using MedCalc 15.8 (MedCalc Software, Ostend, Belgium). For group comparison involving binary data (or for several subgroups), we used either the chi square test or Fisher’s exact test. Comparisons involving continuous data were performed using (1) Student’s t-test when distribution of variables was normal and (2) Mann–Whitney test in other cases. The results were regarded as significant when the p value was less than 0.05. Furthermore, receiver operating characteristic (ROC) curve was constructed to examine the predictive value of the FC level in detecting specific clinical conditions (occurrence of SSc, presence of SIBO using glucose H2/CH4 breath test, etc.); the overall diagnostic accuracy of this test was assessed using the area under the ROC curve.
3. Results
The study cohort included 39 women (97.5%) and one man (2.5%) with a median age of 62 years (range: 34–78), weight of 65 kg (range: 50–99), and BMI of 24.9 (range: 19.8–34). The median disease duration was 10.5 years (range: 1–30). The control group included 36 women (92.3%) and three men (7.7%) with a median age of 61 years (range: 33–77), weight of 69 kg (range: 53–110), and BMI of 26.4 (range: 19–38.4). Both groups were comparable in terms of sex, age, and weight. All patient characteristics, including the levels of fecal calprotectin, are presented in Table 1.

Table 1.
Characteristics of the study and control groups including SIBO and fecal calprotectin levels.
In the study group, 19 patients (47.5%) were diagnosed with SIBO, whereas SIBO was diagnosed in five cases in the control group (12.8%; p = 0.0008). Based on LGBT, eradication of SIBO was achieved in 73.3% of SSc patients. We did not perform gastrointestinal symptom questionnaires, LHBT, and fecal calprotectin measurement after eradication treatment in the controls with SIBO diagnosis due to the unrepresentative number of the group.
Significantly higher FC values were observed in the study group compared to controls (97 vs. 20 μg/g; p < 0.0001). FC levels were significantly higher in SSc patients diagnosed with SIBO compared to SSc patients without SIBO (206 vs. 24 μg/g; p = 0.0010). The calprotectin level was lower in SSc patients with eradicated SIBO (156 vs. 137 μg/g). However, the obtained result was statistically insignificant. Detailed characteristics and comparison of the FC level between the control and study group (including measurements prior and after treatment) are presented in Table 1.
Among SSc patients without SIBO diagnosis, a positive correlation between FC level and age (rho = 0.505; p = 0.0275), CRP value (rho = 0.564; p = 0.0120), neutrophils (rho = 0.781; p = 0.0001), and leukocyte level (rho = 0.580; p = 0.0093) was observed, whereas a negative correlation was observed between this marker and alanine transaminase (rho = −0.567; p = 0.0114). A positive correlation between FC levels and neutrophils was also observed among SSc patients with SIBO diagnosis (rho = 0.561; p = 0.0191). Detailed data on Spearman’s rank correlation between fecal calprotectin levels and selected demographic, clinical, and laboratory variables depending on the presence of SIBO are given in Table 2. In both SIBO-positive and SIBO-negative SSc patients, no statistically significant correlations were found between the FC level and selected gastrointestinal symptoms assessed in both the questionnaires. Out of the five SSc patients with gastroesophageal reflux disease (GERD), two of them were negative for SIBO with median FC values remaining within normal values (46 ug/g), whereas the remaining three patients with GERD who were positive for SIBO had highly elevated FC levels (371.67 ug/g).

Table 2.
Spearman’s rank correlations between fecal calprotectin levels and selected demographic, clinical, and laboratory variables depending on SIBO presence.
In SSc patients, positive correlation was found between calprotectin and CRP levels (rho = 0.330; p = 0.0483). In addition, the FC level assessed after eradication correlated with the level of the quality of life index depending on skin symptoms (rho = −0.711; p = 0.0317). Detailed data on Spearman’s rank correlation between FC levels and selected demographic, clinical, and biochemical variables are presented in Table 3.

Table 3.
Spearman;s rank correlations of fecal calprotectin levels and selected demographic, clinical, and biochemical variables.
FC was able to differentiate patients from the control and study group with 69.44% sensitivity and 87.18% specificity (AUC = 0.83, 95% CI = 0.73–0.91; p < 0.0001; Figure 1). Additionally, FC showed 76.47% sensitivity and 80% specificity in detecting SSc in patients with SIBO (AUC = 0.81, 95% CI = 0.59–0.94; p = 0.0054; Figure 2). FC was also 94.12% sensitive and 73.68% specific in the detection of SIBO in patients with SSc (AUC = 0.82, 95% CI = 0.66–0.93; p < 0.0001; Figure 3). FC also proved to be a sensitive (75.86%) and specific (66.67%) marker to detect a specific disease subtype (dcSCC) (AUC = 0.72, 95% CI = 0.54–0.86; p = 0.0477). FC showed 90% sensitivity and 61.54% specificity in ACA antibody detection (AUC = 0.74, 95% CI = 0.56–0.88; p = 0.0095). Detailed FC characteristics, including its usefulness in detecting SIBO, predicting eradication efficacy, and differentiating different clinical conditions, are presented in Table 4.
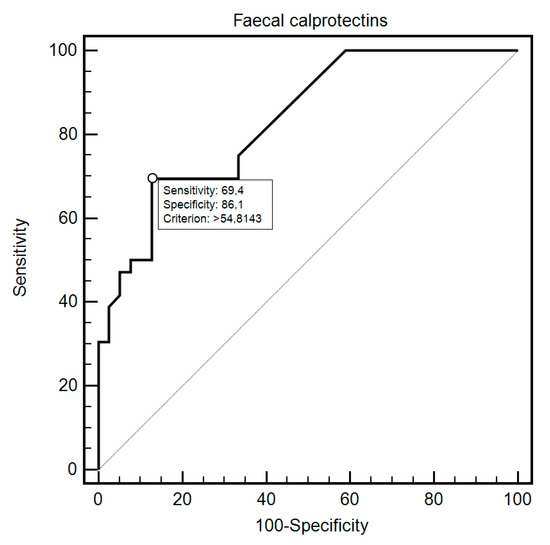
Figure 1.
Receiver operating characteristic (ROC) curve illustrating the diagnostic utility of fecal calprotectin in SSc detection.
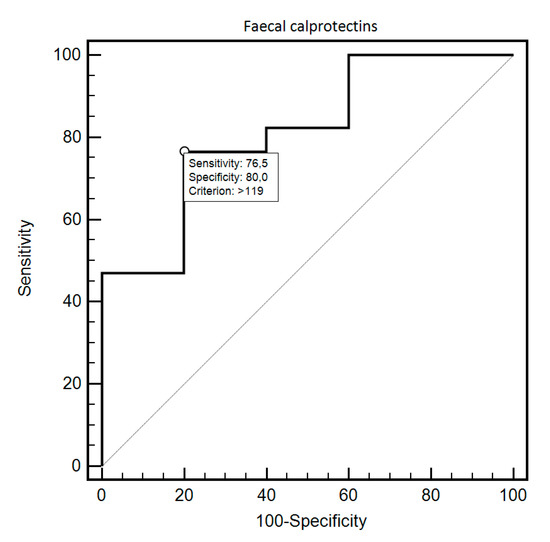
Figure 2.
ROC curve illustrating the diagnostic usefulness of fecal calprotectin in SSc detection in individuals with SIBO.
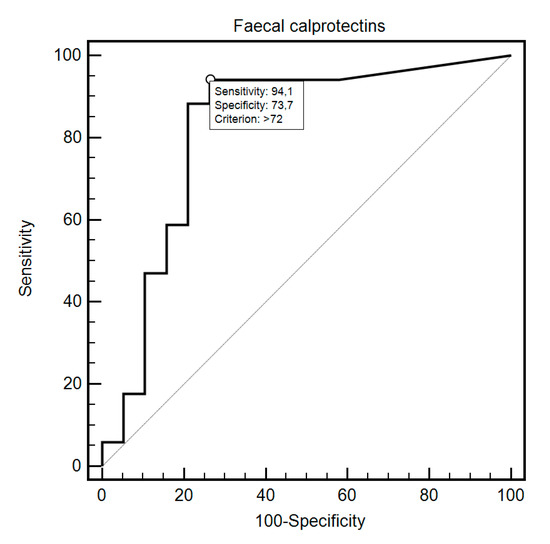
Figure 3.
ROC curve illustrating the diagnostic usefulness of fecal calprotectin in SIBO detection in SSc patients.

Table 4.
The clinical value of fecal calprotectin in detecting SIBO, predicting eradication efficacy, and differentiating different clinical characteristics.
4. Discussion
Approximately 90% of SSc patients develop variously intensified fibrosis within the gastrointestinal tract (GIT) with subsequent motility disturbances [21]. GIT distortions may have a severe impact on quality of life, morbidity, and eventually mortality. Taking this into account, early identification of the GI involvement is a vital step in SSc management and quality of life improvement [33,34]. Quick diagnosis gives healthcare professionals the means to decelerate disease progression and improve the patient’s prognosis [35]. After esophagus, the small bowel is the second most commonly affected organ in SSc [36]. Up to 50% of patients with esophageal disease and 20% of those with small bowel involvement remain asymptomatic [37,38]. Intestinal hypomotility originating from vasculopathy, smooth muscles atrophy, and fibrosis promotes extensive bacterial colonization and may lead to SIBO [21,24,39].
SIBO remains a diagnostic challenge due to lack of standardization, limited validation, cost-effectiveness, complexity, and low accessibility of small intestinal aspirate culture. Considering acceptable specificity, LHBT is an attractive alternative, although it is characterized by lower sensitivity compared to duodenal aspiration and culture [27,40,41]. In our study, we used LHBT to determine SIBO presence. Most researchers, however, use glucose hydrogen breath test (GHBT) for SIBO diagnosis in SSc patients [39]. Nevertheless, LHBT has been suggested as a first-line diagnostic option by the Gastrolyzer manufacturer. Additionally, in spite of the moderately better diagnostic efficacy of GHBT, we chose LHBT to avoid underdiagnosis and undertreatment of patients with SIBO.
Nevertheless, the search for an ideal diagnostic method or an additional SIBO marker to enhance LHBT value requires further research. Some studies have reported the potential diagnostic role of short-chain fatty acids in small intestine aspiration, unconjugated bile acids in serum, urinary excretion of p-aminobenzoic acid or indican, and a 72 h test for fecal fat evaluation. However, their clinical usefulness has not been clearly confirmed [42].
In our study, significantly higher FC values were observed in the study group compared to controls (97 vs. 20 μg/g; p < 0.0001). This indicates frequent gastrointestinal involvement in SSc patients. FC levels were significantly higher in SSc patients diagnosed with SIBO compared to SSc patients without SIBO (206 vs. 24 μg/g; p = 0.0010). This stands in agreement with Andréasson et al., who reported higher FC values in SSc patients with GI involvement (130 vs. 57 μg/g; p = 0.013). In the study by Marie et al., the presence of SIBO was more frequent in the group of SSc patients with abnormal FC levels (46.2 vs. 3.1%; p = 0.000003) [28,29].
Marie et al. also found FC to be a useful instrument in the follow-up and eradication control in SSc patients with SIBO, observing a correlation between decreased FC levels and eradication of SIBO after three months of rotating courses of alternative antibiotic therapy in SSc patients [28]. In our study, the FC level was insignificantly lower in patients with successfully eradicated SIBO (156 vs. 137 μg/g). Nevertheless, therapy efficacy is best evaluated by symptom relief rather than breath testing and FC measures [35]. The fact that there was no significant decrease in FC values in patients with successfully eradicated SIBO and relatively high FC values after the treatment with rifaximin compared with healthy controls imply that SIBO is not independently associated with elevated FC levels in SSc patients. Approximately 90% of SSc patients present variously intensified involvement of the gastrointestinal tract followed by hypomotility and other disturbances [21]. The pathology of the intestinal wall, along with the hypomotility and stasis of the luminal content, may also lead to elevated FC levels. The limitation of our study was the lack of synchronous endoscopic assessment of the GIT of SSc patients. Therefore, the search for other underlying intestinal pathologies behind elevated FC levels in the course of SSc should be the subject of further research.
Similar to Andreasson et al., we failed to find any relationship between FC levels and patients’ GI symptoms as assessed by the abovementioned questionnaires [30]. This is in contrast to Marie et al.’s findings, which described higher median value for the global symptom score (GSS) of digestive symptoms in SSc patients with abnormal FC levels than in those without (median value of 3 vs. 2; p = 0.0006) [28]. The observations concerning the FC levels in SSc patients with reflux disease should exclude the possibility of GERD being independently associated with elevated FC levels and rather imply the role of SIBO in this matter. In hitherto studies, laboratory results of SSc patients with FC levels above 50 μg/g have shown higher median levels of C-reactive protein, while those of patients with FC level above 200 μg/g have shown significantly lower values of ferritin and plasma folic acid [28]. In our study, we found a positive correlation between elevated FC levels and higher neutrophils count among SSc patients with SIBO diagnosis. In SSc patients without SIBO diagnosis, a positive correlation was observed between FC level and age, CRP value, neutrophils, and leukocyte level.
Contrary to our expectations, higher FC values were associated with the presence of ACA antibodies. Studies to date have not described correlation of ACA antibodies with gastrointestinal involvement; therefore, the correlation between high FC values and ACA remains surprising [43]. As expected, FC was also a sensitive and specific marker to detect diffuse SSc subtype. Gut involvement in systemic sclerosis occurs more commonly in the diffuse than the limited variant [35].
5. Conclusions
FC turned out to be a sensitive and specific marker differentiating patients from the control and study group (SSc detection). Additionally, this marker showed high sensitivity and specificity in detecting SIBO in SSc patients. Therefore, we find FC a valuable diagnostic tool, which may enhance the validity of LHBT. Nevertheless, FC alone is not sufficient to detect SIBO. On the basis of our research, we find FC a helpful tool in monitoring the course of the disease in SSc patients and reflecting the gastrointestinal impairment and possible need for more serious and invasive gastrointestinal diagnostics.
The development of SIBO in SSc patients, when associated with intense gastrointestinal symptoms and frequent diarrhoea often leads to malabsorption and malnutrition. This puts SSc patients at risk of further complications, disease progression and morbidity and mortality rates increase. Therefore the diagnostic approach and management of SSc gastrointestinal manifestations should remain active areas of research.
Author Contributions
Conceptualization, B.P.-P., A.G., K.R.-P. and D.K.; Data curation, B.P.-P., A.G. and K.R.-P.; Formal analysis, B.P.-P. and K.R.-P.; Funding acquisition, B.P.-P., K.R.-P. and D.K.; Investigation, B.P.-P. and D.K.; Methodology, B.P.-P., A.G. and D.K.; Project administration, B.P.-P. and A.G; Resources, B.P.-P., A.G. and D.K.; Software, B.P.-P., K.R.-P. and A.G.; Supervision, A.G. and D.K.; Validation, B.P.-P. and A.G.; Visualization, B.P.-P.; Writing—original draft, B.P.-P. and K.R.-P.; Writing—review & editing, B.P.-P., A.G. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to express our special appreciation and thanks to Wojciech Polkowski, whose courtesy we were able to perform lactulose hydrogen breath tests with the Gastrolyzer.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Ayling, R.M.; Kok, K. Fecal Calprotectin. Adv. Clin. Chem 2018, 87, 161–190. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.W. A soluble protein characteristic of the nervous system. Biochem. Biophys. Res. Commun 1965, 19, 739–744. [Google Scholar] [CrossRef]
- Røseth, A.G.; Fagerhol, M.K.; Aadland, E.; Schjønsby, H. Assessment of the neutrophil dominating protein calprotectin in feces a methodological study. Scand. J. Gastroenterol 1992, 27, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Besold, A.N.; Culbertson, E.M.; Nam, L.; Hobbs, R.P.; Boyko, A.; Maxwell, C.N.; Chazin, W.J.; Marques, A.R.; Culotta, V.C. Antimicrobial action of calprotectin that does not involve metal withholding. Metallomics 2018, 10, 1728–1742. [Google Scholar] [CrossRef] [PubMed]
- Kehl-Fie, T.E.; Chitayat, S.; Hood, M.; Damo, S.; Restrepo, N.; Garcia, C.; Munro, K.A.; Chazin, W.J.; Skaar, E.P. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe 2011, 10, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Nakashige, T.G.; Zygiel, E.M.; Drennan, C.L.; Nolan, E.M. Nickel sequestration by the host-defence protein human calprotectin. J. Am. Chem. Soc. 2017, 139, 8828–8836. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.; Felley, C.; Frossard, J.L. Calprotectin in Daily Practice: Where Do We Stand in 2017? Digestion 2017, 95, 293–301. [Google Scholar] [CrossRef]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.D.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef]
- D’Haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef]
- Sipponen, T.; Kolho, K.L. Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. Scand J. Gastroenterol. 2015, 50, 74–80. [Google Scholar] [CrossRef]
- Tibble, J.; Teahon, K.; Thjodleifsson, B.; Roseth, A.; Sigthorsson, G.; Bridger, S.; Foster, R.; Sherwood, R.; Fagerhol, M.; Bjarnason, I. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut 2000, 47, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Iacono, G.; Cottone, M.; Di Prima, L.; Cartabellotta, F.; Cavataio, F.; Scalici, C.; Montalto, G.; Di Fede, G.; Rini, G.B.; et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: A prospective study in adults and children. Clin. Chem. 2003, 49, 861–867. [Google Scholar] [PubMed]
- Costa, F.; Mumolo, M.G.; Ceccarelli, L.; Bellini, M.; Romano, M.R.; Sterpi, C.; Ricchiuti, A.; Marchi, S.; Bottai, M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 2005, 54, 364–368. [Google Scholar] [CrossRef]
- Dolwani, S.; Metzner, M.; Wassell, J.J.; Yong, A.; Hawthorne, A.B. Diagnostic accuracy of faecal calprotectin estimation in prediction of abnormal small bowel radiology. Aliment. Pharm. Ther. 2004, 20, 615–621. [Google Scholar] [CrossRef] [PubMed]
- D’Inca, R.; Dal Pont, E.; Di Leo, V.; Ferronato, A.; Fries, W.; Vettorato, M.G.; Martines, D.; Sturniolo, G.C. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J. Colorectal Dis. 2007, 22, 429–437. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Trummler, M.; Seeholzer, P.; Seibold-Schmid, B.; Seibold, F. Discriminating IBD from IBS: Comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008, 14, 32–39. [Google Scholar] [CrossRef]
- Schroder, O.; Naumann, M.; Shastri, Y.; Povse, N.; Stein, J. Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: Combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment. Pharm. Ther. 2007, 26, 1035–1042. [Google Scholar] [CrossRef]
- Manceau, H.; Chicha-Cattoir, V.; Puy, H.; Peoc’h, K. Fecal calprotectin in inflammatory bowel diseases: Update and perspectives. Clin. Chem Lab. Med. 2017, 55, 474–483. [Google Scholar] [CrossRef]
- Davidson, F.; Lock, R.J. Paediatric reference ranges for faecal calprotectin: A UK study. Ann. Clin. Biochem. 2017, 54, 214–218. [Google Scholar] [CrossRef]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Bures, J.; Cyrany, J.; Kohoutova, D.; Förstl, M.; Rejchrt, S.; Kvetina, J.; Vorisek, V.; Kopacova, M. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 2010, 16, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, L.I.; Simopoulou, T.; Daoussis, D.; Liossis, S.N.; Potamianos, S. Intestinal Involvement in Systemic Sclerosis: A Clinical Review. Dig. Dis Sci. 2018, 63, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, V.K.; Wirz, E.G.; Allanore, Y.; Rossbach, P.; Riemekasten, G.; Hachulla, E.; Distler, O.; Airo, P.; Carreira, P.E.; Gurman, A.B.; et al. Incidences and Risk Factors of Organ Manifestations in the Early Course of Systemic Sclerosis: A Longitudinal EUSTAR Study. PLoS ONE 2016, 11, e0163894. [Google Scholar] [CrossRef] [PubMed]
- Miazga, A.; Osiński, M.; Cichy, W.; Żaba, R. Current views on the etiopathogenesis, clinical manifestation, diagnostics, treatment and correlation with other nosological entities of SIBO. Adv. Med. Sci. 2015, 60, 118–124. [Google Scholar] [CrossRef]
- Quigley, E.M.M. The Spectrum of Small Intestinal Bacterial Overgrowth (SIBO). Curr Gastroenterol Rep. 2019, 21, 3. [Google Scholar] [CrossRef]
- Quigley, E.M. Small intestinal bacterial overgrowth: What it is and what it is not. Curr. Opin. Gastroenterol. 2014, 30, 141–146. [Google Scholar] [CrossRef]
- Kaye, S.A.; Lim, S.G.; Taylor, M.; Patel, S.; Gillespie, S.; Black, C.M. Small bowel bacterial overgrowth in systemic sclerosis: Detection using direct and indirect methods and treatment outcome. Br. J. Rheumatol. 1995, 34, 265–269. [Google Scholar] [CrossRef]
- Marie, I.; Leroi, A.M.; Menard, J.F.; Levesque, H.; Quillard, M.; Ducrotte, P. Fecal calprotectin in systemic sclerosis and review of the literature. Autoimmun. Rev. 2015, 14, 547–554. [Google Scholar] [CrossRef]
- Andréasson, K.; Saxne, T.; Scheja, A.; Bartosik, I.; Mandl, T.; Hesselstrand, R. Faecal levels of calprotectin in systemic sclerosis are stable over time and are higher compared to primary Sjögren’s syndrome and rheumatoid arthritis. Arthritis Res. 2014, 16, R46. [Google Scholar] [CrossRef]
- Andréasson, K.; Scheja, A.; Saxne, T.; Ohlsson, B.; Hesselstrand, R. Faecal calprotectin: A biomarker of gastrointestinal disease in systemic sclerosis. J. Intern. Med. 2011, 270, 50–57. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.; Riemekasten, G.; Carreira, P.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Nagaraja, V.; Gladue, H.; Chey, W.; Pimentel, M.; Frech, T. Measuring Response in the Gastrointestinal Tract in systemic sclerosis. Curr. Opin. Rheumatol. 2013, 25, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Gyger, G.; Baron, M. Systemic sclerosis: Gastrointestinal disease and its management. Rheum. Dis. Clin. N. Am. 2015, 41, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, D.; Li, M.T.; Yao, Y.; Jin, M.; Zeng, X.F.; Qian, J. Gastrointestinal manifestations on impaired quality of life in systemic sclerosis. J. Dig. Dis. 2019, 20, 256–261. [Google Scholar] [CrossRef]
- Emmanuel, A. Current management of the gastrointestinal complications of systemic sclerosis. Nat. Rev. Gastroenterol Hepatol. 2016, 13, 461–472. [Google Scholar] [CrossRef]
- Marie, I.; Ducrotté, P.; Denis, P.; Hellot, M.F.; Levesque, H. Outcome of small-bowel motor impairment in systemic sclerosis—A prospective manometric 5-yr follow-up. Rheumatol. Oxf. 2007, 46, 150–153. [Google Scholar] [CrossRef]
- McFarlane, I.M.; Bhamra, M.S.; Kreps, A.; Iqbal, S.; Al-Ani, F.; Aponte, C.S.; Grant, C.; Singh, S.; Awwal, K.; Koci, K.; et al. Gastrointestinal Manifestations of Systemic Sclerosis. Rheumatol. Sunnyvale 2018, 8, 235. [Google Scholar] [CrossRef]
- Akesson, A.; Wollheim, F.A. Organ manifestations in 100 patients with progressive systemic sclerosis: A comparison between the CREST syndrome and diffuse scleroderma. Br. J. Rheumatol. 1989, 28, 81–86. [Google Scholar] [CrossRef]
- Polkowska-Pruszyńska, B.; Gerkowicz, A.; Szczepanik-Kułak, P.; Krasowska, D. Small intestinal bacterial overgrowth in systemic sclerosis: A review of the literature. Arch. Derm. Res. 2019, 311, 1–8. [Google Scholar] [CrossRef]
- Erdogan, A.; Rao, S.S.; Gulley, D.; Jacobs, C.; Lee, Y.Y.; Badger, C. Small intestinal bacterial overgrowth: Duodenal aspiration vs. glucose breath test. Neurogastroenterol Motil. 2015, 27, 481–489. [Google Scholar] [CrossRef]
- McMahan, Z.H. Gastrointestinal involvement in systemic sclerosis: An update. Curr. Opin. Rheumatol. 2019, 31, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Coss, A.E.; Attaluri, A.; Valestin, J.; Rao, S.S.C. Dysmotility and ppi use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment. Pharm. Ther. 2013, 37, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Walecka, I. Systemic sclerosis and the gastrointestinal tract. Prz. Gastroenterol. 2017, 12, 163–168. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
