Design, Development, and Multi-Characterization of an Integrated Clinical Transrectal Ultrasound and Photoacoustic Device for Human Prostate Imaging
Abstract
1. Introduction
2. Materials and Methods
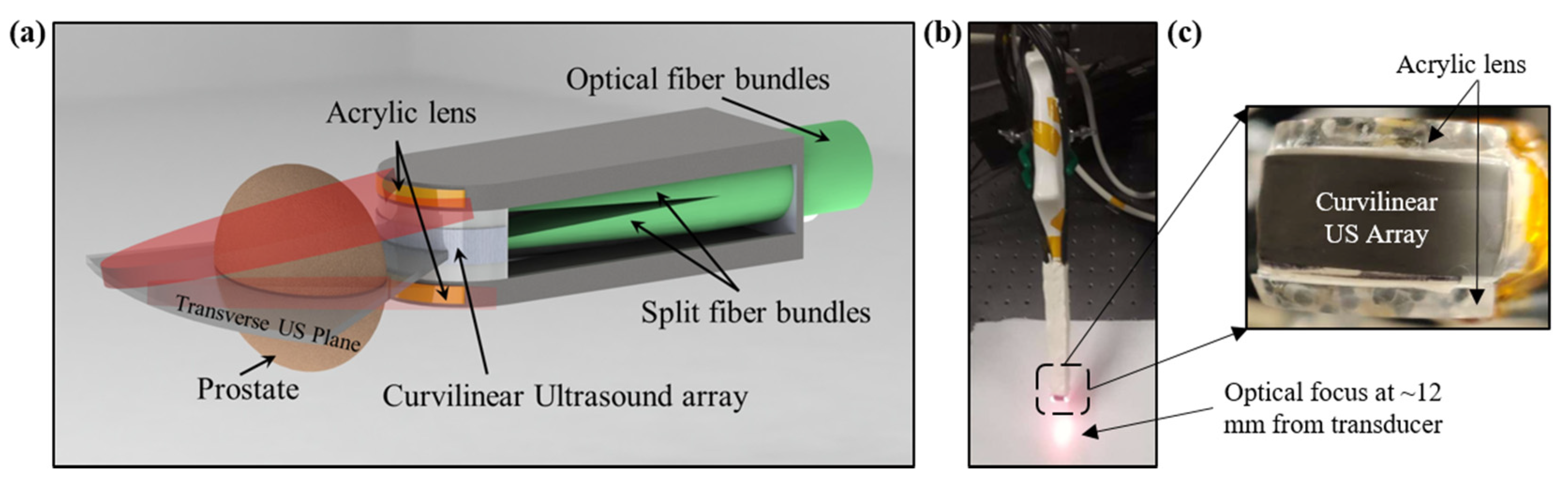
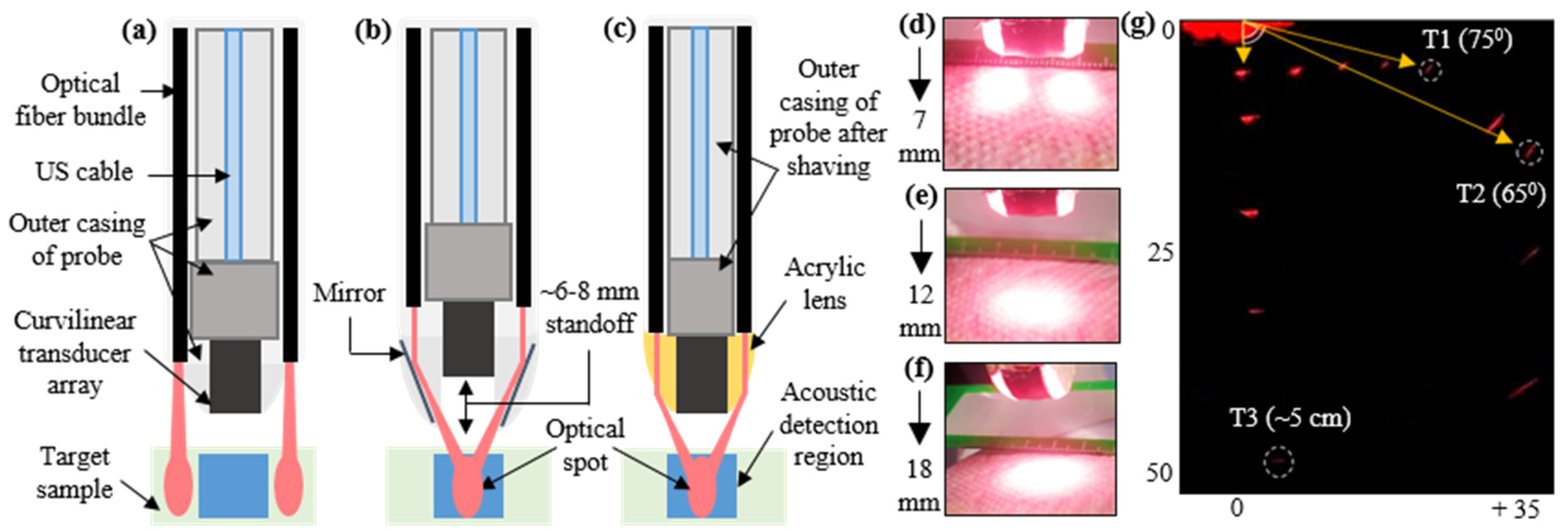
2.1. Design and Development of TRUSPA Device
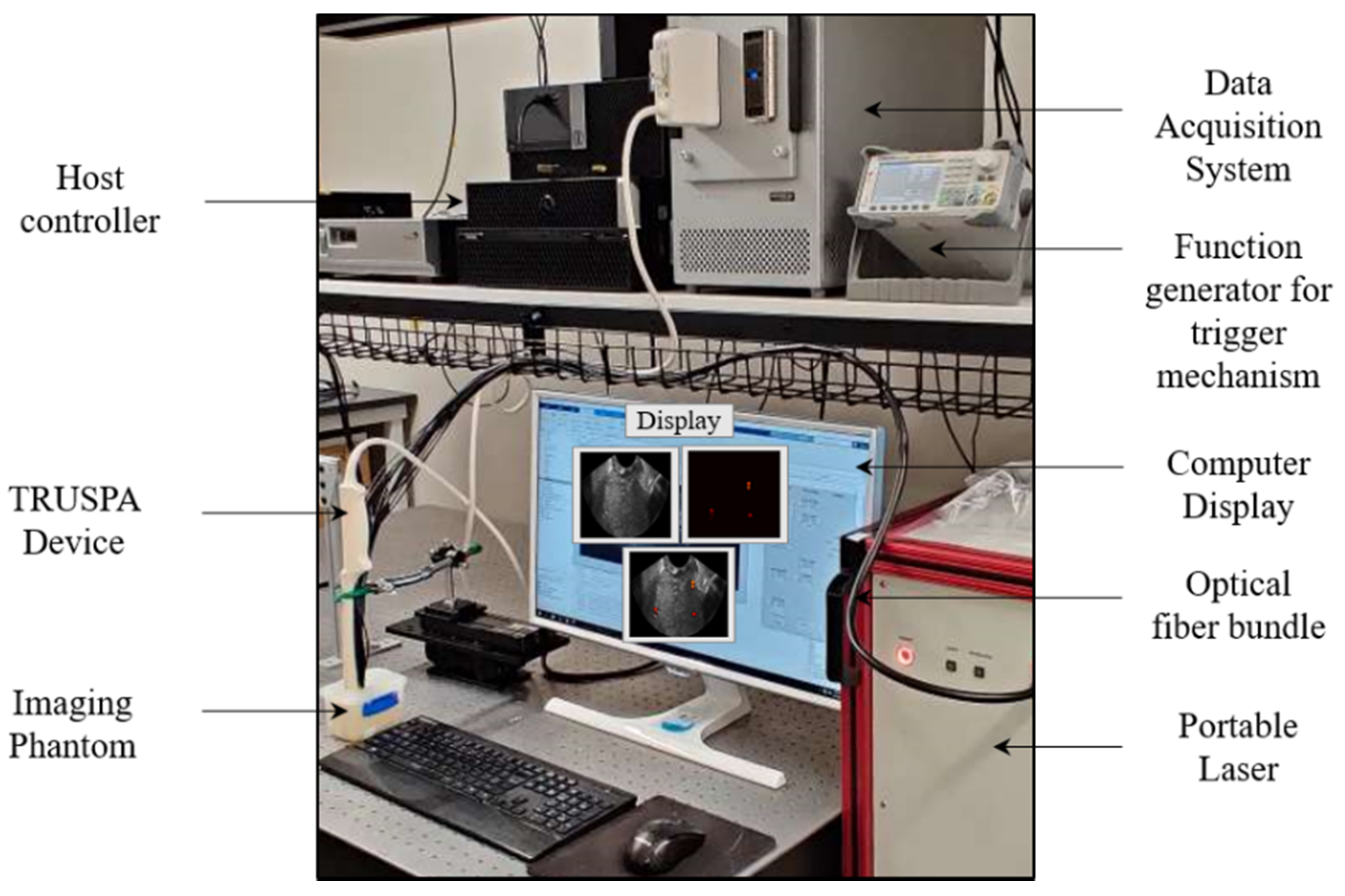
2.2. TRUSPA Imaging System: Experimental Setup
3. Experiments and Results
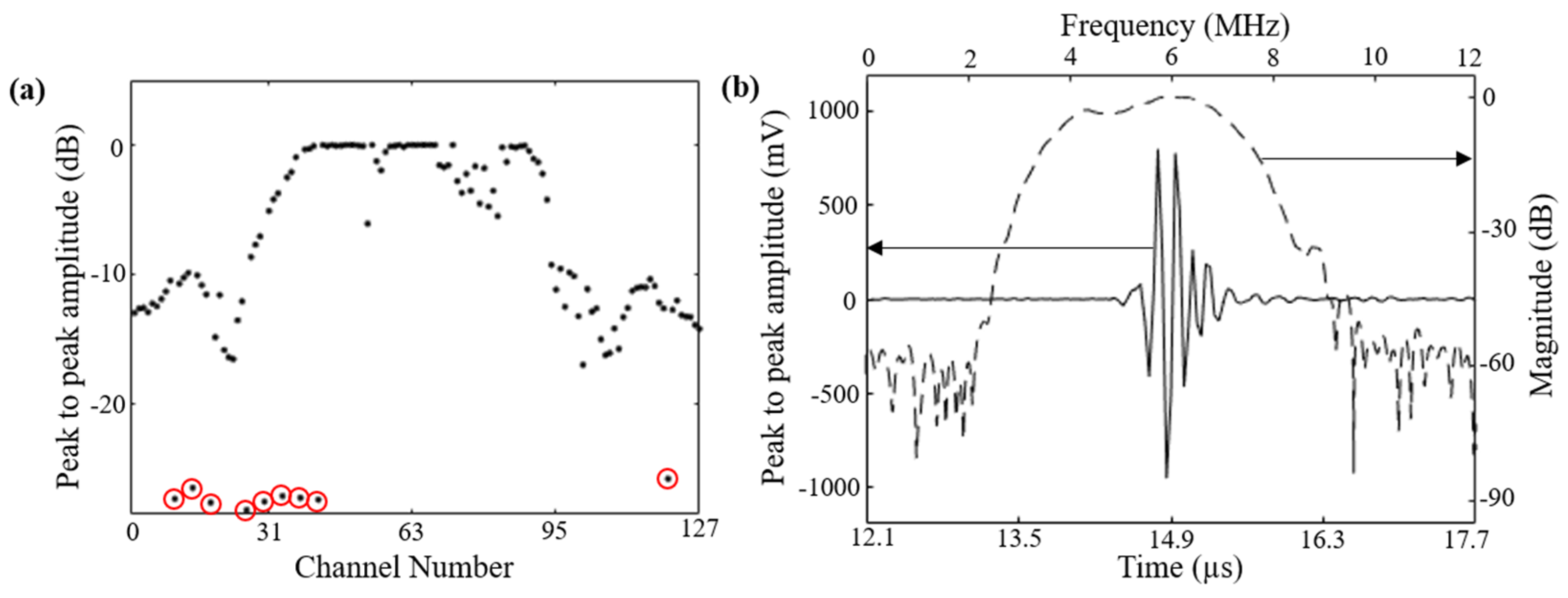
3.1. Characterization: Evaluation of 128-Element Curviliear Transducer Array
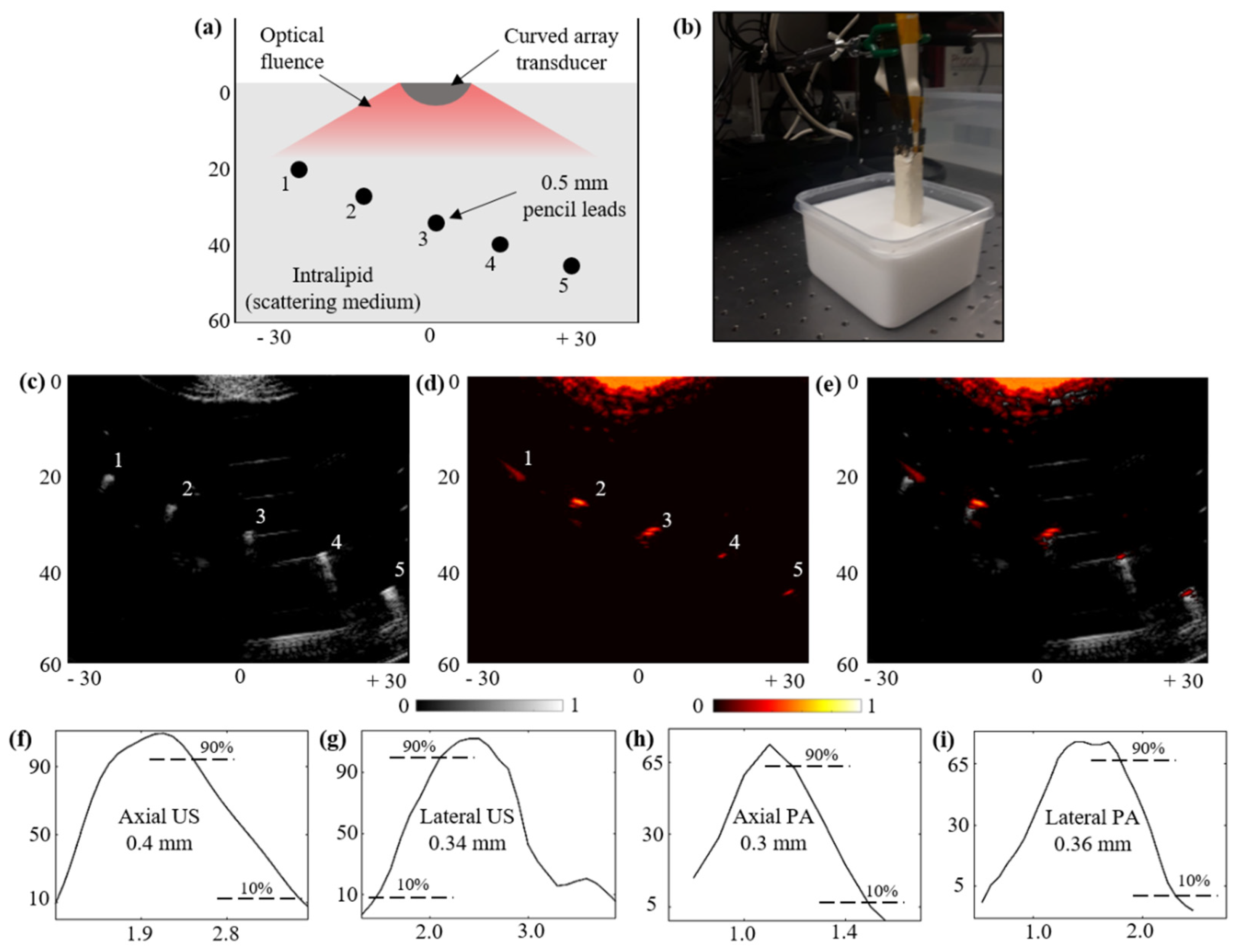
3.2. Characterization: Ultrasound Field Characterizations
3.3. Evaluation of Safety Parameters
3.4. Optical Fluence and PAI Field-of-View Characterization
3.5. Structural Imaging Capabilities over a Scattering Phantom:
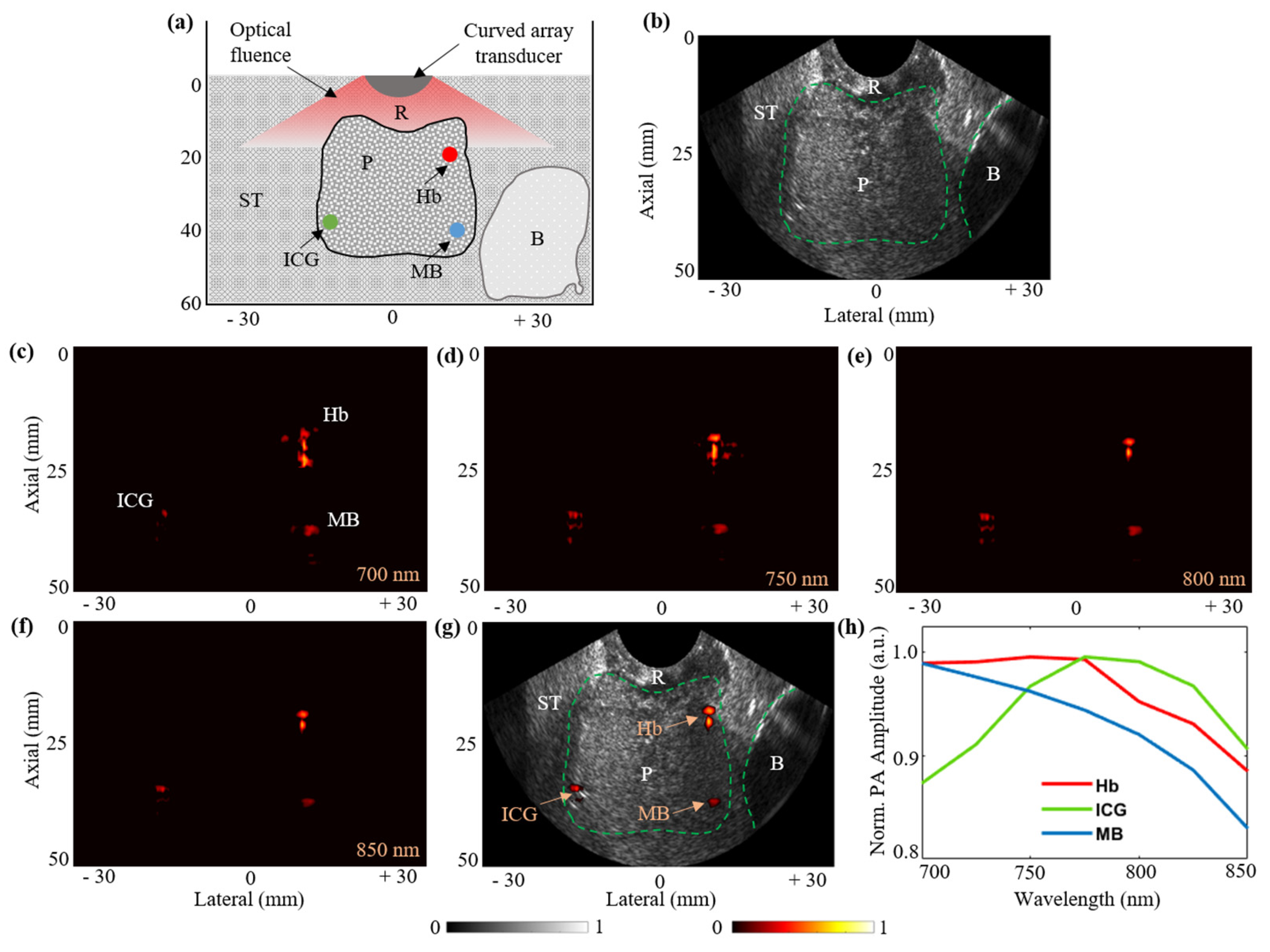
3.6. Functional Imaging Capabilities with Prostate Tissue-Mimicking Phantom:
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | Photoacoustic |
| US | Ultrasound |
| PAI | Photoacoustic Imaging |
| PCa | Prostate Cancer |
| FOV | Field of View |
| TRUS | Transrectal Ultrasound |
| TRUSPA | Transrectal Ultrasound and Photoacoustic |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Deuker, M.; Stolzenbach, L.F.; Pecoraro, A.; Rosiello, G.; Luzzago, S.; Tian, Z.; Saad, F.; Felix, K.H.; Karakiewicz, P.I. PSA, stage, grade and prostate cancer specific mortality in Asian American patients relative to Caucasians according to the United States Census Bureau race definitions. World J. Urol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fleshner, N.E.; O’Sullivan, M.; Fair, W.R. Prevalence and predictors of a positive repeat transrectal ultrasound guided needle biopsy of the prostate. J. Urol. 1997, 158, 505–509. [Google Scholar] [CrossRef]
- Rabbani, F.; Stroumbakis, N.; Kava, B.R.; Cookson, M.S.; Fair, W.R. Incidence and clinical significance of false-negative sextant prostate biopsies. J. Urol. 1998, 159, 1247–1250. [Google Scholar] [CrossRef]
- Taira, A.V.; Merrick, G.S.; Galbreath, R.W.; Andreini, H.; Taubenslag, W.; Curtis, R.; Butler, W.M.; Adamovich, E.; Wallner, K.E. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Ehdaie, B.; Shariat, S.F. Magnetic Resonance Imaging–Targeted Prostate Biopsy: Back to the Future. Eur. Urol. 2013, 63, 141–142. [Google Scholar] [CrossRef]
- Moore, C.M.; Robertson, N.L.; Arsanious, N.; Middleton, T.; Villers, A.; Klotz, L.; Taneja, S.S.; Emberton, M. Image-guided prostate biopsy using magnetic resonance imaging–derived targets: A systematic review. Eur. Urol. 2013, 63, 125–140. [Google Scholar] [CrossRef]
- Siddiqui, M.M.; Rais-Bahrami, S.; Truong, H.; Stamatakis, L.; Vourganti, S.; Nix, J.; Hoang, A.N.; Walton-Diaz, A.; Shuch, B.; Weintraub, M.; et al. Magnetic resonance imaging/ultrasound–fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur. Urol. 2013, 64, 713–719. [Google Scholar] [CrossRef]
- Cheikh, A.B.; Girouin, N.; Colombel, M.; Maréchal, J.M.; Gelet, A.; Bissery, A.; Rabilloud, M.; Lyonnet, D.; Rouvière, O. Evaluation of T2-weighted and dynamic contrast-enhanced MRI in localizing prostate cancer before repeat biopsy. Eur. Radiol. 2009, 19, 770–778. [Google Scholar] [CrossRef]
- Padhani, A.R.; Gapinski, C.J.; Macvicar, D.A.; Parker, G.J.; Suckling, J.; Revell, P.B.; Leach, M.O.; Dearnaley, D.P.; Husband, J.E. Dynamic contrast enhanced MRI of prostate cancer: Correlation with morphology and tumour stage, histological grade and PSA. Clin. Radiol. 2000, 55, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.A.; Day, J.R.; Lonigro, R.J.; Hovelson, D.H.; Siddiqui, J.; Kunju, L.P.; Dunn, R.L.; Meyer, S.; Hodge, P.; Groskopf, J.; et al. Urine TMPRSS2: ERG plus PCA3 for individualized prostate cancer risk assessment. Eur. Urol. 2016, 70, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Cann, G.M.; Gulzar, Z.G.; Cooper, S.; Li, R.; Luo, S.; Tat, M.; Stuart, S.; Schroth, G.; Srinivas, S.; Ronaghi, M.; et al. mRNA-Seq of single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PLoS ONE 2012, 7, e49144. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.L.; Glass, T.J.; Miller, Z.A.; Rosenzweig, S.J.; Buck, A.; Polascik, T.J.; Gupta, R.T.; Brown, A.F.; Madden, J.; Nightingale, K.R. Identifying clinically significant prostate cancers using 3-D in vivo acoustic radiation force impulse imaging with whole-mount histology validation. Ultrasound Med. Biol. 2016, 42, 1251–1262. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.; Harzstark, A.L.; Ferrone, M.; Van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C] pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef] [PubMed]
- Khansa, Z.; Haidar, M.B.; Neaimeh, N.; Korek, M. Comparison of PET imaging with a 68Ga-labelled PSMA ligand versus 18F-choline PET/CT for the diagnosis of Prostate Cancer & Radioprotection for involved personnel. Health Technol. 2019, 9, 607–613. [Google Scholar]
- Levi, J.; Sathirachinda, A.; Gambhir, S.S. A high-affinity, high-stability photoacoustic agent for imaging gastrin-releasing peptide receptor in prostate cancer. Clin. Cancer Res. 2014, 20, 3721–3729. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef]
- Huang, W.; Chen, R.; Peng, Y.; Duan, F.; Huang, Y.; Guo, W.; Chen, X.; Nie, L. In vivo quantitative photoacoustic diagnosis of gastric and intestinal dysfunctions with a broad pH-responsive sensor. ACS Nano 2019, 13, 9561–9570. [Google Scholar] [CrossRef]
- Wang, X.; Roberts, W.W.; Carson, P.L.; Wood, D.P.; Fowlkes, J.B. Photoacoustic tomography: A potential new tool for prostate cancer. Biomed. Opt. Express 2010, 1, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.A.L.; Kuo, N.P.; Song, D.Y.; Kang, J.U.; Boctor, E.M. In vivo visualization of prostate brachytherapy seeds with photoacoustic imaging. J. Biomed. Opt. 2014, 19, 126011. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Tsujita, K.; Irisawa, K.; Kasamatsu, T.; Hirota, K.; Kawaguchi, M.; Shinchi, M.; Ito, K.; Asano, T.; Shinmoto, H.; et al. A pilot study of photoacoustic imaging system for improved real-time visualization of neurovascular bundle during radical prostatectomy. Prostate 2016, 76, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xing, M.; Cong, B.; Qiu, C.; He, D.; Wang, C.; Xiao, Y.; Yin, T.; Shao, M.; Qiu, W.; et al. In vivo transrectal imaging of canine prostate with a sensitive and compact handheld transrectal array photoacoustic probe for early diagnosis of prostate cancer. Biomed. Opt. Express 2019, 10, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, S.R.; Sonn, G.A.; Choe, J.W.; Nikoozadeh, A.; Bhuyan, A.; Park, K.K.; Cristman, P.; Fan, R.; Moini, A.; Lee, B.C.; et al. Simultaneous transrectal ultrasound and photoacoustic human prostate imaging. Sci. Transl. Med. 2019, 11, eaav2169. [Google Scholar] [CrossRef]
- Kothapalli, S.R.; Ma, T.J.; Vaithilingam, S.; Oralkan, Ö.; Khuri-Yakub, B.T.; Gambhir, S.S. Deep tissue photoacoustic imaging using a miniaturized 2-D capacitive micromachined ultrasonic transducer array. IEEE Trans. Biomed. Eng. 2012, 59, 1199–1204. [Google Scholar] [CrossRef]
- Wu, X.; Sanders, J.L.; Zhang, X.; Yamaner, F.Y.; Oralkan, Ö. An FPGA-based backend system for intravascular photoacoustic and ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 66, 45–56. [Google Scholar] [CrossRef]
- Dangi, A.; Cheng, C.Y.; Agrawal, S.; Tiwari, S.; Datta, G.R.; Benoit, R.R.; Pratap, R.; Trolier-Mckinstry, S.; Kothapalli, S.R. A Photoacoustic Imaging Device using Piezoelectric Micromachined Ultrasound Transducers (PMUTs). IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 67, 801–809. [Google Scholar] [CrossRef]
- Dangi, A.; Agrawal, S.; Tiwari, S.; Jadhav, S.; Cheng, C.; Datta, G.R.; Trolier-McKinstry, S.; Pratap, R.; Kothapalli, S.R. Ring PMUT array based miniaturized photoacoustic endoscopy device. In Photons Plus Ultrasound: Imaging and Sensing; International Society for Optics and Photonics: Bellingham, WA, USA, 2019; Volume 10878, p. 1087811. [Google Scholar]
- Şen, T.; Tüfekçioğlu, O.; Koza, Y. Mechanical index. Anatol. J. Cardiol. 2015, 15, 334. [Google Scholar] [CrossRef]
- Nelson, T.R.; Fowlkes, J.B.; Abramowicz, J.S.; Church, C.C. Ultrasound Biosafety Considerations for the Practicing Sonographer and Sonologist. J Ultrasound Med. 2009, 28, 139–150. [Google Scholar] [CrossRef]
- Fomenko, A.; Neudorfer, C.; Dallapiazza, R.F.; Kalia, S.K.; Lozano, A.M. Low-intensity ultrasound neuromodulation: An overview of mechanisms and emerging human applications. Brain Stimul. 2018, 11, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, T.A.; Church, C.C.; Sandstrom, K.; Abbott, J.G.; Ziskin, M.C.; Edmonds, P.D.; Herman, B.; Thomenius, K.E.; Teo, T.J. The thermal index: Its strengths, weaknesses, and proposed improvements. J. Ultrasound Med. 2011, 30, 714–734. [Google Scholar] [CrossRef] [PubMed]
- Laser Institute of America. American National Standard for Safe Use of Lasers; American National Standards Institute, Inc.: New York, NY, USA, 2000. [Google Scholar]
- OMLC Website. Available online: http://omlc.org/spectra/index.html (accessed on 15 June 2020).
- Martino, P.; Galosi, A.B. Practical recommendations for performing ultrasound scanning in the urological and andrological fields. In Atlas of Ultrasonography in Urology, Andrology, and Nephrology; Springer: Cham, Switzerland, 2017; pp. 695–728. [Google Scholar]
- Martin, C.J.; Sutton, D.G. Practical radiation protection in healthcare. In Medical Physics; Oxford University Press: New York NY, USA, 2015; p. 995. [Google Scholar]

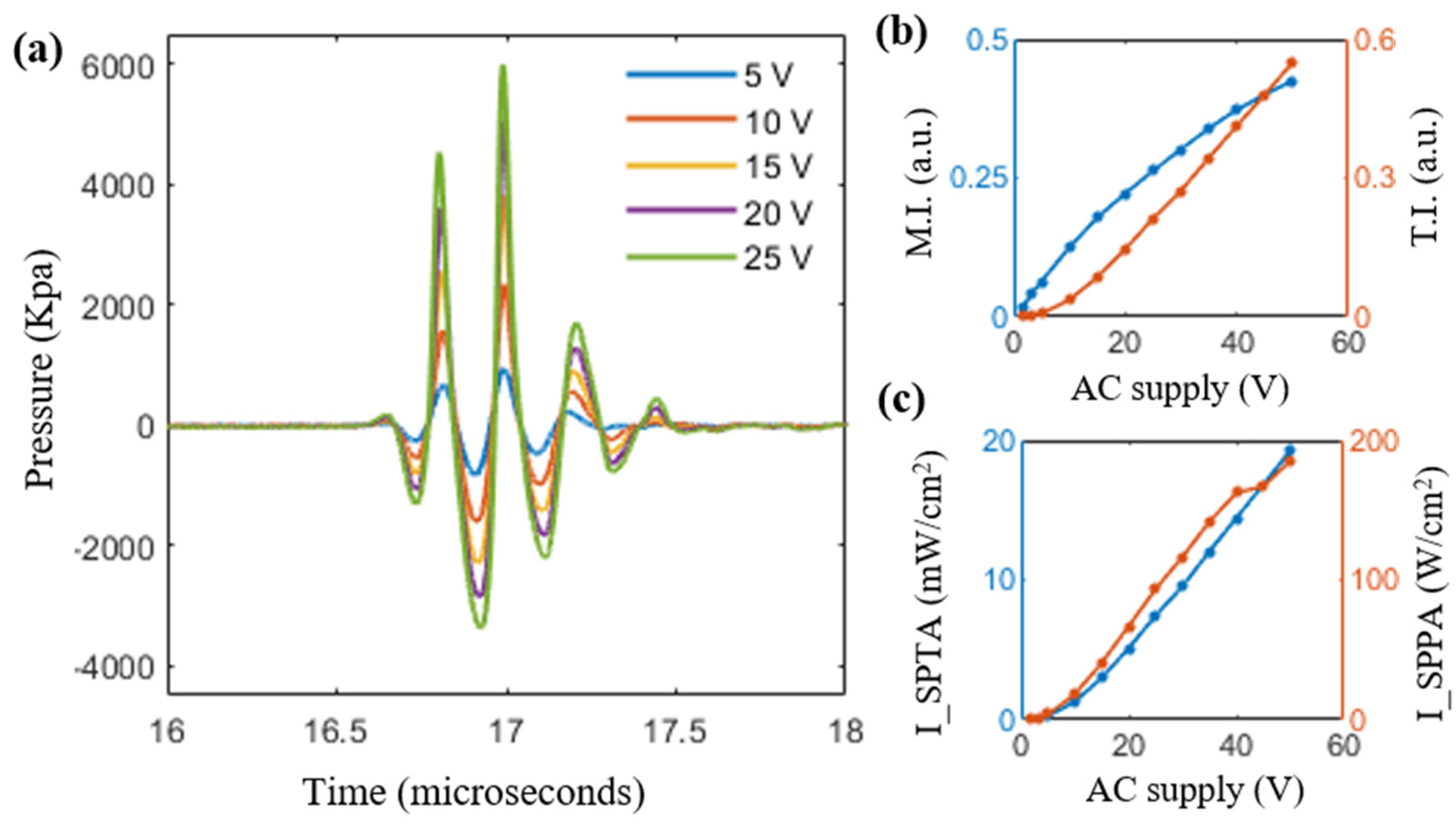
| Supplied AC Voltage (Volts) | Peak Negative Voltage (mV) | Peak Negative Pressure (MPa) | De-Rated Pressure (MPa) | Mechanical Index (MI) | ISPTA (mW/cm2) | ISPPA (W/cm2) | Thermal Index (TI) |
|---|---|---|---|---|---|---|---|
| 1.6 | 10.7 | 0.2192 | 0.0418 | 0.0170 | 0.01 | 0.22 | 0.0003 |
| 3.2 | 25.1 | 0.5172 | 0.0986 | 0.0402 | 0.09 | 1.43 | 0.0026 |
| 5.0 | 40.0 | 0.8234 | 0.1569 | 0.0641 | 0.27 | 3.97 | 0.0077 |
| 10.0 | 78.2 | 1.6085 | 0.3065 | 0.1251 | 1.3 | 18.26 | 0.0371 |
| 15.0 | 111.7 | 2.2981 | 0.4379 | 0.1788 | 2.99 | 40.64 | 0.0854 |
| 20.0 | 138.9 | 2.8578 | 0.5446 | 0.2223 | 5.12 | 66.88 | 0.1463 |
| 25.0 | 164.3 | 3.3800 | 0.6440 | 0.2629 | 7.44 | 93.50 | 0.2126 |
| 30.0 | 188.6 | 3.8805 | 0.7394 | 0.3019 | 9.54 | 116.54 | 0.2726 |
| 35.0 | 212.0 | 4.3628 | 0.8313 | 0.3394 | 12.04 | 141.61 | 0.3440 |
| 40.0 | 233.0 | 4.7936 | 0.9134 | 0.3729 | 14.43 | 162.45 | 0.4123 |
| 45.0 | 250.2 | 5.1479 | 0.9809 | 0.4005 | 16.82 | 167.01 | 0.4806 |
| 50.0 | 265.3 | 5.4585 | 1.0401 | 0.4246 | 19.23 | 184.91 | 0.54940 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, S.; Johnstonbaugh, K.; Clark, J.Y.; Raman, J.D.; Wang, X.; Kothapalli, S.-R. Design, Development, and Multi-Characterization of an Integrated Clinical Transrectal Ultrasound and Photoacoustic Device for Human Prostate Imaging. Diagnostics 2020, 10, 566. https://doi.org/10.3390/diagnostics10080566
Agrawal S, Johnstonbaugh K, Clark JY, Raman JD, Wang X, Kothapalli S-R. Design, Development, and Multi-Characterization of an Integrated Clinical Transrectal Ultrasound and Photoacoustic Device for Human Prostate Imaging. Diagnostics. 2020; 10(8):566. https://doi.org/10.3390/diagnostics10080566
Chicago/Turabian StyleAgrawal, Sumit, Kerrick Johnstonbaugh, Joseph Y. Clark, Jay D. Raman, Xueding Wang, and Sri-Rajasekhar Kothapalli. 2020. "Design, Development, and Multi-Characterization of an Integrated Clinical Transrectal Ultrasound and Photoacoustic Device for Human Prostate Imaging" Diagnostics 10, no. 8: 566. https://doi.org/10.3390/diagnostics10080566
APA StyleAgrawal, S., Johnstonbaugh, K., Clark, J. Y., Raman, J. D., Wang, X., & Kothapalli, S.-R. (2020). Design, Development, and Multi-Characterization of an Integrated Clinical Transrectal Ultrasound and Photoacoustic Device for Human Prostate Imaging. Diagnostics, 10(8), 566. https://doi.org/10.3390/diagnostics10080566






