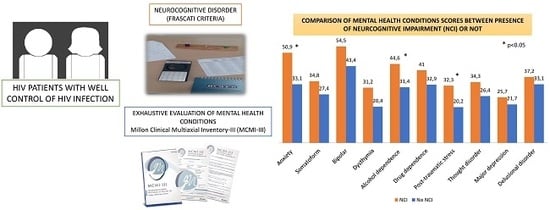
The Role of Mental Health Conditions in the Diagnosis of Neurocognitive Impairment in People Living with HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables
Procedure
2.3. Data Analysis
3. Results
3.1. Sociodemographic and Clinical Characteristics of the Sample
3.2. Prevalence of NCI and Mental Health Conditions
3.3. Association between Sociodemographic, Clinical-HIV Variables and Mental Health Conditions with NCI Diagnosis
3.4. Relation of Mental Health Conditions Score and NCI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ances, B.M.; Letendre, S.L. CROI 2019: Neurologic complications of HIV disease. Top. Antivir. Med. 2019, 27, 26–33. [Google Scholar] [PubMed]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Thaler, N.S.; Sayegh, P.; Arentoft, A.; Thames, A.D.; Castellon, S.A.; Hinkin, C.H. Increased neurocognitive intra-individual variability is associated with declines in medication adherence in HIV-infected adults. Neuropsychology 2015, 29, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.C.; Woods, S.P.; Vigil, O.; Heaton, R.K.; Schweinsburg, B.C.; Ellis, R.J.; Grant, I.; Marcotte, T.D.; The San Diego HIV Neurobehavioral Research Center (HNRC) Group. A neuropsychological investigation of multitasking in HIV infection: Implications for everyday functioning. Neuropsychology 2011, 25, 511–519. [Google Scholar] [CrossRef]
- Rueda, S.; Raboud, J.; Mustard, C.; Bayoumi, A.; Lavis, J.N.; Rourke, S.B. Employment status is associated with both physical and mental health quality of life in people living with HIV. AIDS Care 2011, 23, 435–443. [Google Scholar] [CrossRef]
- Alford, K.; Vera, J.H. Cognitive Impairment in people living with HIV in the ART era: A Review. Br. Med. Bull. 2018, 127, 55–68. [Google Scholar] [CrossRef]
- Lescure, F.-X.; Omland, L.H.; Engsig, F.N.; Roed, C.; Gerstoft, J.; Pialoux, G.; Kronborg, G.; Larsen, C.S.; Obel, N. Incidence and Impact on Mortality of Severe Neurocognitive Disorders in Persons With and Without HIV Infection: A Danish Nationwide Cohort Study. Clin. Infect. Dis. 2011, 52, 235–243. [Google Scholar] [CrossRef]
- González-Baeza, A.; Carvajal, F.; Bayón, C.; Pérez-Valero, I.; Estébanez, M.; Bernardino, J.I.; Monge, S.; Lagarde, M.; Hernando, A.; Arnalich, F.; et al. Pattern of neurocognitive function in patients receiving boosted protease inhibitor monotherapy: A detailed neuropsychological study. J. Neurovirol. 2014, 20, 362–370. [Google Scholar] [CrossRef]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef]
- Muñoz-Moreno, J.A.; Prats, A.; Pérez-Álvarez, N.; Fumaz, C.R.; Garolera, M.; Doval, E.; Negredo, E.; Ferrer, M.J.; Clotet, B. A Brief and Feasible Paper-Based Method to Screen for Neurocognitive Impairment in HIV-Infected Patients: The NEU Screen. J. Acquir. Immune Defic. Syndr. 2013, 63, 585–592. [Google Scholar] [CrossRef]
- Portilla, I.; Reus, S.; León, R.; van-der Hofstadt, C.; Sánchez, J.; López, N.; Boix, V.; Merino, E.; Portilla, J. Neurocognitive Impairment in Well-Controlled HIV-Infected Patients: A Cross-Sectional Study. AIDS Res. Hum. Retrovir. 2019, 35, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.; Bayon, C.; Molina, J.-M.; McNamara, P.; Resch, C.; Muñoz-Moreno, J.A.; Kulasegaram, R.; Schewe, K.; Burgos-Ramirez, A.; De Alvaro, C.; et al. Screening for neurocognitive impairment, depression, and anxiety in HIV-infected patients in Western Europe and Canada. AIDS Care 2014, 26, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Sacktor, N.; Skolasky, R.L.; Seaberg, E.; Munro, C.; Becker, J.T.; Martin, E.; Ragin, A.; Levine, A.; Miller, E. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016, 86, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Simioni, S.; Cavassini, M.; Annoni, J.-M.; Rimbault Abraham, A.; Bourquin, I.; Schiffer, V.; Calmy, A.; Chave, J.-P.; Giacobini, E.; Hirschel, B.; et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2009, 24, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Winston, A.; Arenas-Pinto, A.; Stöhr, W.; Fisher, M.; Orkin, C.M.; Aderogba, K.; De Burgh-Thomas, A.; O’Farrell, N.; Lacey, C.J.N.; Leen, C.; et al. Neurocognitive Function in HIV Infected Patients on Antiretroviral Therapy. PLoS ONE 2013, 8, e61949. [Google Scholar] [CrossRef] [PubMed]
- Tegger, M.K.; Crane, H.M.; Tapia, K.A.; Uldall, K.K.; Holte, S.E.; Kitahata, M.M. The Effect of Mental Illness, Substance Use, and Treatment for Depression on the Initiation of Highly Active Antiretroviral Therapy among HIV-Infected Individuals. AIDS Patient Care STDs 2008, 22, 233–243. [Google Scholar] [CrossRef]
- Lopes, M.; Olfson, M.; Rabkin, J.; Hasin, D.S.; Alegría, A.A.; Lin, K.-H.; Grant, B.F.; Blanco, C. Gender, HIV Status, and Psychiatric Disorders: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 2012, 73, 384–391. [Google Scholar] [CrossRef]
- Turan, B.; Budhwani, H.; Fazeli, P.L.; Browning, W.R.; Raper, J.L.; Mugavero, M.J.; Turan, J.M. How Does Stigma Affect People Living with HIV? The Mediating Roles of Internalized and Anticipated HIV Stigma in the Effects of Perceived Community Stigma on Health and Psychosocial Outcomes. AIDS Behav. 2017, 21, 283–291. [Google Scholar] [CrossRef]
- Chambers, L.A.; Rueda, S.; Baker, D.N.; Wilson, M.G.; Deutsch, R.; Raeifar, E.; Rourke, S.B.; Team, T.S.R. Stigma, HIV and health: A qualitative synthesis. BMC Public Health 2015, 15, 848. [Google Scholar] [CrossRef]
- Underwood, J.; Winston, A. Guidelines for Evaluation and Management of Cognitive Disorders in HIV-Positive Individuals. Curr. HIV/AIDS Reports 2016, 13, 235–240. [Google Scholar] [CrossRef]
- Fehr, J.; Nicca, D.; Langewitz, W.; Haerry, D. Guidelines of European AIDS Clinical Society (EACS). 2018. Available online: https://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf (accessed on 14 July 2020).
- Carey, C.L.; Paul Woods, S.; Rippeth, J.D.; Heaton, R.K.; Grant, I.; the HIV Neurobehavioral Research Center (HNRC) Group. Prospective Memory in HIV-1 Infection. J. Clin. Exp. Neuropsychol. 2006, 28, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Lin, H.; Liu, X.; Wong, F.Y.; Sun, Y.V.; Marconi, V.C.; He, N. Higher Prevalence of Frailty Among a Sample of HIV-Infected Middle-aged and Older Chinese Adults Is Associated With Neurocognitive Impairment and Depressive Symptoms. J. Infect. Dis. 2017, 215, 687–692. [Google Scholar] [CrossRef] [PubMed][Green Version]
- For the Cognitive Impairment in People with HIV in the European Region (CIPHER) Study Group; Haddow, L.J.; Laverick, R.; Daskalopoulou, M.; McDonnell, J.; Lampe, F.C.; Gilson, R.; Speakman, A.; Antinori, A.; Balestra, P.; et al. Multicenter European Prevalence Study of Neurocognitive Impairment and Associated Factors in HIV Positive Patients. AIDS Behav. 2018, 22, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Marin-Webb, V.; Jessen, H.; Kopp, U.; Jessen, A.B.; Hahn, K. Validation of the International HIV Dementia Scale as a Screening Tool for HIV-Associated Neurocognitive Disorders in a German-Speaking HIV Outpatient Clinic. PLoS ONE 2016, 11, e0168225. [Google Scholar] [CrossRef]
- Giancola, M.L.; Balestra, P.; Ammassari, A.; Ricottini, M.; Lorenzini, P.; Angeletti, C.; Bellagamba, R.; Tommasi, C.; Tempestilli, M.; Zaccarelli, M.; et al. Prevalence and Associated Factors of Neurocognitive Impairment in HIV-Positive Patients on Effective Efavirenz/Emtricitabine/Tenofovir Disoproxil Fumarate Treatment. AIDS Res. Hum. Retrovir. 2018, 34, 907–908. [Google Scholar] [CrossRef]
- Hoenigl, M.; de Oliveira, M.F.; Pérez-Santiago, J.; Zhang, Y.; Morris, S.; McCutchan, A.J.; Finkelman, M.; Marcotte, T.D.; Ellis, R.J.; Gianella, S. (1→3)-β-D-Glucan Levels Correlate With Neurocognitive Functioning in HIV-Infected Persons on Suppressive Antiretroviral Therapy: A Cohort Study. Medicine 2016, 95, e3162. [Google Scholar] [CrossRef]
- Zimmerman, M.; Posternak, M.A.; Chelminski, I. Is It Time to Replace the Hamilton Depression Rating Scale as the Primary Outcome Measure in Treatment Studies of Depression? J. Clin. Psychopharmacol. 2005, 25, 105–110. [Google Scholar] [CrossRef]
- Fialho, R.M.; Pereira, M.; Mendonça, N.; Ouakinin, S. Depressive Symptoms and Neurocognitive Performance Among HIV-Infected Women. Women Health 2013, 53, 117–134. [Google Scholar] [CrossRef]
- Cysique, L.A.; Deutsch, R.; Atkinson, J.H.; Young, C.; Marcotte, T.D.; Dawson, L.; Grant, I.; Heaton, R.K.; THE HNRC GROUP. Incident major depression does not affect neuropsychological functioning in HIV-infected men. J. Int. Neuropsychol. Soc. 2007, 13, 1–11. [Google Scholar] [CrossRef]
- Pinheiro, C.A.T.; Souza, L.D.M.; Motta, J.V.S.; Kelbert, E.F.; Souza, M.S.; Martins, C.S.R.; Coelho, F.M.C.; Pinheiro, K.A.T.; Pinheiro, R.T. Depression and diagnosis of neurocognitive impairment in HIV-positive patients. Braz. J. Med. Biol. Res. 2016, 49, e5344. [Google Scholar] [CrossRef]
- The Mind Exchange Working Group; Antinori, A.; Arendt, G.; Grant, I.; Letendre, S.; Chair; Munoz-Moreno, J.A.; Eggers, C.; Brew, B.; Brouillette, M.-J.; et al. Assessment, Diagnosis, and Treatment of HIV-Associated Neurocognitive Disorder: A Consensus Report of the Mind Exchange Program. Clin. Infect. Dis. 2013, 56, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Cardenal, V.; y Sánchez, M.P. Adaptación y Baremación al Español del Inventario Clínico Multiaxial de Millon-III (MCMI-III); Pearson: Madrid, Spain, 2007. [Google Scholar]
- Millon, T.; Davis, R.; Millon, C. Millon Clinical Multiaxial Inventory (MCMI-III); Pearson: Minneapolis, MN, USA, 1997. [Google Scholar]
- Whetten, K.; Reif, S.; Whetten, R.; Murphy-McMillan, L.K. Trauma, Mental Health, Distrust, and Stigma Among HIV-Positive Persons: Implications for Effective Care. Psychosom. Med. 2008, 70, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Gore-Felton, C.; Koopman, C. Behavioral Mediation of the Relationship Between Psychosocial Factors and HIV Disease Progression. Psychosom. Med. 2008, 70, 569–574. [Google Scholar] [CrossRef]
- Schuster, R.; Bornovalova, M.; Hunt, E. The Influence of Depression on the Progression of HIV: Direct and Indirect Effects. Behav. Modif. 2012, 36, 123–145. [Google Scholar] [CrossRef]
- Ironson, G.; Balbin, E.; Stuetzle, R.; Fletcher, M.A.; O’Cleirigh, C.; Laurenceau, J.P.; Schneiderman, N.; Solomon, G. Dispositional optimism and the mechanisms by which it predicts slower disease progression in HIV: Proactive behavior, avoidant coping, and depression. Int. J. Behav. Med. 2005, 12, 86–97. [Google Scholar] [CrossRef]
- Lima, V.D.; Geller, J.; Bangsberg, D.R.; Patterson, T.L.; Daniel, M.; Kerr, T.; Montaner, J.S.; Hogg, R.S. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS 2007, 21, 1175–1183. [Google Scholar] [CrossRef]
- Latkin, C.A.; Curry, A.D.; Hua, W.; Davey, M.A. Direct and Indirect Associations of Neighborhood Disorder With Drug Use and High-Risk Sexual Partners. Am. J. Prev. Med. 2007, 32, S234–S241. [Google Scholar] [CrossRef]
- Murphy, D.A.; Durako, S.J.; Moscicki, A.B.; Vermund, S.H.; Ma, Y.; Schwarz, D.F.; Muenz, L.R.; Adolescent Medicine HIV/AIDS Research Network. No change in health risk behaviors over time among HIV infected adolescents in care: Role of psychological distress. J. Adolesc. Health 2001, 29, 57–63. [Google Scholar] [CrossRef]
- Galvan, F.H.; Burnam, M.A.; Bing, E.G. Co-occurring Psychiatric Symptoms and Drug Dependence or Heavy Drinking Among HIV-Positive People. J. Psychoact. Drugs 2003, 35, 153–160. [Google Scholar] [CrossRef]
- Samet, J.H.; Horton, N.J.; Meli, S.; Freedberg, K.A.; Palepu, A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol. Clin. Exp. Res. 2004, 28, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.H.; Horton, N.J.; Traphagen, E.T.; Lyon, S.M.; Freedberg, K.A. Alcohol Consumption and HIV Disease Progression: Are They Related? Alcohol. Clin. Exp. Res. 2003, 27, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Dingle, G.A.; Oei, T.P.S. Is alcohol a cofactor of HIV and AIDS? Evidence from immunological and behavioral studies. Psychol. Bull. 1997, 122, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Kresina, T.F.; Flexner, C.W.; Sinclair, J.; Correia, M.A.; Stapleton, J.T.; Adeniyi-Jones, S.; Cargill, V.; Cheever, L.W. Alcohol Use and HIV Pharmacotherapy. AIDS Res. Hum. Retrovir. 2002, 18, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Stevens, O.; Moncrieff, M.; Gafos, M. Chemsex-related drug use and its association with health outcomes in men who have sex with men: A cross-sectional analysis of Antidote clinic service data. Sex. Transm. Infect. 2020, 96, 124–130. [Google Scholar] [CrossRef]
- Bloch, M.; Kamminga, J.; Jayewardene, A.; Bailey, M.; Carberry, A.; Vincent, T.; Quan, D.; Maruff, P.; Brew, B.; Cysique, L.A. A Screening Strategy for HIV-Associated Neurocognitive Disorders That Accurately Identifies Patients Requiring Neurological Review. Clin. Infect. Dis. 2016, 63, 687–693. [Google Scholar] [CrossRef]
- Shrestha, R.; Weikum, D.; Copenhaver, M.; Altice, F.L. The Influence of Neurocognitive Impairment, Depression, and Alcohol Use Disorders on Health-Related Quality of Life among Incarcerated, HIV-Infected, Opioid Dependent Malaysian Men: A Moderated Mediation Analysis. AIDS Behav. 2017, 21, 1070–1081. [Google Scholar] [CrossRef]
- Solé, B.; Jiménez, E.; Torrent, C.; Reinares, M.; del Mar Bonnin, C.; Torres, I.; Varo, C.; Grande, I.; Valls, E.; Salagre, E.; et al. Cognitive Impairment in Bipolar Disorder: Treatment and Prevention Strategies. Int. J. Neuropsychopharmacol. 2017, 20, 670–680. [Google Scholar] [CrossRef]
- Meade, C.S.; Towe, S.L.; Skalski, L.M.; Robertson, K.R. Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug Alcohol Depend. 2015, 149, 128–135. [Google Scholar] [CrossRef]
- Díaz-Caneja, C.M.; Cervilla, J.A.; Haro, J.M.; Arango, C.; de Portugal, E. Cognition and functionality in delusional disorder. Eur. Psychiatry 2019, 55, 52–60. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ho, W.; Ramirez, S.H.; Potula, R.; Abood, M.E.; Unterwald, E.; Tuma, R. HIV-1 infection and alcohol abuse: Neurocognitive impairment, mechanisms of neurodegeneration and therapeutic interventions. Brain Behav. Immun. 2011, 25, S61–S70. [Google Scholar] [CrossRef] [PubMed]
| Sociodemographic and Clinical Characteristics | Variables | ||
|---|---|---|---|
| Male, n (%) | 77.5 (62) | ||
| Age (years), mean ± SD | 46.1 ± 7.6 | ||
| Age (≥44 years), n (%) | 56.3 (45) | ||
| Educational levels, n (%): | |||
| Primary or no education | 33.8 (27) | ||
| Secondary education | 41.3 (33) | ||
| University education | 25.0 (20) | ||
| Clinical HIV variables | |||
| HIV transmission mechanism, n (%) | |||
| Former-IDU 1 | 7.5 (6) | ||
| Sexual: | 92.4 (74) | ||
| MSM 2 | 58.8 (47) | ||
| HTX 3 | 33.8 (27) | ||
| Time since HIV diagnosis (years), mean ± SD | 12.4 ± 7.1 | ||
| ≥10 years since HIV diagnosis, n (%) | 61.3 (49) | ||
| Current CD4+ lymphocytes (cells/µL.), mean ± SD | 653.5 ± 219.5 | ||
| Current CD4+ count < 500 cells/µL., n (%) | 23.8 (19) | ||
| Nadir CD4+ lymphocyte (cells/µL.), mean ± SD | 246.0 ± 151.6 | ||
| Nadir CD4+ count ≤ 350 cells/µL, n (%) | 76.3 (61) | ||
| Mental Health Conditions | Prevalence (95% CI) 1 | n |
|---|---|---|
| Anxiety | 21.3 (11.7–30.8) | 17 |
| Somatoform | 5.0 (1.4–12.3) | 4 |
| Bipolar | 11.3 (3.7–18.8) | 9 |
| Dysthymia | 2.5 (0.3–8.7) | 2 |
| Alcohol dependence | 2.5 (0.3–8.7) | 2 |
| Drug dependence | 8.8 (1.9–15.6) | 7 |
| Post-traumatic stress | - | 0 |
| Thought disorder | 5.0 (1.4–12.3) | 4 |
| Major depression | - | 0 |
| Delusional disorder | 6.3 (2.0–14.0) | 5 |
| At least one mental health condition | 37.5 (26.3–48.7) | 30 |
| Sociodemographic, Clinical HIV Variables and Mental Health Conditions | Neurocognitive Impairment | Orc 3 (95% CI) 4 | pc5 | Ora 6 (95% CI) 4 | pa7 | ||
|---|---|---|---|---|---|---|---|
| % | (n/N) | ||||||
| Gender | |||||||
| Male | 22.6 | (14/62) | 0.5 (0.2–1.5) | 0.224 | - | - | |
| Female | 38.9 | (7/18) | 1 | ||||
| Age | |||||||
| ≥44 years | 31.1 | (14/45) | 1.8 (0.6–5.1) | 0.263 | - | - | |
| <44 years | 20.0 | (7/35) | 1 | ||||
| Education level | |||||||
| Non/Primary | 37.0 | (10/27) | 2.7 (1.0–7.0) | 0.118 | - | - | |
| Secondary/University | 20.8 | (11/53) | 1 | ||||
| HIV mechanism transmission | |||||||
| Former-IDU 1 | 33.3 | (2/6) | 1.5 (0.3–8.6) | 0.650 | - | - | |
| Sexual 2 | 25.7 | (21/80) | 1 | ||||
| Time since diagnosis | |||||||
| ≥10 years | 36.7 | (18/49) | 5.5 (1.4–20.4) | 0.007 | 5.0 (1.2–21.6) | 0.030 | |
| <10 years | 9.7 | (3/31) | 1 | ||||
| Current CD4+ (cells/µL) | |||||||
| <500/µL | 21.1 | (4/19) | 0.7 (0.2–2.4) | 0.767 | - | - | |
| ≥500/µL | 27.9 | (17/61) | 1 | ||||
| Nadir CD4+ (cells/µL) | |||||||
| ≤350/µL | 32.8 | (20/61) | 8.8 (1.1–70.5) | 0.018 | 6.6 (0.7–59.4) | 0.095 | |
| >350/µL | 5.3 | (1/19) | 1 | ||||
| At least one mental health condition | |||||||
| Presence | 46.7 | (14/30) | 5.4 (1.8–15.7) | 0.001 | 6.3 (1.9–20.8) | 0.002 | |
| Absence | 14.0 | (7/50) | 1 | ||||
| Mental Health Conditions | Neurocognitive Impairment | t 3 | p 4 | d 5 | |||
|---|---|---|---|---|---|---|---|
| Yes (n = 21) | No (n = 59) | ||||||
| M 1 | SD 2 | M 1 | SD 2 | ||||
| Anxiety | 50.9 | 32.6 | 33.1 | 30.8 | 2.2 | 0.028 | 0.56 |
| Somatoform | 34.8 | 27.7 | 27.4 | 26.5 | 1.1 | 0.285 | 0.28 |
| Bipolar | 54.5 | 22.9 | 43.4 | 24.4 | 1.8 | 0.072 | 0.47 |
| Dysthymia | 31.2 | 25.3 | 20.4 | 24.1 | 1.8 | 0.085 | 0.44 |
| Alcohol dependence | 44.6 | 25.3 | 31.4 | 22.8 | 2.2 | 0.029 | 0.55 |
| Drug dependence | 41.0 | 27.9 | 32.9 | 25.2 | 1.2 | 0.222 | 0.30 |
| Post-traumatic stress | 32.3 | 22.2 | 20.2 | 22.9 | 2.1 | 0.039 | 0.53 |
| Thought disorder | 34.3 | 28.7 | 26.4 | 24.6 | 1.2 | 0.229 | 0.30 |
| Major depression | 25.7 | 28.6 | 21.7 | 23.0 | 0.6 | 0.569 | 0.15 |
| Delusional disorder | 37.2 | 34.5 | 33.1 | 31.6 | 0.5 | 0.618 | 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portilla-Tamarit, I.; Ruiz-Robledillo, N.; Díez-Martínez, M.; Ferrer-Cascales, R.; Alcocer-Bruno, C.; Portilla, J. The Role of Mental Health Conditions in the Diagnosis of Neurocognitive Impairment in People Living with HIV. Diagnostics 2020, 10, 543. https://doi.org/10.3390/diagnostics10080543
Portilla-Tamarit I, Ruiz-Robledillo N, Díez-Martínez M, Ferrer-Cascales R, Alcocer-Bruno C, Portilla J. The Role of Mental Health Conditions in the Diagnosis of Neurocognitive Impairment in People Living with HIV. Diagnostics. 2020; 10(8):543. https://doi.org/10.3390/diagnostics10080543
Chicago/Turabian StylePortilla-Tamarit, Irene, Nicolás Ruiz-Robledillo, Marcos Díez-Martínez, Rosario Ferrer-Cascales, Cristian Alcocer-Bruno, and Joaquín Portilla. 2020. "The Role of Mental Health Conditions in the Diagnosis of Neurocognitive Impairment in People Living with HIV" Diagnostics 10, no. 8: 543. https://doi.org/10.3390/diagnostics10080543
APA StylePortilla-Tamarit, I., Ruiz-Robledillo, N., Díez-Martínez, M., Ferrer-Cascales, R., Alcocer-Bruno, C., & Portilla, J. (2020). The Role of Mental Health Conditions in the Diagnosis of Neurocognitive Impairment in People Living with HIV. Diagnostics, 10(8), 543. https://doi.org/10.3390/diagnostics10080543