Decreased Cytoplasmic Expression of ADAMTS14 Is Correlated with Reduced Survival Rates in Oral Squamous Cell Carcinoma Patients
Abstract
1. Introduction
2. Results
2.1. Clinical Prameters of OSCC Patients
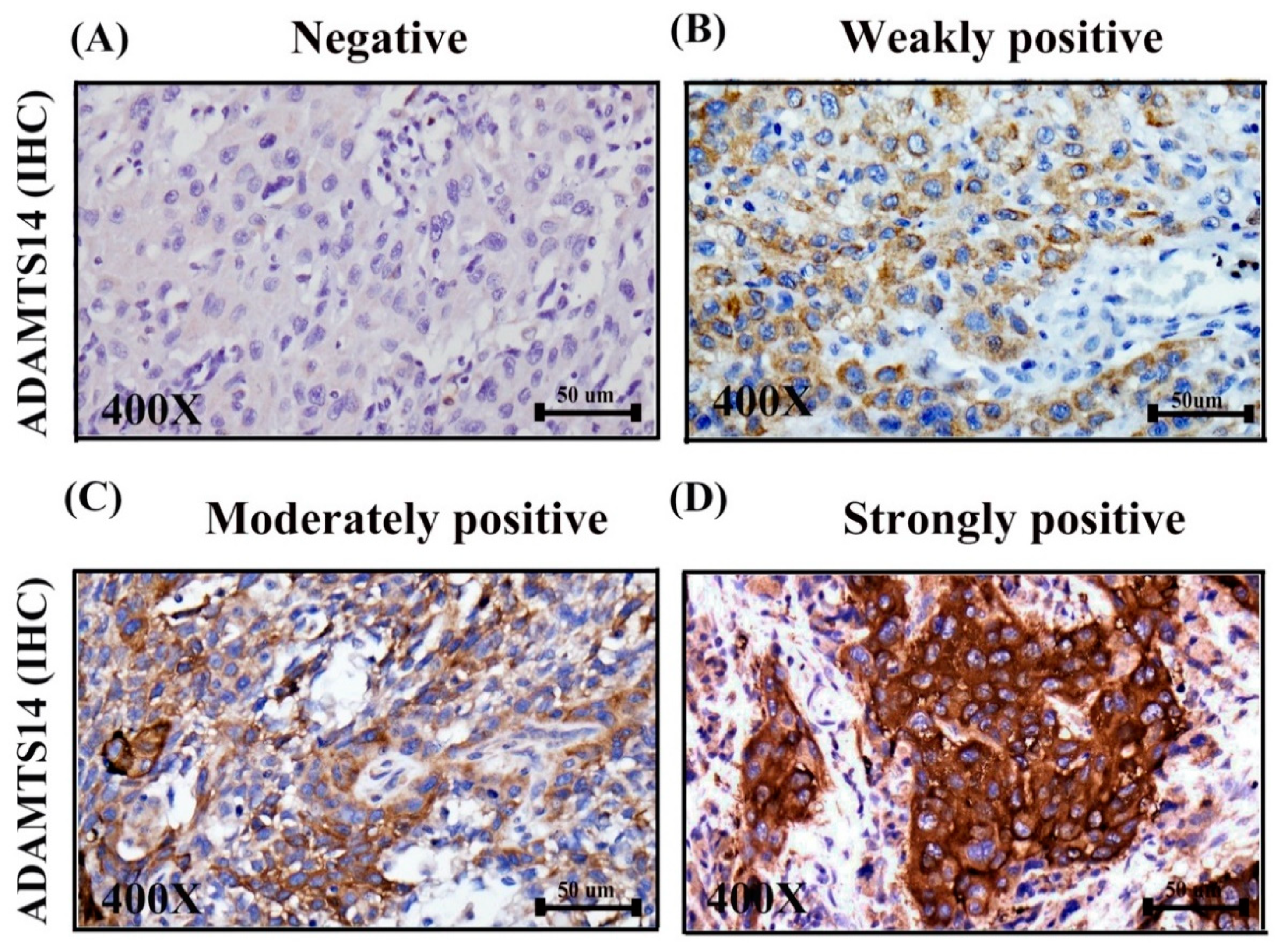
2.2. Correlations between ADAMTS14 Expression and ClinicopathologicF of OSCC
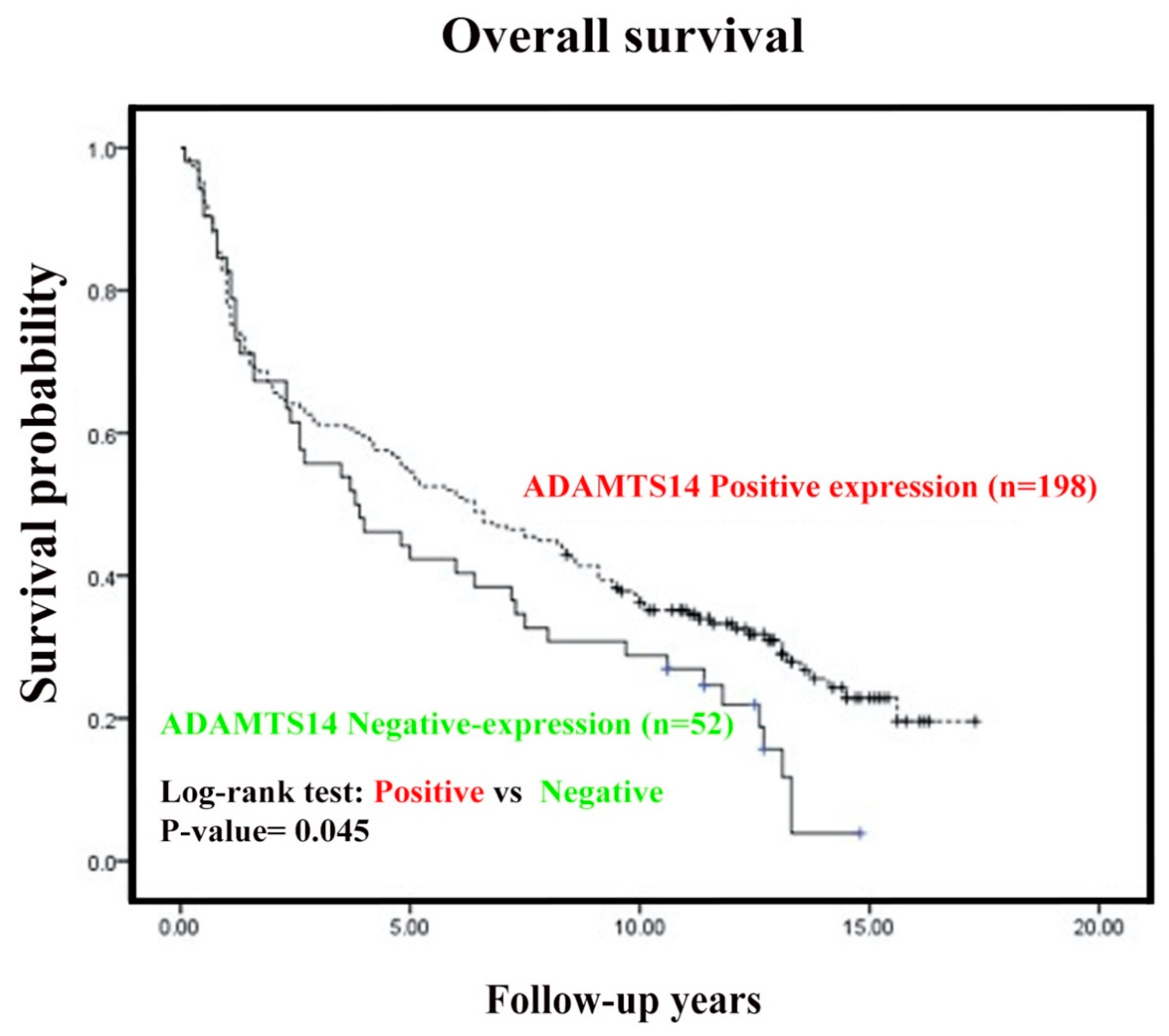
2.3. Negative Cytoplasmic Expression of ADAMTS14 was Associated with Short Overall Survival in OSCC Patients
2.4. Prognostic Variables in OSCC Patients Determined According to Cox Proportional Hazard Model Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Statement and Tissue Microarrays (TMAs)
4.2. Immunohistochemistry Analysis
4.3. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Kang, H.; Kiess, A.; Chung, C.H. Emerging biomarkers in head and neck cancer in the era of genomics. Nat. Rev. Clin. Oncol. 2015, 12, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.Y.; Lim, E. An overview of detection and screening of oral cancer in taiwan. Chin. J. Dent. Res. 2015, 18, 7–12. [Google Scholar] [PubMed]
- Shah, J.P.; Singh, B. Keynote comment: Why the lack of progress for oral cancer? Lancet Oncol. 2006, 7, 356–357. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Adel, M.; Liao, C.T.; Lee, L.Y.; Hsueh, C.; Lin, C.Y.; Fan, K.H.; Wang, H.M.; Ng, S.H.; Lin, C.H.; Tsao, C.K.; et al. Incidence and outcomes of patients with oral cavity squamous cell carcinoma and fourth primary tumors: A long-term follow-up study in a betel quid chewing endemic area. Medicine (Baltimore) 2016, 95, e2950. [Google Scholar] [CrossRef]
- Singh, J.P.; Zhang, K.; Wu, J.; Yang, X. O-glcnac signaling in cancer metabolism and epigenetics. Cancer Lett. 2015, 356, 244–250. [Google Scholar] [CrossRef]
- Krueger, K.E.; Srivastava, S. Posttranslational protein modifications: Current implications for cancer detection, prevention, and therapeutics. Mol. Cell Proteomics 2006, 5, 1799–1810. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of oral cancer. Int J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Warnakulasuriya, S. Causes of oral cancer--an appraisal of controversies. Br. Dent. J. 2009, 207, 471–475. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver mir-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The adamts (a disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Apte, S.S. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (adamts) superfamily: Functions and mechanisms. J. Biol Chem 2009, 284, 31493–31497. [Google Scholar] [CrossRef] [PubMed]
- Stanton, H.; Melrose, J.; Little, C.B.; Fosang, A.J. Proteoglycan degradation by the adamts family of proteinases. Biochim. Biophys Acta 2011, 1812, 1616–1629. [Google Scholar] [CrossRef]
- Lin, E.A.; Liu, C.J. The role of adamtss in arthritis. Protein Cell 2010, 1, 33–47. [Google Scholar] [CrossRef]
- Rocks, N.; Paulissen, G.; El Hour, M.; Quesada, F.; Crahay, C.; Gueders, M.; Foidart, J.M.; Noel, A.; Cataldo, D. Emerging roles of adam and adamts metalloproteinases in cancer. Biochimie 2008, 90, 369–379. [Google Scholar] [CrossRef]
- Wagstaff, L.; Kelwick, R.; Decock, J.; Edwards, D.R. The roles of adamts metalloproteinases in tumorigenesis and metastasis. Front. Biosci. (Landmark Ed.) 2011, 16, 1861–1872. [Google Scholar] [CrossRef]
- Kumar, S.; Rao, N.; Ge, R. Emerging roles of adamtss in angiogenesis and cancer. Cancers (Basel) 2012, 4, 1252–1299. [Google Scholar] [CrossRef]
- Noel, A.; Gutierrez-Fernandez, A.; Sounni, N.E.; Behrendt, N.; Maquoi, E.; Lund, I.K.; Cal, S.; Hoyer-Hansen, G.; Lopez-Otin, C. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front. Pharmacol. 2012, 3, 140. [Google Scholar] [CrossRef]
- Cal, S.; Lopez-Otin, C. Adamts proteases and cancer. Matrix Biol. 2015, 44–46, 77–85. [Google Scholar] [CrossRef]
- Salter, R.C.; Ashlin, T.G.; Kwan, A.P.; Ramji, D.P. Adamts proteases: Key roles in atherosclerosis? J. Mol. Med. (Berl) 2010, 88, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, C.; Cormier-Daire, V. The adamts(l) family and human genetic disorders. Hum. Mol. Genet. 2011, 20, R163–R167. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, P.E.; Howell, M.D. Adamts expression and function in central nervous system injury and disorders. Matrix Biol. 2015, 44–46, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Colige, A.; Vandenberghe, I.; Thiry, M.; Lambert, C.A.; Van Beeumen, J.; Li, S.W.; Prockop, D.J.; Lapiere, C.M.; Nusgens, B.V. Cloning and characterization of adamts-14, a novel adamts displaying high homology with adamts-2 and adamts-3. J. Biol. Chem. 2002, 277, 5756–5766. [Google Scholar] [CrossRef] [PubMed]
- Bolz, H.; Ramirez, A.; von Brederlow, B.; Kubisch, C. Characterization of adamts14, a novel member of the adamts metalloproteinase family. Biochim. Biophys Acta 2001, 1522, 221–225. [Google Scholar] [CrossRef]
- Kay, B.K.; Williamson, M.P.; Sudol, M. The importance of being proline: The interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000, 14, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Opitz, R.; Muller, M.; Reuter, C.; Barone, M.; Soicke, A.; Roske, Y.; Piotukh, K.; Huy, P.; Beerbaum, M.; Wiesner, B.; et al. A modular toolkit to inhibit proline-rich motif-mediated protein-protein interactions. Proc. Natl. Acad. Sci. USA 2015, 112, 5011–5016. [Google Scholar] [CrossRef]
- Dubail, J.; Kesteloot, F.; Deroanne, C.; Motte, P.; Lambert, V.; Rakic, J.M.; Lapiere, C.; Nusgens, B.; Colige, A. Adamts-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity. Cell Mol. Life Sci. 2010, 67, 4213–4232. [Google Scholar] [CrossRef]
- Bekhouche, M.; Colige, A. The procollagen n-proteinases adamts2, 3 and 14 in pathophysiology. Matrix Biol. 2015, 44–46, 46–53. [Google Scholar] [CrossRef]
- Goertsches, R.; Comabella, M.; Navarro, A.; Perkal, H.; Montalban, X. Genetic association between polymorphisms in the adamts14 gene and multiple sclerosis. J. Neuroimmunol. 2005, 164, 140–147. [Google Scholar] [CrossRef]
- El Khoury, L.; Posthumus, M.; Collins, M.; Handley, C.J.; Cook, J.; Raleigh, S.M. Polymorphic variation within the adamts2, adamts14, adamts5, adam12 and timp2 genes and the risk of achilles tendon pathology: A genetic association study. J. Sci. Med. Sport 2013, 16, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Poonpet, T.; Honsawek, S.; Tammachote, N.; Kanitnate, S.; Tammachote, R. Adamts14 gene polymorphism associated with knee osteoarthritis in thai women. Genet. Mol. Res. 2013, 12, 5301–5309. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lopez, J.; Pombo-Suarez, M.; Loughlin, J.; Tsezou, A.; Blanco, F.J.; Meulenbelt, I.; Slagboom, P.E.; Valdes, A.M.; Spector, T.D.; Gomez-Reino, J.J.; et al. Association of a nssnp in adamts14 to some osteoarthritis phenotypes. Osteoarthritis Cartilage 2009, 17, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hsieh, M.J.; Liu, Y.F.; Chou, Y.E.; Lin, C.W.; Yang, S.F. Adamts14 gene polymorphism and environmental risk in the development of oral cancer. PLoS ONE 2016, 11, e0159585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Porter, S.; Scott, S.D.; Sassoon, E.M.; Williams, M.R.; Jones, J.L.; Girling, A.C.; Ball, R.Y.; Edwards, D.R. Dysregulated expression of adamalysin-thrombospondin genes in human breast carcinoma. Clin. Cancer Res. 2004, 10, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.J.; Hsieh, M.J.; Chou, Y.E.; Wang, P.H.; Yeh, C.B.; Yang, S.F.; Lee, H.L.; Liu, Y.F. Effects of adamts14 genetic polymorphism and cigarette smoking on the clinicopathologic development of hepatocellular carcinoma. PLoS ONE 2017, 12, e0172506. [Google Scholar] [CrossRef] [PubMed]
- Looijenga, L.H.; Stoop, H.; de Leeuw, H.P.; de Gouveia Brazao, C.A.; Gillis, A.J.; van Roozendaal, K.E.; van Zoelen, E.J.; Weber, R.F.; Wolffenbuttel, K.P.; van Dekken, H.; et al. Pou5f1 (oct3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003, 63, 2244–2250. [Google Scholar]
- Ho, Y.J.; Shih, C.P.; Yeh, K.T.; Shi, B.; Gong, Z.; Lin, Y.M.; Lu, J.W. Correlation between high expression levels of jumonji domain-containing 4 and short survival in cases of colon adenocarcinoma. Biochem. Biophys Res. Commun. 2018, 503, 1442–1449. [Google Scholar] [CrossRef]
- Tao, R.; Li, Q.; Gao, X.; Ma, L. Overexpression of grk6 associates with the progression and prognosis of colorectal carcinoma. Oncol. Lett. 2018, 15, 5879–5886. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, Y.; Wang, K.; He, Z.; Gong, Z.; Zhao, Z.; Yang, Y.; Gao, X.; Li, F.; Wu, H.; et al. Biomarkers: Paving stones on the road towards the personalized precision medicine for oral squamous cell carcinoma. BMC Cancer 2018, 18, 911. [Google Scholar] [CrossRef]
- Rocks, N.; Paulissen, G.; Quesada Calvo, F.; Polette, M.; Gueders, M.; Munaut, C.; Foidart, J.M.; Noel, A.; Birembaut, P.; Cataldo, D. Expression of a disintegrin and metalloprotease (adam and adamts) enzymes in human non-small-cell lung carcinomas (nsclc). Br. J. Cancer 2006, 94, 724–730. [Google Scholar] [CrossRef]
- Held-Feindt, J.; Paredes, E.B.; Blomer, U.; Seidenbecher, C.; Stark, A.M.; Mehdorn, H.M.; Mentlein, R. Matrix-degrading proteases adamts4 and adamts5 (disintegrins and metalloproteinases with thrombospondin motifs 4 and 5) are expressed in human glioblastomas. Int. J. Cancer 2006, 118, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Filou, S.; Korpetinou, A.; Kyriakopoulou, D.; Bounias, D.; Stavropoulos, M.; Ravazoula, P.; Papachristou, D.J.; Theocharis, A.D.; Vynios, D.H. Adamts expression in colorectal cancer. PLoS ONE 2015, 10, e0121209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Huang, J.; Yang, Z. The roles of adamts in angiogenesis and cancer. Tumour Biol. 2015, 36, 4039–4051. [Google Scholar] [CrossRef] [PubMed]
- Casal, C.; Torres-Collado, A.X.; Plaza-Calonge Mdel, C.; Martino-Echarri, E.; Ramon, Y.C.S.; Rojo, F.; Griffioen, A.W.; Rodriguez-Manzaneque, J.C. Adamts1 contributes to the acquisition of an endothelial-like phenotype in plastic tumor cells. Cancer Res. 2010, 70, 4676–4686. [Google Scholar] [CrossRef] [PubMed]
- Lind, G.E.; Kleivi, K.; Meling, G.I.; Teixeira, M.R.; Thiis-Evensen, E.; Rognum, T.O.; Lothe, R.A. Adamts1, crabp1, and nr3c1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell Oncol. 2006, 28, 259–272. [Google Scholar]
- Rosendahl, M.S.; Ko, S.C.; Long, D.L.; Brewer, M.T.; Rosenzweig, B.; Hedl, E.; Anderson, L.; Pyle, S.M.; Moreland, J.; Meyers, M.A.; et al. Identification and characterization of a pro-tumor necrosis factor-alpha-processing enzyme from the adam family of zinc metalloproteases. J. Biol. Chem. 1997, 272, 24588–24593. [Google Scholar] [CrossRef]
- Hinkle, C.L.; Mohan, M.J.; Lin, P.; Yeung, N.; Rasmussen, F.; Milla, M.E.; Moss, M.L. Multiple metalloproteinases process protransforming growth factor-alpha (protgf-alpha). Biochemistry 2003, 42, 2127–2136. [Google Scholar] [CrossRef]
- Atfi, A.; Dumont, E.; Colland, F.; Bonnier, D.; L’Helgoualc’h, A.; Prunier, C.; Ferrand, N.; Clement, B.; Wewer, U.M.; Theret, N. The disintegrin and metalloproteinase adam12 contributes to tgf-beta signaling through interaction with the type ii receptor. J. Cell Biol. 2007, 178, 201–208. [Google Scholar] [CrossRef]
- Dupont, L.; Ehx, G.; Chantry, M.; Monseur, C.; Leduc, C.; Janssen, L.; Cataldo, D.; Thiry, M.; Jerome, C.; Thomassin, J.M.; et al. Spontaneous atopic dermatitis due to immune dysregulation in mice lacking adamts2 and 14. Matrix Biol. 2018, 70, 140–157. [Google Scholar] [CrossRef]
- Fernandes, R.J.; Hirohata, S.; Engle, J.M.; Colige, A.; Cohn, D.H.; Eyre, D.R.; Apte, S.S. Procollagen ii amino propeptide processing by adamts-3. Insights on dermatosparaxis. J. Biol. Chem. 2001, 276, 31502–31509. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhou, Y.; Huang, Y.; Wang, Y.; Wang, W.; Kuai, X. Overexpression of adamts-2 in tumor cells and stroma is predictive of poor clinical prognosis in gastric cancer. Hum. Pathol. 2019, 84, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.H.; Yang, W.J.; Liu, S.W.; Li, J.; Zhang, C.Y.; Zhu, Y.; Zhang, C.P. Gene expression profiling of craniofacial fibrous dysplasia reveals adamts2 overexpression as a potential marker. Int. J. Clin. Exp. Pathol. 2014, 7, 8532–8541. [Google Scholar] [PubMed]
- Aydemir, A.T.; Alper, M.; Kockar, F. Sp1-mediated downregulation of adamts3 gene expression in osteosarcoma models. Gene 2018, 659, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, D.; Liu, H.; Sun, H.; Liu, Z.; Zhang, L.; Hu, Y. The competing endogenous circular rna adamts14 suppressed hepatocellular carcinoma progression through regulating microrna-572/regulator of calcineurin 1. J. Cell Physiol. 2019, 234, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.; Span, P.N.; Sweep, F.C.; Tjan-Heijnen, V.C.; Pennington, C.J.; Pedersen, T.X.; Johnsen, M.; Lund, L.R.; Romer, J.; Edwards, D.R. Adamts8 and adamts15 expression predicts survival in human breast carcinoma. Int. J. Cancer 2006, 118, 1241–1247. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, T.; Zhang, F.B.; He, C. Expression of adamts12 in colorectal cancer-associated stroma prevents cancer development and is a good prognostic indicator of colorectal cancer. Dig. Dis. Sci. 2011, 56, 3281–3287. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, P. Downregulation of adamts18 may serve as a poor prognostic biomarker for cervical cancer patients. Appl Immunohistochem Mol. Morphol. 2018, 26, 670–675. [Google Scholar] [CrossRef]
- Guo, R.; Yang, J.; Liu, X.; Wu, J.; Chen, Y. Increased von willebrand factor over decreased adamts-13 activity is associated with poor prognosis in patients with advanced non-small-cell lung cancer. J. Clin. Lab. Anal. 2018, 32. [Google Scholar] [CrossRef]
- Liu, C.; Han, M.; Zhao, L.; Zhu, M.; Xu, Q.; Song, Y.; Wang, H. Adamts-13 activity reduction in plasma of acute myeloid leukemia predicts poor prognosis after bone marrow transplantation. Hematology 2019, 24, 129–133. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.; Ni, W.; Xuan, Y. Adamts-6 is a predictor of poor prognosis in patients with esophageal squamous cell carcinoma. Exp. Mol. Pathol. 2018, 104, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Hsing, M.T.; Yeh, C.M.; Chen, C.J.; Yang, J.S.; Yeh, K.T. Decreased cytoplasmic x-box binding protein-1 expression is associated with poor prognosis and overall survival in patients with oral squamous cell carcinoma. Clin. Chim. Acta 2018, 479, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Yeh, C.M.; Hsieh, M.J.; Lin, Y.M.; Chen, M.W.; Chen, C.J.; Lin, C.Y.; Hung, H.F.; Yeh, K.T.; Yang, S.F. Low cytoplasmic casein kinase 1 epsilon expression predicts poor prognosis in patients with hepatocellular carcinoma. Tumour Biol. 2016, 37, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.K.; Yeh, K.T.; Hsieh, M.J.; Yeh, C.M.; Lin, C.C.; Kao, C.Y.; Huang, L.R.; Lin, S.H. UNC13C suppress tumor progression via inhibiting EMT pathway and improves survival in oral squamous cell carcinoma. Front Oncol. 2019, 8, 728. [Google Scholar]
| Factors | Total Number (n = 250) | % |
|---|---|---|
| Gender | ||
| Female | 12 | 4.8 |
| Male | 238 | 95.2 |
| Age (Year) | ||
| Range | 31–88 | |
| Mean | 54.9 | |
| Median | 53.0 | |
| T (Tumor size) | ||
| I | 60 | 24.0 |
| II | 78 | 31.2 |
| III | 20 | 8.0 |
| IV | 92 | 36.8 |
| N (Lymph node) | ||
| N0 | 155 | 62.0 |
| N1 | 95 | 38.0 |
| M (Metastasis) | ||
| No | 248 | 92.2 |
| Yes | 2 | 0.8 |
| AJCC cancer stage | ||
| I | 46 | 18.4 |
| II | 54 | 21.6 |
| III | 28 | 11.2 |
| IV | 122 | 49.0 |
| Histological grade | ||
| Well | 42 | 16.8 |
| Moderate | 201 | 80.4 |
| Poor | 7 | 2.8 |
| Clinical therapy | ||
| Radiotherapy | 152 | 60.8 |
| Chemotherapy | 60 | 24.0 |
| Cytoplasmic Staining of ADAMTS14 Total | ||||
|---|---|---|---|---|
| Variable | Negative | Positive | (n = 250) | p-Value |
| Age | 55.23 ± 11.6 | 55.52 ± 10.6 | 0.471 | |
| Gender | ||||
| Female | 3(5.8) | 9(4.5) | 12 | |
| Male | 49(94.2) | 189(95.5) | 238 | 0.718 a |
| Histological grade | ||||
| Well | 7(13.5) | 35(17.7) | 42 | |
| Moderate, Poor | 45(86.5) | 163(82.3) | 208 | 0.469 |
| T status | ||||
| T1, T2 | 24(46.1) | 114(57.6) | 138 | |
| T3, T4 | 28(53.8) | 84(42.4) | 112 | 0.140 |
| Lymph Node Metastasis | ||||
| No | 23(44.2) | 132(66.7) | 155 | |
| Yes | 29(55.8) | 66(33.3) | 95 | 0.003 ** |
| Distance Metastasis | ||||
| No | 51(98.1) | 197(99.5) | 248 | |
| Yes | 1(1.9) | 1(0.5) | 2 | 0.373 a |
| Stage | ||||
| I, II | 14(26.9) | 86(43.4) | 100 | |
| III, IV | 38(73.1) | 112(56.6) | 150 | 0.031 * |
| Drinking | ||||
| No | 21(45.7) | 62(39.0) | 83 | |
| Yes | 25(54.3) | 97(61.0) | 122 | 0.418 |
| Betel nut chewing | ||||
| No | 16(45.7) | 49(39.2) | 65 | |
| Yes | 19(54.3) | 76(60.8) | 95 | 0.488 |
| Survival | ||||
| ≤3 year | 23(44.2) | 77(38.9) | 100 | |
| >3 year | 29(55.8) | 121(61.1) | 150 | 0.484 |
| ≤5 year | 30(57.7) | 90(45.5) | 120 | |
| >5 year | 22(42.3) | 108(54.5) | 130 | 0.116 |
| Univariate Multivariate. | ||||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Expression of ADAMTS14 | ||||||
| Negative | 1.4 | 1.2 | ||||
| Positive | 1.0 | 0.507–0.996 | 0.047 * | 1.0 | 0.601–1.199 | 0.353 |
| Lymph Node Metastasis | ||||||
| No | 1.0 | 1.0 | ||||
| Yes | 1.9 | 1.451–2.566 | <0.001 *** | 1.4 | 1.004–20.42 | 0.048 * |
| Stage | ||||||
| I, II | 1.0 | 1.0 | ||||
| II, IV | 1.8 | 1.312–2.361 | <0.001 *** | 1.5 | 1.065–2.183 | 0.021 * |
| Histological grade | ||||||
| Well | 1.0 | 1.0 | ||||
| Moderate, Poor | 1.9 | 1.216–2.860 | 0.004 ** | 1.9 | 1.191–2.943 | 0.007 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-M.; Lin, C.-W.; Lu, J.-W.; Yeh, K.-T.; Lin, S.-H.; Yang, S.-F. Decreased Cytoplasmic Expression of ADAMTS14 Is Correlated with Reduced Survival Rates in Oral Squamous Cell Carcinoma Patients. Diagnostics 2020, 10, 122. https://doi.org/10.3390/diagnostics10020122
Lin Y-M, Lin C-W, Lu J-W, Yeh K-T, Lin S-H, Yang S-F. Decreased Cytoplasmic Expression of ADAMTS14 Is Correlated with Reduced Survival Rates in Oral Squamous Cell Carcinoma Patients. Diagnostics. 2020; 10(2):122. https://doi.org/10.3390/diagnostics10020122
Chicago/Turabian StyleLin, Yueh-Min, Chiao-Wen Lin, Jeng-Wei Lu, Kun-Tu Yeh, Shu-Hui Lin, and Shun-Fa Yang. 2020. "Decreased Cytoplasmic Expression of ADAMTS14 Is Correlated with Reduced Survival Rates in Oral Squamous Cell Carcinoma Patients" Diagnostics 10, no. 2: 122. https://doi.org/10.3390/diagnostics10020122
APA StyleLin, Y.-M., Lin, C.-W., Lu, J.-W., Yeh, K.-T., Lin, S.-H., & Yang, S.-F. (2020). Decreased Cytoplasmic Expression of ADAMTS14 Is Correlated with Reduced Survival Rates in Oral Squamous Cell Carcinoma Patients. Diagnostics, 10(2), 122. https://doi.org/10.3390/diagnostics10020122






