Role of pH Value in Clinically Relevant Diagnosis
Abstract
1. Introduction
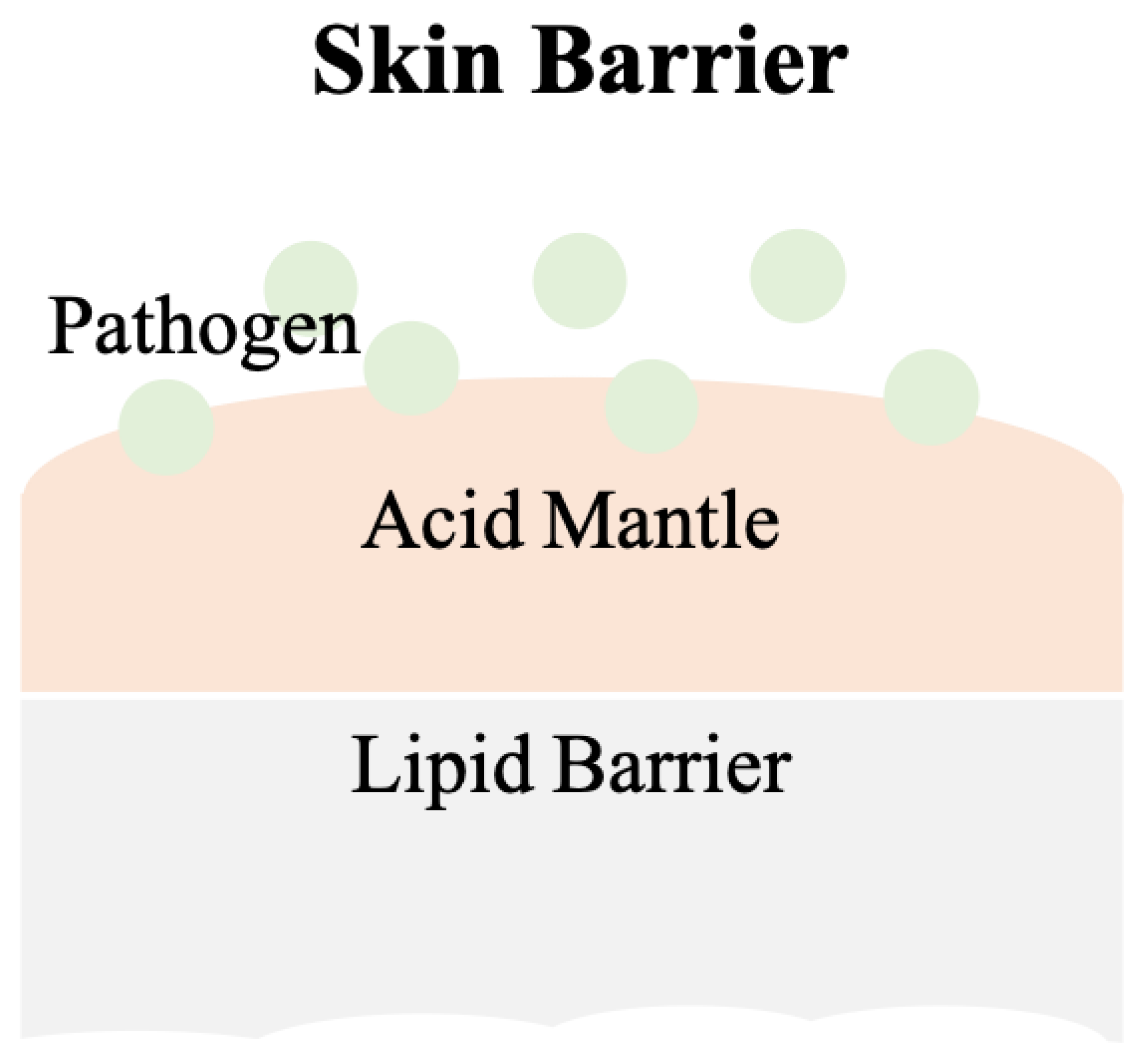
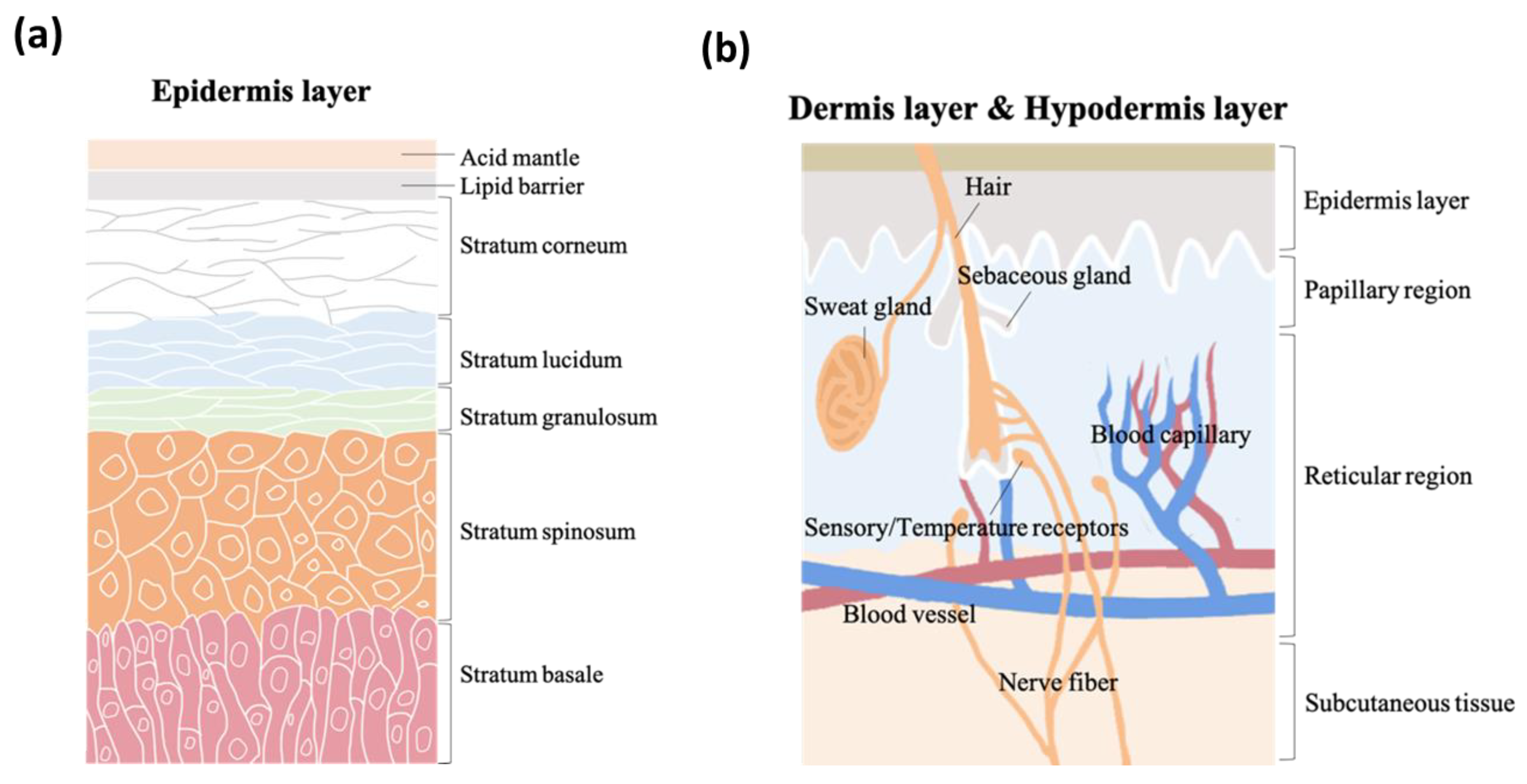
2. Skin
2.1. pH Values on Skin Surfaces
2.2. Factors That Affects Skin pH
2.3. Skin Diseases and pH Values
2.4. Skin Care
3. Wounds
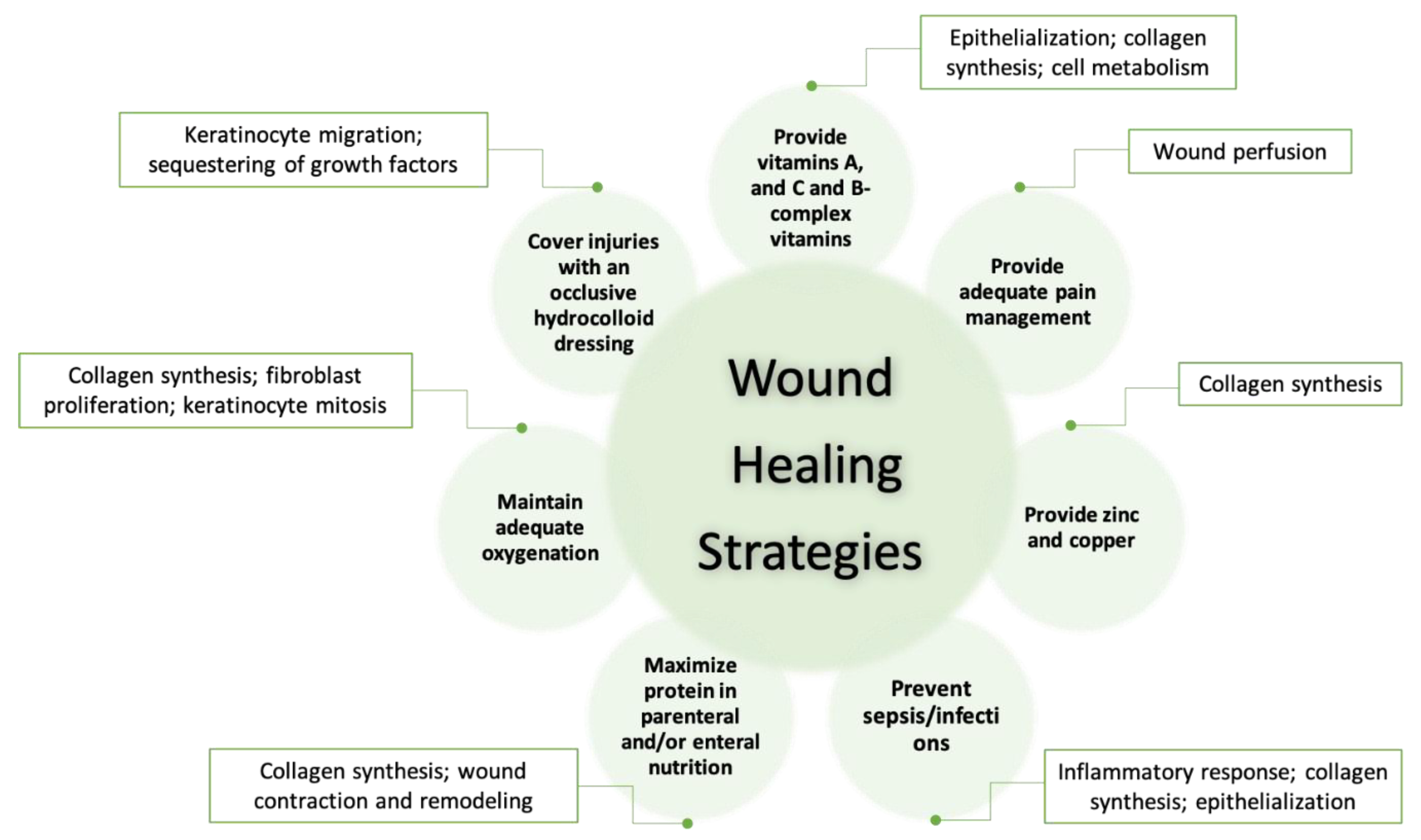
3.1. The Role of pH Value in Wound Healing
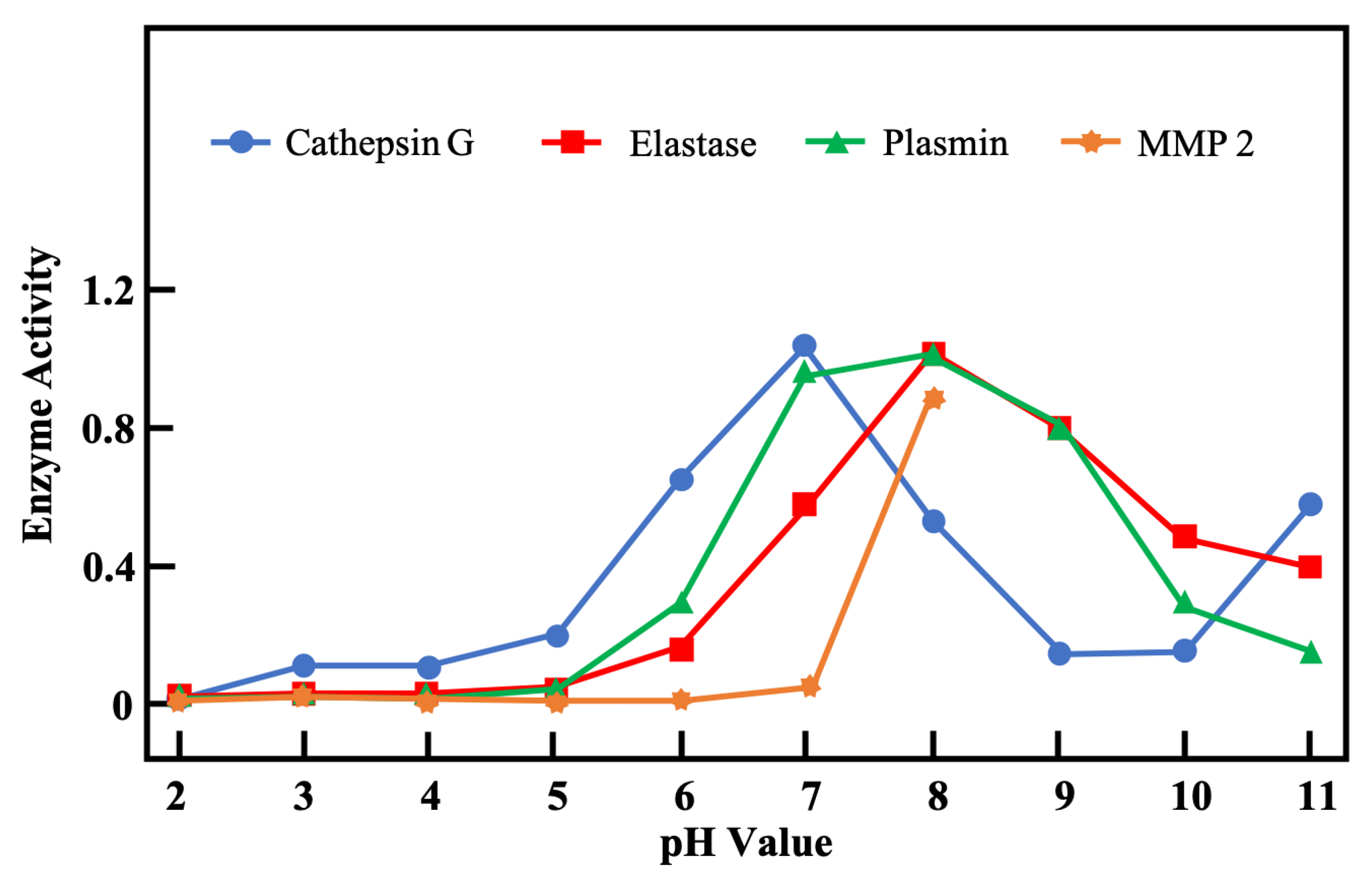
3.2. pH in Infected Wounds
3.3. Wound Dressings
3.4. Wound Care
4. Future
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwa, C.; Bauer, E.A.; Cohen, D.E. Skin Biology. Dermatol. Ther. 2011, 24, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Amsden, B.G.; Goosen, M.F.A. Transdermal Delivery of Peptide and Protein Drugs: An Overview. AIChE J. 1995, 41, 1972–1997. [Google Scholar] [CrossRef]
- Schade, H.; Marchionini, A. Der Säuremantel Der Haut (Nach Gaskettenmessungen). Klin. Wochenschr. 1928, 7, 12–14. [Google Scholar] [CrossRef]
- Sochorová, M.; Staňková, K.; Pullmannová, P.; Kováčik, A.; Zbytovská, J.; Vávrová, K. Permeability Barrier and Microstructure of Skin Lipid Membrane Models of Impaired Glucosylceramide Processing. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Epidermal Lipids, Barrier Function, and Desquamation. J. Invest. Dermatol. 1983, 80, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.R.; Harris, K.L.; Jubin, K.; Bainbridge, N.J.; Jordan, N.R. The Effect of PH in Modulating Skin Cell Behaviour. Br. J. Dermatol. 2009, 161, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G. The Significance of Surface PH in Chronic Wounds. Wounds UK 2007, 3, 52–56. [Google Scholar]
- Pipelzadeh, M.H.; Naylor, I.L. The in Vitro Enhancement of Rat Myofibroblast Contractility by Alterations to the PH of the Physiological Solution. Eur. J. Pharmacol. 1998, 357, 257–259. [Google Scholar] [CrossRef]
- Greener, B.; Hughes, A.A.; Bannister, N.P.; Douglass, J. Proteases and PH in Chronic Wounds Proteolytic. J. Wound Care 2005, 14, 59–61. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of PH on Wound-Healing: A New Perspective for Wound-Therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ziaie, B.; Ochoa, M.; Zhou, J.; Jiang, H.; Yoon, C.K.; Oscai, M.; Jain, V.; Morken, T.; Oliveira, R.H.; Maddiplata, D.; et al. A Manufacturable Smart Dressing with Oxygen Delivery and Sensing Capability for Chronic Wound Management. In Proc. SPIE 10639, Micro- and Nanotechnology Sensors, Systems, and Applications X; SPIE: Orlando, FL, USA, 2018; pp. 106391C1–106391C10. [Google Scholar]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, 1703509. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Nagao, M.; Elias, A.L.; Narain, R.; Chung, H.J. A PH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings. Polymers (Basel) 2017, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Kiaee, G.; Mostafalu, P.; Samandari, M.; Sonkusale, S. A PH-Mediated Electronic Wound Dressing for Controlled Drug Delivery. Adv. Healthc. Mater. 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Lahoti, A.; Pandey, K.; Rao, P.K. Evaluation of Skin and Subcutaneous Tissue Thickness at Insulin Injection Sites in Indian, Insulin Naïve, Type-2 Diabetic Adult Population. Indian J. Endocrinol. Metab. 2013, 17, 864. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, H.; Green, M. Skin PH Pattern in the Newborn Infant. Am. J. Dis. Child. 1958, 95, 35–41. [Google Scholar] [CrossRef]
- Garcia Bartels, N.; Scheufele, R.; Prosch, F.; Schink, T.; Proquitté, H.; Wauer, R.R.; Blume-Peytavi, U. Effect of Standardized Skin Care Regimens on Neonatal Skin Barrier Function in Different Body Areas. Pediatr. Dermatol. 2010, 27, 1–8. [Google Scholar] [CrossRef]
- Giusti, F.; Martella, A.; Bertoni, L.; Seidenari, S. Skin Barrier, Hydration, and PH of the Skin of Infants under 2 Years of Age. Pediatr. Dermatol. 2001, 18, 93–96. [Google Scholar] [CrossRef]
- Cleminson, J.; McGuire, W. Topical Emollient for Preventing Infection in Preterm Infants. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef]
- Hans Behrendt, M.D.; Marvin Green, M.P. Patterns of Skin PH from Birth through Adolescence, with a Synopsis on Skin Growth. Amer J. Dis. Child. 1971, 122, 184. [Google Scholar]
- Blank, I.H. Patterns of Skin PH from Birth through Adolescence: With a Synopsis on Skin. Arch Dermatol. 1971, 104, 224–225. [Google Scholar] [CrossRef]
- Zlotogorski, A. Distribution of Skin Surface PH on the Forehead and Cheek of Adults. Arch. Dermatol. Res. 1987, 279, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Blank, I.H. Measurement of PH of the Skin Surface. J. Invest. Dermatol. 1938, 2, 75–79. [Google Scholar] [CrossRef][Green Version]
- Ehlers, C.; Ivens, U.I.; Senderovitz, T.; Serup, J. Females Have Lower Skin Surface PH than Men A. Ski. Res. Technol. 2001, 9, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Krueger, N.; Kerscher, M. Skin Physiology in Men and Women: In Vivo Evaluation of 300 People Including TEWL, SC Hydration, Sebum Content and Skin Surface PH. Int. J. Cosmet. Sci. 2013, 35, 477–483. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Korting, H.C. The PH of the Skin Surface and Its Impact on the Barrier Function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Issachar, N.; Gall, Y.; Borell, M.T.; Poelman, M.C. PH Measurements during Lactic Acid Stinging Test in Normal and Sensitive Skin. Contact Dermat. 1997, 36, 152–155. [Google Scholar] [CrossRef]
- Itoh, S.; Nakayama, T. Ammonia in Human Sweat and Its Origin. Jpn. J. Physiol. 1952, 3, 133–137. [Google Scholar] [CrossRef][Green Version]
- Matousek, J.L.; Campbell, K.L. A Comparative Review of Cutaneous PH. Vet. Dermatol. 2002, 13, 293–300. [Google Scholar] [CrossRef]
- Hans, C.K.; Otto, B.-F. The Effect of Detergents on Skin PH and Its Consequence. Clin. Dermatol. 1996, 14, 23–27. [Google Scholar]
- Barel, A.O.; Lambrecht, R.; Clarys, P.; Morrison, B.M.; Paye, M. A Comparative Study of the Effects on the Skin of a Classical Bar Soap and a Syndet Cleansing Bar in Normal Use Conditions. Skin (Los. Angeles) 2001, 7, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Gfatter, R.; Hackl, P.; Braun, F. Effects of Soap and Detergents on Skin Surface PH, Stratum Corneum Hydration and Fat Content in Infants. Dermatology 1997, 195, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.H.; Korting, H.C. The Concept of the Acid Mantle of the Skin: Its Relevance for the Choice of Skin Cleansers. Dermatology 1995, 191, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, A.M.; Gloor, M.; Gehring, W.; Kleesz, P. Damage to the Skin by Repetitive Washing. Contact Dermatitis 1995, 32, 225–232. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Grunewald, A.M.; Gloor, M.; Kleesz, P. Barrier Recompensation Mechanisms. Curr. Probl. Dermatol. 1996, 25, 206–213. [Google Scholar]
- Hartmann, A.A. Effect of Occlusion on Resident Flora, Skin-Moisture and Skin-PH. Dermatol. Res. 1983, 275, 251–254. [Google Scholar] [CrossRef]
- Aly, R.; Shirley, C.; Cunico, B.; Maibach, H. Effect of Prolonged Occlusion on the Microbial Flora, PH, Carbon Dioxide and Transepidermal Water Loss on Human Skin. J. Invest. Dermatol. 1978, 31, 378–381. [Google Scholar] [CrossRef]
- Elsner, P.; Maibach, H.I.; Francisco, S. The Effect of Prolonged Drying on Transepidermal Water Loss, Capacitance and PH of Human Vulvar and Forearm Skin. Acta Derm. Venereol. 1990, 70, 105–109. [Google Scholar]
- Waller, J.M.; Maibach, H.I. Age and Skin Structure and Function, a Quantitative Approach (I): Blood Flow, PH, Thickness, and Ultrasound Echogenicity. Ski. Res. Technol. 2005, 11, 221–235. [Google Scholar] [CrossRef]
- Warrier, A.G.; Kligman, A.M.; Harper, R.A.; Bowman, J.; Wickett, R.R. A Comparison of Black and White Skin Using Noninvasive Methods. J. Cosmet. Sci. 1996, 47, 229–240. [Google Scholar]
- Alexis, A.F.; Anigbogu, A.; Baldeweck, T.; Barbosa, V.H.; Bargo, P.R.; Batisse, D.; Berardesca, E.; Brauner, G.J.; de Lacharrière, O.; de Rigal, J.; et al. Ethnic Skin and Hair; CRC Press: Boca Raton, FL, USA, 2007; pp. 116–117. [Google Scholar]
- Rawlings, A.V. Ethnic Skin Types: Are There Differences in Skin Structure and Function? Int. J. Cosmet. Sci. 2006, 28, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Choi, S.Y.; Byun, H.J.; Huh, C.H.; Park, K.C.; Patel, R.A.; Shinn, A.H.; Youn, S.W. Comparison of Sebum Secretion, Skin Type, PH in Humans with and without Acne. Arch. Dermatol. Res. 2006, 298, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.S.; van Heyningen, R.E. Observations on Lactate Content of Sweat. J. Appl. Physiol. 1952, 4, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.; Robinson, S. Chemical Composition of Sweat. Physiol. Rev. 1954, 34, 202–220. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Mark, V.; Dahl, M. Staphylococcus Aureus and Atopic Dermatitis. Arch Dermatol. 1983, 119, 840–846. [Google Scholar]
- Cole, G.W.; Silverberg, N.L. The Adherence of Staphylococcus Aureus to Human Corneocytes. Arch. Dermatol. 1986, 122, 166–169. [Google Scholar] [CrossRef]
- Kumaran, D.; Eswaramoorthy, S.; Furey, W.; Sax, M.; Swaminathan, S. Structure of Staphylococcal Enterotoxin C2 at Various PH Levels. Acta Crystallogr. Sect. D Biol. Crystallogr. 2001, 57, 1270–1275. [Google Scholar] [CrossRef]
- Anderson, D.S. The Acid-Base Balance of the Skin. Br. J. Dermatol. 1951, 63, 283–295. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Elias, P.M. Stratum Corneum PH: Formation and Function of the “Acid Mantle”. Exog. Dermatol. 2002, 1, 163–175. [Google Scholar] [CrossRef]
- Beare, J.M.; Cheeseman, E.A.; Gailey, A.A.H.; Neill, D.W. The pH of the Skin Surface of Children with Seborrhoeic Dermatitis Compared with Unaffected Children. Br. J. Dermatol. 1958, 70, 233–241. [Google Scholar] [CrossRef] [PubMed]
- MacKay, B.J.; Denepitiya, L.; Iacono, V.J.; Krost, S.B.; Pollock, J.J. Growth-Inhibitory and Bactericidal Effects of Human Parotid Salivary Histidine-Rich Polypeptides on Streptococcus Mutans. Infect. Immun. 1984, 44, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Ladra, K.M.; Hake, R.B. Nonoxidative Fungicidal Mechanisms of Mammalian Granulocytes: Demonstration of Components with Candidacidal Activity in Human, Rabbit, and Guinea Pig Leukocytes. Infect. Immun. 1975, 11, 1226–1234. [Google Scholar] [CrossRef]
- Community, D. Mechanisms for the Microbicidal Activity of Cationic Proteins of Human Granulocytes. Infect. Immun. 1976, 14, 1269–1275. [Google Scholar]
- Chikakane, K.; Takahashi, H. Measurement of Skin PH and Its Significance in Cutaneous Diseases. Clin. Dermatol. 1995, 13, 299–306. [Google Scholar] [CrossRef]
- Berg, R.W.; Milligan, M.C.; Sarbaugh, F.C. Association of Skin Wetness and PH With Diaper Dermatitis. Pediatr. Dermatol. 1994, 11, 18–20. [Google Scholar] [CrossRef]
- Zimmerer, R.E.; Lawson, K.D.; Calvert, C.J. The Effects of Wearing Diapers on Skin. Pediatr. Dermatol. 1986, 3, 95–101. [Google Scholar] [CrossRef]
- Buckingham, K.W.; Berg, R.W. Etiologic Factors in Diaper Dermatitis: The Role of Feces. Pediatr. Dermatol. 1986, 3, 107–112. [Google Scholar] [CrossRef]
- Buffo, J.; Herman, M.A.; Soll, D.R. A Characterization of PH-Regulated Dimorphism in Candida Albicans. Mycopathologia 1984, 85, 21–30. [Google Scholar] [CrossRef]
- Whiteway, M.; Bachewich, C. Morphogenesis in Candida Albicans. Annu. Rev. Microbiol. 2007, 61, 529–553. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Van De Veerdonk, F.L.; Brown, A.J.P.; Netea, M.G. Candida Albicans Morphogenesis and Host Defence: Discriminating Invasion from Colonization. Nat. Rev. Microbiol. 2012, 10, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Whiteway, M.; Oberholzer, U. Candida Morphogenesis and Host-Pathogen Interactions. Curr. Opin. Microbiol. 2004, 7, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Scherwitz, C. Ultrastructure of Human Cutaneous Candidosis. J. Invest. Dermatol. 1982, 78, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Tur, E.; Cohen, O.; Rusecki, Y. Skin Surface PH in Intertriginous Areas in NIDDM Patients. Diabetes Care 1993, 16, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S. Newborn Skin Care Revisited. Indian J. Dermatol. 2007, 52, 1–4. [Google Scholar] [CrossRef]
- Tyebkhan, G. Skin Cleansing in Neonates and Infants-Basics of Cleansers. Indian J. Pediatr. 2002, 69, 767–769. [Google Scholar] [CrossRef]
- Nguyen, T.H. Textbook of Dermatologic Surgery. Mayo Clin. Proc. 1998, 73, 709. [Google Scholar] [CrossRef]
- SARKAR, R.; BASU, S.; AGRAWAL, R.; GUPTA, P. Skin Care for the Newborn Principles of Skin Care of the Newborn. Indian Periaterics 2010, 47, 593–598. [Google Scholar] [CrossRef]
- Jung, Y.C.; Kim, E.J.; Cho, J.C.; Suh, K.D.; Nam, G.W. Effect of Skin PH for Wrinkle Formation on Asian: Korean, Vietnamese and Singaporean. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 328–332. [Google Scholar] [CrossRef]
- Youn, S.H.; Choi, C.W.; Choi, J.W.; Youn, S.W. The Skin Surface PH and Its Different Influence on the Development of Acne Lesion According to Gender and Age. Ski. Res. Technol. 2013, 19, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Yosipovitch, G. Skin PH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Nix, D.H. Factors to Consider When Selecting Skin Cleansing Products. J. Wound Cont. Nurses 2000, 27, 260–268. [Google Scholar]
- Reiber, G.E.; Vileikyte, L.; Boyko, E.J.; del Aguila, M.; Smith, D.G.; Lavery, L.A.; Boulton, A.J. Causal Pathways for Incident L o w e R- E x t Remity Ulcers in Patients. Diabetes Care 1999, 22, 157–162. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Ducker, S. New Study Documents Cost and Impact of Chronic Wounds; Demonstrates Imperative for Wound-Specific Quality Measures, Payment Models and Federal Research Funding. Alliance of Wound Care Stakeholders. 2017. Available online: https://www.woundsource.com/blog/alliance-update-new-study-documents-cost-and-impact-chronic-wounds-demonstrates-imperative (accessed on 14 February 2020).
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; daVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Heal. 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The Effects of PH on Wound Healing, Biofilms, and Antimicrobial Efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Lönnqvist, S.; Emanuelsson, P.; Kratz, G. Influence of Acidic PH on Keratinocyte Function and Re-Epithelialisation of Human in Vitro Wounds. J. Plast. Surg. Hand Surg. 2015, 49, 346–352. [Google Scholar] [CrossRef]
- Liu, Y.; Kalén, A.; Risto, O.; Wahlström, O. Fibroblast Proliferation Due to Exposure to a Platelet Concentrate in Vitro Is PH Dependent. Wound Repair Regen. 2002, 10, 336–340. [Google Scholar] [CrossRef]
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The Effect of PH on Cell Viability, Cell Migration, Cell Proliferation, Wound Closure, and Wound Reepithelialization: In Vitro and in Vivo Study. Wound Repair Regen. 2017, 25, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Karrer, S.; Heinlin, J.; Landthaler, M.; Babilas, P. The Impact of the PH Value on Skin Integrity and Cutaneous Wound Healing. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, W.C.; Trager, S. Quantitative Culture Technique and Infection in Complex Wounds of the Extremities Closed with Free Flaps. Plast. Reconstr. Surg. 1995, 95, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Thomas, J.G.; Williams, D.W. Biofilms and Bacterial Imbalances in Chronic Wounds: Anti-Koch. Int. Wound J. 2010, 7, 169–175. [Google Scholar] [CrossRef]
- Leveen, H.H.; Falk, G.; Borek, B.; Diaz, C.; Lynfield, Y.; Wynkoop, B.J.; Mabunda, G.A.; Rubricius, J.L.; Christoudias, G.C. Chemical Acidification of Wounds. An Adjuvant to Healing and the Unfavorable Action of Alkalinity and Ammonia. Ann. Surg. 1973, 178, 745–753. [Google Scholar] [CrossRef]
- Percival, S.L.; Finnegan, S.; Donelli, G.; Vuotto, C.; Rimmer, S.; Lipsky, B.A. Antiseptics for Treating Infected Wounds: Efficacy on Biofilms and Effect of PH. Crit. Rev. Microbiol. 2016, 42, 293–309. [Google Scholar] [CrossRef]
- Percival, S.L.; Thomas, J.G.; Slone, W.; Linton, S.; Corum, L.; Okel, T. The Efficacy of Silver Dressings and Antibiotics on MRSA and MSSA Isolated from Burn Patients. Wound Repair Regen. 2011, 19, 767–774. [Google Scholar] [CrossRef]
- Wutzler, P.; Sauerbrei, A.; Klöcking, R.; Brögmann, B.; Reimer, K. Virucidal Activity and Cytotoxicity of the Liposomal Formulation of Povidone-Iodine. Antivir. Res. 2002, 54, 89–97. [Google Scholar] [CrossRef]
- Broxton, P.; Woodcock, P.M.; Heatley, F.; Gilbert, P. Interaction of Some Polyhexamethylene Biguanides and Membrane Phospholipids in Escherichia Coli. J. Appl. Microbiol. 1984, 57, 115–124. [Google Scholar]
- Russell, A.D.; Path, F.R.C. Chlorhexidine: Antibacterial Action and Bacterial Resistance. Infection 1986, 14, 212–215. [Google Scholar] [CrossRef]
- Hierner, R.; Degreef, H.; Vranckx, J.J.; Garmyn, M.; Massagé, P.; van Brussel, M. Skin Grafting and Wound Healing—The “Dermato-Plastic Team Approach”. Clin. Dermatol. 2005, 23, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Lavery, L.A. Negative Pressure Wound Therapy after Partial Diabetic Foot Amputation: A Multicentre, Randomised Controlled Trial. Lancet 2005, 366, 1704–1710. [Google Scholar] [CrossRef]
- Clinmicrorevs, M.; Bowler, P.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of Silver on Burn Wound Infection Control and Healing: Review of the Literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Fong, J.; Wood, F. Nanocrystalline Silver Dressings in Wound Management: A Review. Int. J. Nanomed. 2006, 1, 441–449. [Google Scholar] [CrossRef]
- Wilkinson, L.J.; White, R.J.; Chipman, J.K. Silver and Nanoparticles of Silver in Wound Dressings: A Review of Efficacy and Safety. J. Wound Care 2011, 20, 543–549. [Google Scholar] [CrossRef]
- Leaper, D.J. Silver Dressings: Their Role in Wound Management. Int. Wound J. 2006, 3, 282–294. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial Resistance to Silver in Wound Care. J. Hosp. Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef]
- Strodtbeck, F. Physiology of Wound Healing. Nat. Immunol. 2001, 1, 43–52. [Google Scholar] [CrossRef]
- Stahl, R.S.; Kopf, G.S. Reconstruction of Infant Thoracic Wounds. Plast. Reconstr. Surg. 1988, 82, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Bookout, K.; McCord, S.; McLane, K. Case Studies of an Infant, a Toddler, and an Adolescent Treated with a Negative Pressure Wound Treatment System. J. Wound Ostomy Cont. Nurs. 2004, 31, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Gergelé, L.; Perrot, J.L.; Dominé, A.; Labeille, B.; Borelli, P.; Cambazard, F. Quantification of Capillary Blood Cell Flow Using Reflectance Confocal Microscopy. Ski. Res. Technol. 2014, 20, 373–378. [Google Scholar] [CrossRef]
- Hindelang, B.; Aguirre, J.; Schwarz, M.; Berezhnoi, A.; Eyerich, K.; Ntziachristos, V.; Biedermann, T.; Darsow, U. Non-Invasive Imaging in Dermatology and the Unique Potential of Raster-Scan Optoacoustic Mesoscopy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1051–1061. [Google Scholar] [CrossRef] [PubMed]

| AG I | AG II | AG III | AG IV | AG V | Mean | |||||||
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | |
| F | 5.01 ± 0.42 | 4.31 ± 0.28 | 4.98 ± 0.50 | 4.47 ± 0.33 | 4.92 ± 0.40 | 4.50 ± 0.36 | 5.10 ± 0.49 | 4.56 ± 0.30 | 4.67 ± 0.32 | 4.57 ± 0.35 | 4.94 ± 0.45 | 4.48 ± 0.33 |
| C | 5.33 ± 0.32 | 4.66 ± 0.38 | 5.27 ± 0.44 | 4.77 ± 0.33 | 5.19 ± 0.29 | 4.74 ± 0.36 | 5.35 ± 0.46 | 4.82 ± 0.27 | 5.21 ± 0.41 | 4.93 ± 0.32 | 5.27 ± 0.39 | 4.78 ± 0.34 |
| N | 5.09 ± 0.32 | 4.62 ± 0.37 | 5.09 ± 0.36 | 4.72 ± 0.36 | 5.08 ± 0.34 | 4.63 ± 0.34 | 5.37 ± 0.49 | 4.76 ± 0.28 | 5.05 ± 0.41 | 4.73 ± 0.29 | 5.13 ± 0.40 | 4.69 ± 0.33 |
| A | 5.12 ± 0.31 | 4.49 ± 0.42 | 5.22 ± 0.40 | 4.50 ± 0.36 | 5.17 ± 0.38 | 4.53 ± 0.30 | 5.44 ± 0.45 | 4.62 ± 0.37 | 5.12 ± 0.43 | 4.58 ± 0.37 | 5.21 ± 0.41 | 4.54 ± 0.36 |
| H | 5.10 ± 0.37 | 4.33 ± 0.36 | 5.19 ± 0.40 | 4.41 ± 0.32 | 5.03 ± 0.35 | 4.38 ± 0.31 | 5.25 ± 0.38 | 4.55 ± 0.42 | 4.88 ± 0.41 | 4.46 ± 0.40 | 5.09 ± 0.40 | 4.42 ± 0.37 |
| Female (Age ± SD) | Male (Age ± SD) | |||||||||||
| AG I | 24.73 ± 2.59 | 25.70 ± 2.48 | ||||||||||
| AG II | 33.30 ± 2.92 | 33.23 ± 2.90 | ||||||||||
| AG III | 43.63 ± 2.81 | 44.23 ± 3.09 | ||||||||||
| AG IV | 55.17 ± 2.84 | 54.20 ± 3.13 | ||||||||||
| AG V | 66.57 ± 4.31 | 66.63 ± 2.61 | ||||||||||
| Factors | Influences | References |
|---|---|---|
| Age | Increased age is associated with skin pH values that are in steady fluctuation. This contributes to difficulties in making comparisons. Females in [26] show significantly higher skin pH values than males. | [23,26,41] |
| Ethnic | Whites have slightly higher pH than blacks, but there are mostly no statistically significant differences between ethnic groups. | [42,43,44] |
| Sebum | There is a negative correlation between sebum secretion and skin pH that is statistically significant in only the skin U-zone with acne and in the skin T-zone without acne, yet the correlation is not strong enough to explain the impact of sebum secretion on skin pH. | [45] |
| Sweat | Sweat is predominantly composed of sodium chloride, lactic acid, urea, and fatty acid. The acidic nature of human skin is partially due to lactic acid in sweat. As the rate of eccrine sweat increases, the concentrations of ammonia, pyruvic acid and lactate decrease, hence the pH increases. | [28,29,30,46,47] |
| Soaps and detergents | The use of soaps, synthetic detergents and even tap water will raise skin pH in a short-term effect. | [27,31,32,33,34,35] |
| Cosmetic products | Skin pH values drop after the application of cosmetic products, which can buffer the impact of tap water washing. | [36,37] |
| Occlusive dressings | Under occlusion, skin pH increases, but after removing occlusive dressings, the pH milieu drops back to an acidic nature. The application of occlusion has a short period effect on skin pH values. | [38,39,40] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, S.-H.; Shen, C.-J.; Shen, C.-F.; Cheng, C.-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics 2020, 10, 107. https://doi.org/10.3390/diagnostics10020107
Kuo S-H, Shen C-J, Shen C-F, Cheng C-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics. 2020; 10(2):107. https://doi.org/10.3390/diagnostics10020107
Chicago/Turabian StyleKuo, Shu-Hua, Ching-Ju Shen, Ching-Fen Shen, and Chao-Min Cheng. 2020. "Role of pH Value in Clinically Relevant Diagnosis" Diagnostics 10, no. 2: 107. https://doi.org/10.3390/diagnostics10020107
APA StyleKuo, S.-H., Shen, C.-J., Shen, C.-F., & Cheng, C.-M. (2020). Role of pH Value in Clinically Relevant Diagnosis. Diagnostics, 10(2), 107. https://doi.org/10.3390/diagnostics10020107







