The Diagnostic Performance of Interleukin-6 and C-Reactive Protein for Early Identification of Neonatal Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Period
2.2. Classification of Study Participants
2.3. Blood Culture
2.4. IL-6 and CRP Determinations
2.5. Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hofer, N.; Zacharias, E.; Müller, W.; Resch, B. An Update on the Use of C-Reactive Protein in Early-Onset Neonatal Sepsis: Current Insights and New Tasks. Neonatology 2012, 102, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Barnett, E.D.; Klein, J.O. Infectious Diseases of the Fetus and Newborn, 6th ed.; Remington, J.S., Klein, J.O., Eds.; Elsevier Health Sciences: Philadelphia, PA, USA, 2011; ISBN 9781416064008. [Google Scholar]
- Lemons, J.A.; Bauer, C.R.; Oh, W.; Korones, S.B.; Papile, L.-A.; Stoll, B.J.; Verter, J.; Temprosa, M.; Wright, L.L.; Ehrenkranz, R.A.; et al. Very Low Birth Weight Outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics 2001, 107, e1. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, I.M.; Ehrenkranz, R.A.; Edberg, S.C.; Baltimore, R.S. A ten-year review of neonatal sepsis and comparison with the previous fifty-year experience. Pediatr. Infect. Dis. J. 1990, 9, 819–890. [Google Scholar] [CrossRef] [PubMed]
- Calil, R.; Marba, S.T.M.; Von Nowakonski, A.; Tresoldi, A.T. Reduction in colonization and nosocomial infection by multiresistant bacteria in a neonatal unit after institution of educational measures and restriction in the use of cephalosporins. Am. J. Infect. Control 2001, 29, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Munoz, K.; Xu, J.; Mally, P. Biomarkers for neonatal sepsis: Recent developments. Res. Rep. Neonatol. 2014, 157. [Google Scholar] [CrossRef]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric Antibiotic Treatment Reduces Mortality in Severe Sepsis and Septic Shock From the First Hour. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gilligan, P.H.; Gonzalez, M.D.; Jerris, R.C.; Kehl, S.C.; Patel, R.; et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiologya. Clin. Infect. Dis. 2018, 67, e1–e94. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.C.; Shankar-Hari, M.M.; Annane, D.; Bauer, M.M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.J.; Coopersmith, C.C.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events With Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef]
- Linsenmeyer, K.; Gupta, K.; Strymish, J.M.; Dhanani, M.; Brecher, S.M.; Breu, A.C. Culture if spikes? Indications and yield of blood cultures in hospitalized medical patients. J. Hosp. Med. 2016, 11, 336–340. [Google Scholar] [CrossRef]
- Buck, C.; Bundschu, J.; Gallati, H.; Bartmann, P.; Pohlandt, F. Interleukin-6: A sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics 1994, 93, 54–58. [Google Scholar]
- Silveira, R.; Procianoy, R. Evaluation of interleukin-6, tumour necrosis factor-α and interleukin-1β for early diagnosis of neonatal sepsis. Acta Paediatr. 2007, 88, 647–650. [Google Scholar] [CrossRef]
- Kao, P.C.; Shiesh, S.C.; Wu, T.J. Review: Serum C-reactive protein as a marker for wellness assessment. Ann. Clin. Lab. Sci. 2006, 35, 163–169. [Google Scholar]
- Pourcyrous, M.; Bada, H.S.; Korones, S.B.; Baselski, V.; Wong, S.P. Significance of serial C-reactive protein responses in neonatal infection and other disorders. Pediatrics 1993, 92, 431–435. [Google Scholar]
- Doellner, H.; Arntzen, K.J.; Haereid, P.E.; Aag, S.; Austgulen, R. Interleukin-6 concentrations in neonates evaluated for sepsis. J. Pediatr. 1998, 132, 295–299. [Google Scholar] [CrossRef]
- Haque, K.N. Definitions of bloodstream infection in the newborn. Pediatr. Crit. Care Med. 2005, 6, S45–S49. [Google Scholar] [CrossRef]
- Ussat, M.; Vogtmann, C.; Gebauer, C.; Pulzer, F.; Thome, U.; Knüpfer, M. The role of elevated central-peripheral temperature difference in early detection of late-onset sepsis in preterm infants. Early Hum. Dev. 2015, 91, 677–681. [Google Scholar] [CrossRef]
- Franz, A.R.; Bauer, K.; Schalk, A.; Garland, S.M.; Bowman, E.D.; Rex, K.; Nyholm, C.; Norman, M.; Bougatef, A.; Kron, M.; et al. Measurement of Interleukin 8 in Combination with C-Reactive Protein Reduced Unnecessary Antibiotic Therapy in Newborn Infants: A Multicenter, Randomized, Controlled Trial. Pediatrics 2004, 114, 1–8. [Google Scholar] [CrossRef]
- Mishra, U.K.; Jacobs, S.E.; Doyle, L.W.; Garland, S.M. Newer approaches to the diagnosis of early onset neonatal sepsis. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 91, F208–F212. [Google Scholar] [CrossRef]
- Benitz, W.E. Adjunct Laboratory Tests in the Diagnosis of Early-Onset Neonatal Sepsis. Clin. Perinatol. 2010, 37, 421–438. [Google Scholar] [CrossRef]
- Bhandari, V.; Wang, C.; Rinder, C.; Rinder, H. Hematologic Profile of Sepsis in Neonates: Neutrophil CD64 as a Diagnostic Marker. Pediatrics 2008, 121, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.A.; Shahidullah, M.; Noor, M.K.; Islam, F.; Alo, D.; Begum, N.A. Utility of C-reactive protein and hematological parameters in the detection of neonatal sepsis. Mymensingh Med. J. 2010, 19, 259–263. [Google Scholar] [PubMed]
- Sastre, J.B.L.; Solís, D.P.; Serradilla, V.R.; Colomer, B.F.; Cotallo, G.D.; Vidal, X.K.; López, E.N.; del Río, M.G.; Luna, M.S.; Cueto, A.B.; et al. Procalcitonin is not sufficiently reliable to be the sole marker of neonatal sepsis of nosocomial origin. BMC Pediatr. 2006, 6, 16. [Google Scholar] [CrossRef]
- Celik, I.H.; Demirel, F.G.; Uras, N.; Oguz, S.S.; Erdeve, O.; Biyikli, Z.; Dilmen, U. What are the cut-off levels for IL-6 and CRP in neonatal sepsis? J. Clin. Lab. Anal. 2010, 24, 407–412. [Google Scholar] [CrossRef]
- Mehr, S.; Doyle, L.W. Cytokines as markers of bacterial sepsis in newborn infants: A review. Pediatr. Infect. Dis. J. 2000, 19, 879–887. [Google Scholar] [CrossRef]
- Mehr, S.; Doyle, L. Interleukin-6 concentrations in neonatal sepsis. Lancet 1999, 353, 1798–1799. [Google Scholar] [CrossRef]
- Ng, P.C.; Cheng, S.H.; Chui, K.M.; Fok, T.F.; Wong, M.Y.; Wong, W.; Wong, R.P.O.; Cheung, K.L. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 77, F221–F227. [Google Scholar] [CrossRef]
- Mathai, E.; Christopher, U.; Mathai, M.; Jana, A.K.; Rose, D.; Bergstrom, S. Is C-reactive protein level useful in differentiating infected from uninfected neonates among those at risk of infection? Indian Pediatr. 2004, 41, 895–900. [Google Scholar]
- Benitz, W.E.; Han, M.Y.; Madan, A.; Ramachandra, P. Serial Serum C-Reactive Protein Levels in the Diagnosis of Neonatal Infection. Pediatrics 1998, 102, e41. [Google Scholar] [CrossRef]
- Khassawneh, M.; Hayajneh, W.A.; Kofahi, H.; Khader, Y.S.; Amarin, Z.; Daoud, A. Diagnostic Markers for Neonatal Sepsis: Comparing C-reactive Protein, Interleukin-6 and Immunoglobulin, M. Scand. J. Immunol. 2007, 65, 171–175. [Google Scholar] [CrossRef]
- Ganesan, P. Evaluation of IL-6, CRP and hs-CRP as Early Markers of Neonatal Sepsis. J. Clin. Diagn. Res. 2016, 10, DC13–DC17. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.; Aarag, E.; Rønnestad, A.; Sollid, J.E.; Abrahamsen, T.G.; Kjeldsen, G.; Flaegstad, T. Coagulase-Negative Staphylococcal Sepsis in Neonates. Pediatr. Infect. Dis. J. 2005, 24, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Speer, C.P. The role of Staphylococcus epidermidis in neonatal sepsis: Guarding angel or pathogenic devil? Int. J. Med. Microbiol. 2014, 304, 513–520. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Categories | Total n (%) | ||
|---|---|---|---|---|
| Proven Sepsis n (%) | Clinical Sepsis n (%) | Controls n (%) | ||
| Total | 104 (11.6) | 160 (17.8) | 635 (70.6) | 899 (100) |
| Gender | ||||
| Male | 56 (53.8) | 93 (58.1) | 376 (59.2) | 525 (58.4) |
| Female | 48 (46.2) | 67 (41.9) | 259 (40.8) | 374 (41.6) |
| Age groups | ||||
| ≤72 h | 9 (8.7) | 87 (54.4) | 549 (86.5) | 645 (71.7) |
| >72 h | 95 (91.3) | 73 (45.6) | 86 (13.5) | 254 (28.3) |
| Age in days (mean ± SD) | 11.4 ± 7.3 | 7.2 ± 8.6 | 2.5 ± 5.0 | 4.4 ± 6.8 |
| Microorganism | Frequency n (%) | IL-6 pg/mL Median(range) | CRP mg/L Median(range) | ||||
|---|---|---|---|---|---|---|---|
| CRP1 | CRP2 | CRP3 | CRP4 | CRP5 | |||
| CoNS | 53 (51.0) | 777 (67–50000) | 12 (0.6–100) | 40 (3.3–131) | 23 (6.5–154) | 15 (1.7–138) | 8 (1.6–132) |
| Staphylococcus epidermidis | 38 (36.5) | 663 (97–42285) | 11 (1–90) | 33 (8.1–131) | 19 (6.5–91) | 12 (1.7–66) | 7 (1.6–32) |
| Staphylococcus haemolyticus | 12 (11.5) | 2692 (67–50000) | 21 (0.6–100) | 56 (17.7–112) | 33 (13.7–154) | 16 (12.9–138) | 10 (4.4–132) |
| Staphylococcus hominis | 3 (2.9) | 1308 (162–1695) | 4 (0.6–31) | 43 (3.3–83) | 48 (13.6–82) | 35 (19.2–50) | 31 (4.0–45) |
| Non CoNS | 51 (49.0) | 3310 (37–50000) | 14 (0.3–220) | 46 (8.0–200) | 43 (3.6–229) | 28 (3.3–103) | 14 (3.1–105) |
| Escherichia coli | 20 (19.2) | 25264 (54–50000) | 12 (0.4–90) | 45 (8.0–200) | 46 (18.1–144) | 21 (3.3–89) | 20 (6.4–82) |
| Staphylococcus aureus | 11 (10.6) | 600 (193–13074) | 24 (1.1–174) | 43 (13.8–194) | 20 (7.4–229) | 25 (13.1–103) | 7 (3.1–64) |
| Enterococcus faecalis | 5 (4.8) | 1149 (180–50000) | 6 (0.3–24) | 27 (16.0–54) | 37 (3.6–67) | 19 (11.4–76) | 5 (3.9–82) |
| Streptococcus agalactiae | 3 (2.9) | 374 (37–3310) | 6 (5.1–120) | 51 (18.7–61) | 25 (10.4–41) | 28 | 5 |
| Enterobacter cloacae | 2 (1.9) | 35205 (20410–50000) | 3 (0.8–4) | 62 (16.0–108) | 98 (79.0–117) | 40 (32.4–48) | 22 (4.0–40) |
| Listeria monocytogenes | 2 (1.9) | 33915 (17830–50000) | 210 (199.6–220) | 171 (165.2–176) | 86 (80.6–90) | 38 (34.8–40) | n/A |
| Klebsiella oxytoca | 2 (1.7) | 5188 (1049–9326) | 7 (1.9–12) | 43 (27.8–57) | n/A | 8 | n/A |
| Bacillus cereus | 1 (1.0) | 1695 | 31 | 83 | 82 | 50 | 31 |
| Klebsiella pneumoniae | 1 (1.0) | 2216 | 22 | 38 | 18 | 11 | 7 |
| Enterobacter hormaechei | 1 (1.0) | 50000 * | 88 | 160 | 105 | 65 | 50 |
| Morganella morganii | 1 (1.9) | n/A | 169 | 194 | 229 | 85 | 64 |
| Pseudomonas aeruginosa | 1 (1.9) | 50000 * | 2 | n/A | n/A | n/A | n/A |
| Candida albicans | 1 (1.0) | 368.0 | 103 | 137 | 136 | 96 | 105 |
| Total | 104 (100) | 1612 (37–50000) | 12 (0.3–220) | 44 (3.3–200) | 28 (3.6–229) | 19 (1.7–138) | 9 (1.6–132) |
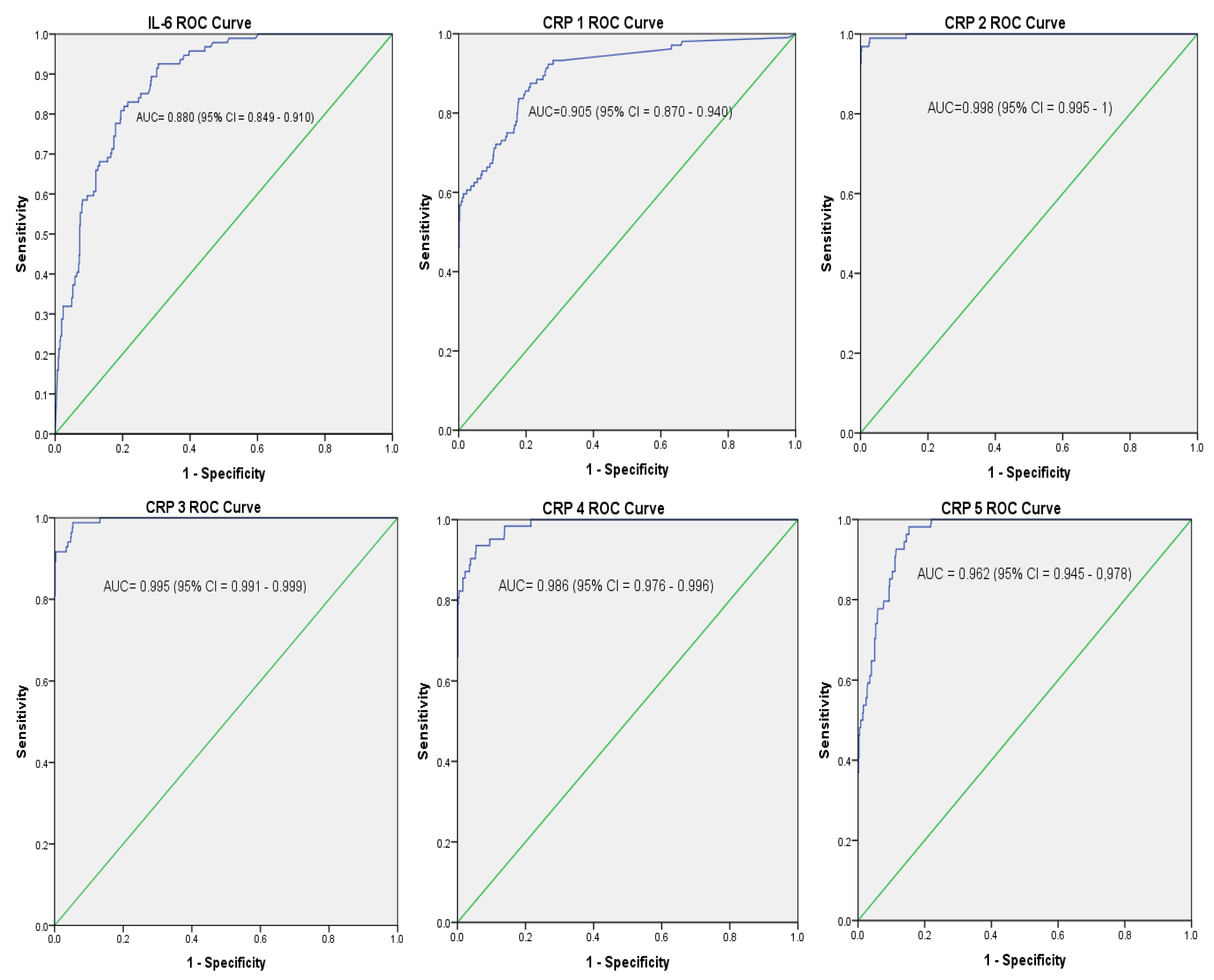
| Patient Category | Single and Combine Test # | Cut-off Value * | AUC (95% CI) | SN (%) | SP (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Proven sepsis versus Controls | IL-6 | 313.5 pg/mL | 0.88 (0.85–0.91) | 73.1 | 80.2 | 37.6 | 94.8 |
| CRP 1 | 2.15 mg/L | 0.91 (0.87–0.94) | 83.7 | 82.2 | 43.5 | 96.8 | |
| IL-6 and CRP 1 | - | - | 98.1 | 69.0 | 34.1 | 99.5 | |
| CRP 2 | 8.01 mg/L | 1.00 | 89.4 | 97.3 | 84.5 | 98.3 | |
| IL-6 and CRP 2 | = | - | 97.1 | 78.1 | 42.1 | 99.4 | |
| CRP 3 | 6.80 mg/L | 1.00 (0.99–1.00) | 76.9 | 95.3 | 72.7 | 96.2 | |
| IL-6 and CRP 3 | - | - | 91.3 | 76.7 | 39.1 | 98.2 | |
| CRP 4 | 5.25 mg/L | 0.99 (0.98–1.00) | 55.8 | 92.3 | 54.2 | 92.7 | |
| IL-6 and CRP 4 | - | - | 88.5 | 74.8 | 36.5 | 97.5 | |
| CRP 5 | 3.72 mg/L | 0.96 (0.95–0.98) | 48.1 | 88.2 | 40.0 | 60.0 | |
| IL-6 and CRP 5 | - | - | 85.6 | 73.1 | 34.2 | 96.9 |
| Patient Category | Single and Combine Test # | SN (%) | SP (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Clinical sepsis vs. Controls | IL-6 (313.5 pg/mL) | 32.3 | 83.6 | 37.5 | 80.2 |
| CRP 1 (2.15 mg/L) | 58.6 | 100 | 100 | 82.2 | |
| CRP 2 (8.01 mg/L) | 90.4 | 100 | 100 | 97.3 | |
| IL-6 and CRP 1 | 44.8 | 100 | 100 | 69 | |
| IL-6 and CRP 2 | 53.5 | 100 | 100 | 78.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessema, B.; Lippmann, N.; Willenberg, A.; Knüpfer, M.; Sack, U.; König, B. The Diagnostic Performance of Interleukin-6 and C-Reactive Protein for Early Identification of Neonatal Sepsis. Diagnostics 2020, 10, 978. https://doi.org/10.3390/diagnostics10110978
Tessema B, Lippmann N, Willenberg A, Knüpfer M, Sack U, König B. The Diagnostic Performance of Interleukin-6 and C-Reactive Protein for Early Identification of Neonatal Sepsis. Diagnostics. 2020; 10(11):978. https://doi.org/10.3390/diagnostics10110978
Chicago/Turabian StyleTessema, Belay, Norman Lippmann, Anja Willenberg, Matthias Knüpfer, Ulrich Sack, and Brigitte König. 2020. "The Diagnostic Performance of Interleukin-6 and C-Reactive Protein for Early Identification of Neonatal Sepsis" Diagnostics 10, no. 11: 978. https://doi.org/10.3390/diagnostics10110978
APA StyleTessema, B., Lippmann, N., Willenberg, A., Knüpfer, M., Sack, U., & König, B. (2020). The Diagnostic Performance of Interleukin-6 and C-Reactive Protein for Early Identification of Neonatal Sepsis. Diagnostics, 10(11), 978. https://doi.org/10.3390/diagnostics10110978






