Association of SARC-F Questionnaire and Mortality in Prevalent Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. SARC-F
2.3. Laboratory Tests
2.4. Assessment of Body Composition, Muscle Strength, and Physical Performance
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail older persons aged 80 years and older: Results from ilsirente study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef]
- Arango-Lopera, V.E.; Arroyo, P.; Gutierrez-Robledo, L.M.; Perez-Zepeda, M.U.; Cesari, M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging 2013, 17, 259–262. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.R.; Choi, M.J.; Kim, S.G.; Lee, Y.K.; Noh, J.W.; Kim, H.J.; Song, Y.R. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin. Nutr. 2014, 33, 64–68. [Google Scholar] [CrossRef]
- Bataille, S.; Serveaux, M.; Carreno, E.; Pedinielli, N.; Darmon, P.; Robert, A. The diagnosis of sarcopenia is mainly driven by muscle mass in hemodialysis patients. Clin. Nutr. 2017, 36, 1654–1660. [Google Scholar] [CrossRef]
- Lamarca, F.; Carrero, J.J.; Rodrigues, J.C.; Bigogno, F.G.; Fetter, R.L.; Avesani, C.M. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: The impact of different diagnostic criteria. J. Nutr. Health Aging 2014, 18, 710–717. [Google Scholar] [CrossRef]
- Fahal, I.H. Uraemic sarcopenia: Aetiology and implications. Nephrol. Dial. Transplant. 2014, 29, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Domanski, M.; Ciechanowski, K. Sarcopenia: A major challenge in elderly patients with end-stage renal disease. J. Aging Res. 2012, 2012, 754739. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2011, 2, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Johansen, K.L.; Lindholm, B.; Stenvinkel, P.; Cuppari, L.; Avesani, C.M. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016, 90, 53–66. [Google Scholar] [CrossRef]
- Kimmel, P.L.; Patel, S.S. Quality of life in patients with chronic kidney disease: Focus on end-stage renal disease treated with hemodialysis. Semin. Nephrol. 2006, 26, 68–79. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Morley, J.E. Sarc-f: A simple questionnaire to rapidly diagnose sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Dupuy, C.; Abellan Van Kan, G.; Cesari, M.; Vellas, B.; Faruch, M.; Dray, C.; de Souto Barreto, P. Sarcopenia screened by the SARC-F questionnaire and physical performances of elderly women: A cross-sectional study. J Am. Med. Dir. Assoc. 2017, 18, 848–852. [Google Scholar] [CrossRef]
- Wu, T.Y.; Liaw, C.K.; Chen, F.C.; Kuo, K.L.; Chie, W.C.; Yang, R.S. Sarcopenia screened with SARC-F questionnaire is associated with quality of life and 4-year mortality. J. Am. Med. Dir. Assoc. 2016, 17, 1129–1135. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Won, C.W. Validation of the Korean version of the SARC-F questionnaire to assess sarcopenia: Korean frailty and aging cohort study. J. Am. Med. Dir. Assoc. 2018, 19, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Leung, J.; Morley, J.E. Defining sarcopenia in terms of incident adverse outcomes. J. Am. Med. Dir. Assoc. 2015, 16, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Yamamoto, S.; Matsuzawa, R.; Harada, M.; Watanabe, T.; Shimoda, T.; Suzuki, Y.; Kamiya, K.; Osada, S.; Yoshida, A.; Matsunaga, A. SARC-F questionnaire: Rapid and easy tool for identifying physical limitations in hemodialysis patients. JCSM Clin. Rep. 2019, 4, 1–12. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Morley, J.E.; Cao, L. Rapid screening for sarcopenia. J. Cachexia Sarcopenia Muscle 2015, 6, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Shinaberger, C.S.; Kilpatrick, R.D.; Regidor, D.L.; McAllister, C.J.; Greenland, S.; Kopple, J.D.; Kalantar-Zadeh, K. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am. J. Kidney Dis. 2006, 48, 37–49. [Google Scholar] [CrossRef]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transpl. 2015, 30, 1718–1725. [Google Scholar] [CrossRef]
- Leal, V.O.; Mafra, D.; Fouque, D.; Anjos, L.A. Use of handgrip strength in the assessment of the muscle function of chronic kidney disease patients on dialysis: A systematic review. Nephrol. Dial. Transpl. 2011, 26, 1354–1360. [Google Scholar] [CrossRef]
- Muff, G.; Dufour, S.; Meyer, A.; Severac, F.; Favret, F.; Geny, B.; Lecocq, J.; Isner-Horobeti, M.-E. Comparative assessment of knee extensor and flexor muscle strength measured using a hand-held vs. Isokinetic dynamometer. J. Phys. Ther. Sci. 2016, 28, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Walker, B.; Phillips, J.K.; Fejer, R.; Beck, R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: A systematic review. PM&R 2011, 3, 472–479. [Google Scholar]
- Jin, M.; Du, H.; Zhang, Y.; Zhu, H.; Xu, K.; Yuan, X.; Pan, H.; Shan, G. Characteristics and reference values of fat mass index and fat free mass index by bioelectrical impedance analysis in an adult population. Clin. Nutr. 2019, 38, 2325–2332. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Morley, J.E. Validating the SARC-F: A suitable community screening tool for sarcopenia? J. Am. Med. Dir. Assoc. 2014, 15, 630–634. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Murata, K. Sarc-f for screening of sarcopenia among older adults: A meta-analysis of screening test accuracy. J. Am. Med. Dir. Assoc. 2018, 19, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Kera, T.; Kawai, H.; Hirano, H.; Kojima, M.; Watanabe, Y.; Motokawa, K.; Fujiwara, Y.; Osuka, Y.; Kojima, N.; Kim, H.; et al. Limitations of SARC-F in the diagnosis of sarcopenia in community-dwelling older adults. Arch. Gerontol. Geriatr. 2020, 87, 103959. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 271) | Men (n = 151) | Women (n = 120) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 64.4 ± 14.3 | 63.0 ± 13.8 | 66.1 ± 14.8 | 0.081 |
| HD duration (years) | 4.0 (1.5–7.9) | 3.4 (1.4–6.0) | 4.7 (1.8–11.4) | 0.003 * |
| Causes of ESRD, n (%) | ||||
| DM | 127 (46.9) | 80 (53.0) | 47 (39.2) | 0.024 * |
| Chronic GN | 79 (29.2) | 35 (23.2) | 44 (36.7) | 0.015 * |
| Others | 65 (24.0) | 36 (23.8) | 29 (24.2) | 0.950 |
| Comorbid conditions, n (%) | ||||
| Cardiovascular disease | 100 (36.9) | 59 (39.1) | 41 (34.2) | 0.406 |
| Chronic liver disease | 46 (17.0) | 25 (16.6) | 21 (17.5) | 0.837 |
| Malignancy | 31 (11.4) | 16 (10.6) | 15 (12.5) | 0.625 |
| Stroke | 20 (7.4) | 11 (7.3) | 9 (7.5) | 0.946 |
| PAD | 21 (7.7) | 14 (9.3) | 7 (5.8) | 0.293 |
| Dementia | 12 (4.4) | 5 (3.3) | 7 (5.8) | 0.316 |
| Charlson comorbidity index | 4 (3–5) | 4 (4–5) | 4 (3–5) | 0.245 |
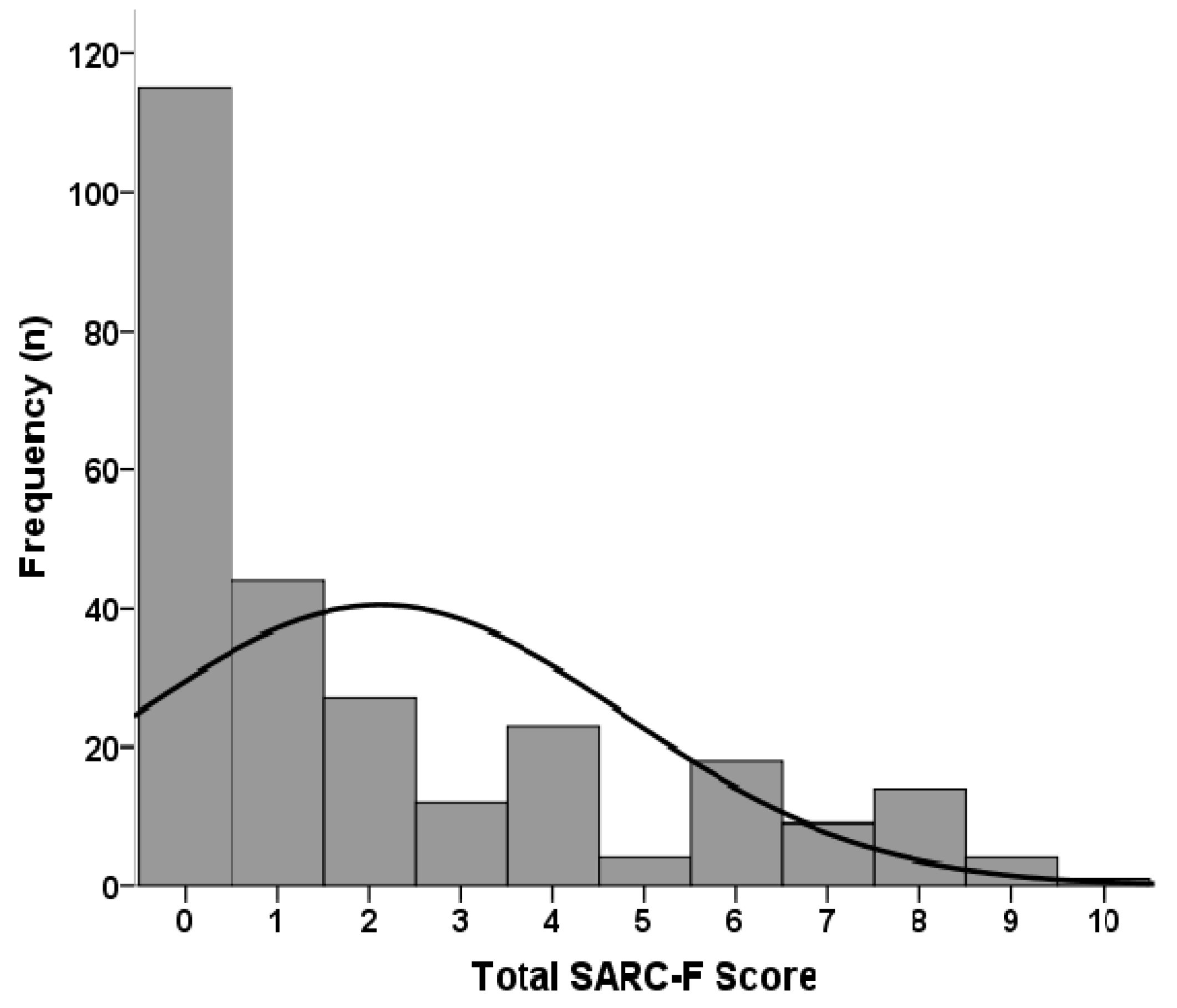
| SARC-F score | 1 (0–4) | 1 (0–2) | 2 (0–4) | 0.016 * |
| Examination | ||||
| BMI (Kg/m2) | 24.7 ± 4.8 | 25.1 ± 4.3 | 24.1 ± 5.4 | 0.126 |
| Kt/V (Gotch) | 1.31 (1.18–1.46) | 1.21 (1.13–1.34) | 1.41 (1.31–1.59) | <0.001 * |
| Hemoglobin (g/dL) | 10.3 (9.6–10.9) | 10.3 (9.5–11.1) | 10.3 (9.6–10.8) | 0.148 |
| Albumin (g/dL) | 4.3 (3.9–4.6) | 4.3 (4.0–4.6) | 4.3 (3.9–4.5) | 0.180 |
| BUN (mg/dL) | 62 (51–74) | 64 (52–74) | 61 (51–72) | 0.291 |
| Creatinine (mg/dL) | 9.2 ± 2.3 | 9.9 ± 2.4 | 8.2 ± 1.6 | <0.001 * |
| Phosphorus (mg/dL) | 4.8 (3.8–5.7) | 4.9 (3.6–5.7) | 4.8 (3.9–5.7) | 0.608 |
| C-reactive protein (mg/dL) | 0.36 (0.09–0.87) | 0.39 (0.10–0.89) | 0.29 (0.08–0.86) | 0.371 |
| nPNA (g/kg/day) | 0.97 (0.82–1.11) | 0.95 (0.80–1.09) | 0.99 (0.85–1.16) | 0.249 |
| Variables | SARC-F | |
|---|---|---|
| r | p | |
| Basic characteristics a | ||
| Age (years) | 0.405 | <0.001 * |
| HD duration (years) | 0.006 | 0.919 |
| Charlson comorbidity index a | 0.273 | <0.001 * |
| Body composition b | ||
| MAMC (cm) | −0.253 | 0.011 * |
| Skeletal muscle index (Kg/m2) | −0.128 | 0.203 |
| Fat tissue index (Kg/m2) | 0.133 | 0.187 |
| Calf circumference (cm) | −0.235 | 0.019 * |
| Muscle strength b | ||
| Handgrip strength (Kg) | ||
| Right | −0.284 | 0.004 * |
| Left | −0.302 | 0.002 * |
| Hip flexion strength (Kg-force) | ||
| Right | −0.236 | 0.018 * |
| Left | −0.301 | 0.002 * |
| Knee extension strength (Kg-force) | ||
| Right | −0.341 | 0.001 * |
| Left | −0.303 | 0.002 * |
| Physical performance | ||
| 6 m gait speed (m/s) b | −0.339 | 0.001 * |
| Repeated sit-to-stand test (s) c | 0.199 | 0.058 |
| AUC (95% CI) | Cut-Off | Sen (%) | Spe (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| Low skeletal muscle index a | 0.658 (0.556–0.750) | ≥1 | 66.7 | 65.9 | 16.2 | 95.2 |
| Handgrip strength weakness b | 0.651 (0.549–0.744) | ≥2 | 36.6 | 91.5 | 75.0 | 67.5 |
| Slow gait speed c | 0.685 (0.584–0.774) | ≥1 | 56.1 | 76.3 | 62.2 | 71.4 |
| Poor sit-to-stand test ≥12 s d | 0.656 (0.554–0.748) | ≥1 | 49.1 | 77.8 | 73.0 | 55.6 |
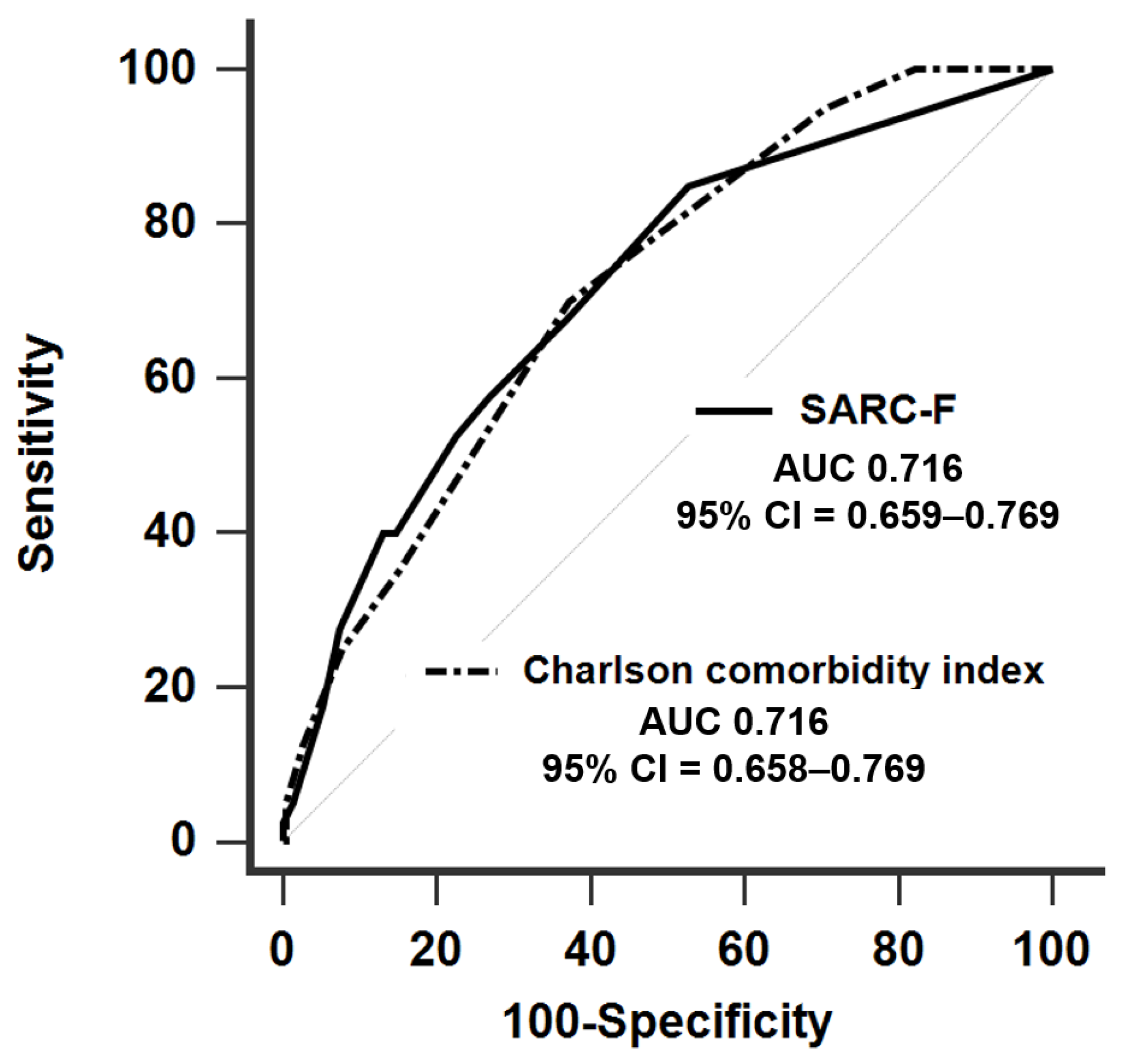
| Possible sarcopenia e | 0.671 (0.570–0.762) | ≥1 | 47.7 | 82.9 | 83.8 | 46.0 |
| Sarcopenia f | 0.694 (0.593–0.782) | ≥1 | 71.4 | 65.6 | 13.5 | 96.8 |
| Characteristics | Survival (n = 231) | Non-Survival (n = 40) | p |
|---|---|---|---|
| Demographics | |||
| Age (years) | 63.1 ± 14.1 | 71.7 ± 13.5 | <0.001 * |
| Male (%) | 129 (55.8) | 22 (55.0) | 0.921 |
| HD duration (years) | 4.1 (1.5–8.2) | 4.0 (1.8–7.2) | 0.715 |
| Causes of ESRD, n (%) | |||
| DM | 105 (45.5) | 22 (55.0) | 0.264 |
| Chronic GN | 69 (29.9) | 10 (25.0) | 0.531 |
| Others | 57 (24.7) | 8 (20.0) | 0.523 |
| Comorbid conditions, n (%) | |||
| Cardiovascular disease | 78 (33.8) | 22 (55.0) | 0.010 * |
| Chronic liver disease | 39 (16.9) | 7 (17.5) | 0.924 |
| Malignancy | 23 (10.0) | 8 (20.0) | 0.065 |
| Stroke | 18 (7.8) | 2 (5.0) | 0.533 |
| PAD | 15 (6.5) | 6 (15.0) | 0.063 |
| Dementia | 8 (3.5) | 4 (10.0) | 0.064 |
| Charlson comorbidity index | 4 (3–5) | 5 (4–7) | <0.001 * |
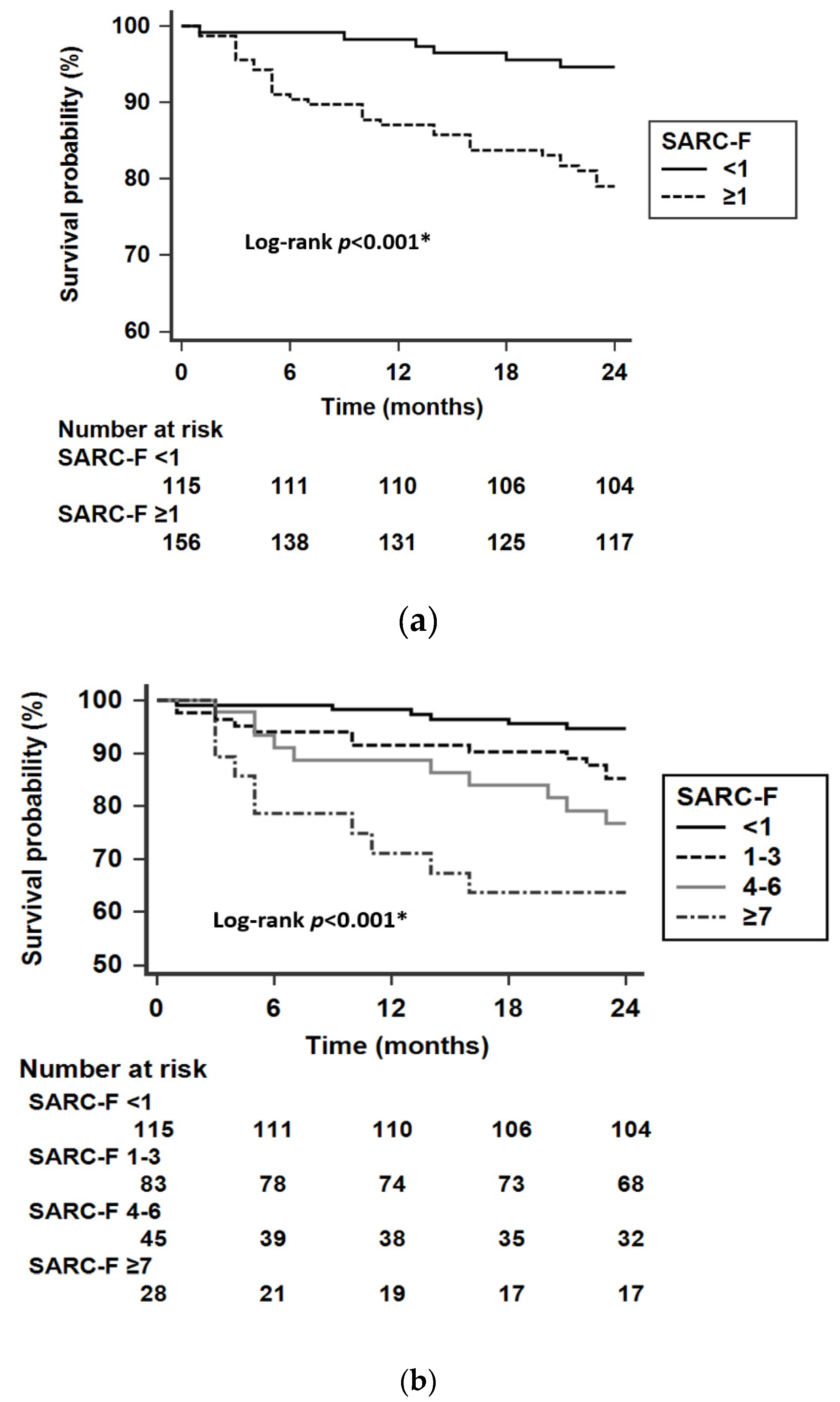
| SARC-F | |||
| Total score | 1 (0–3) | 4 (1–7) | <0.001 * |
| SARC-F ≥ 1, n (%) | 122 (52.8) | 34 (85.0) | <0.001 * |
| Examination | |||
| BMI (Kg/m2) | 25.0 ± 4.8 | 22.6 ± 4.4 | 0.004 * |
| Kt/V (Gotch) | 1.30 (1.18–1.40) | 1.42 (1.23–1.61) | 0.013 * |
| Hemoglobin (g/dL) | 10.3 (9.7–10.9) | 9.8 (9.0–10.6) | 0.076 |
| Albumin (g/dL) | 4.3 (4.0–4.6) | 3.9 (3.7–4.2) | <0.001 * |
| BUN (mg/dL) | 62 (52–74) | 62 (47–72) | 0.403 |
| Creatinine (mg/dL) | 9.4 ± 2.2 | 7.7 ± 1.9 | <0.001 * |
| Phosphorus (mg/dL) | 4.9 (3.9–5.8) | 4.4 (3.2–5.0) | 0.022 * |
| C-reactive protein (mg/dL) | 0.32 (0.08–0.83) | 0.68 (0.29–1.22) | 0.003 * |
| nPNA (g/kg/day) | 0.99 (0.83–1.13) | 0.90 (0.72–1.05) | 0.039 * |
| Model | SARC-F (per 1 Unit of Increase) | SARC-F ≥ 1 | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Crude model | 1.26 (1.15–1.39) | <0.001 * | 4.58 (1.92–10.91) | 0.001 * |
| Model 1 | 1.22 (1.10–1.36) | <0.001 * | 3.55 (1.44–8.74) | 0.006 * |
| Model 2 | 1.15 (1.02–1.29) | 0.020 * | 3.00 (1.21–7.43) | 0.018 * |
| Model 3 | 1.12 (0.98–1.29) | 0.110 | 2.87 (1.11–7.38) | 0.029 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-L.; Hou, J.-S.; Lai, Y.-H.; Wang, C.-H.; Kuo, C.-H.; Liou, H.-H.; Hsu, B.-G. Association of SARC-F Questionnaire and Mortality in Prevalent Hemodialysis Patients. Diagnostics 2020, 10, 890. https://doi.org/10.3390/diagnostics10110890
Lin Y-L, Hou J-S, Lai Y-H, Wang C-H, Kuo C-H, Liou H-H, Hsu B-G. Association of SARC-F Questionnaire and Mortality in Prevalent Hemodialysis Patients. Diagnostics. 2020; 10(11):890. https://doi.org/10.3390/diagnostics10110890
Chicago/Turabian StyleLin, Yu-Li, Jia-Sian Hou, Yu-Hsien Lai, Chih-Hsien Wang, Chiu-Huang Kuo, Hung-Hsiang Liou, and Bang-Gee Hsu. 2020. "Association of SARC-F Questionnaire and Mortality in Prevalent Hemodialysis Patients" Diagnostics 10, no. 11: 890. https://doi.org/10.3390/diagnostics10110890
APA StyleLin, Y.-L., Hou, J.-S., Lai, Y.-H., Wang, C.-H., Kuo, C.-H., Liou, H.-H., & Hsu, B.-G. (2020). Association of SARC-F Questionnaire and Mortality in Prevalent Hemodialysis Patients. Diagnostics, 10(11), 890. https://doi.org/10.3390/diagnostics10110890






