The Association between Plasma Levels of Intact and Cleaved uPAR Levels and the Risk of Biochemical Recurrence after Radical Prostatectomy for Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
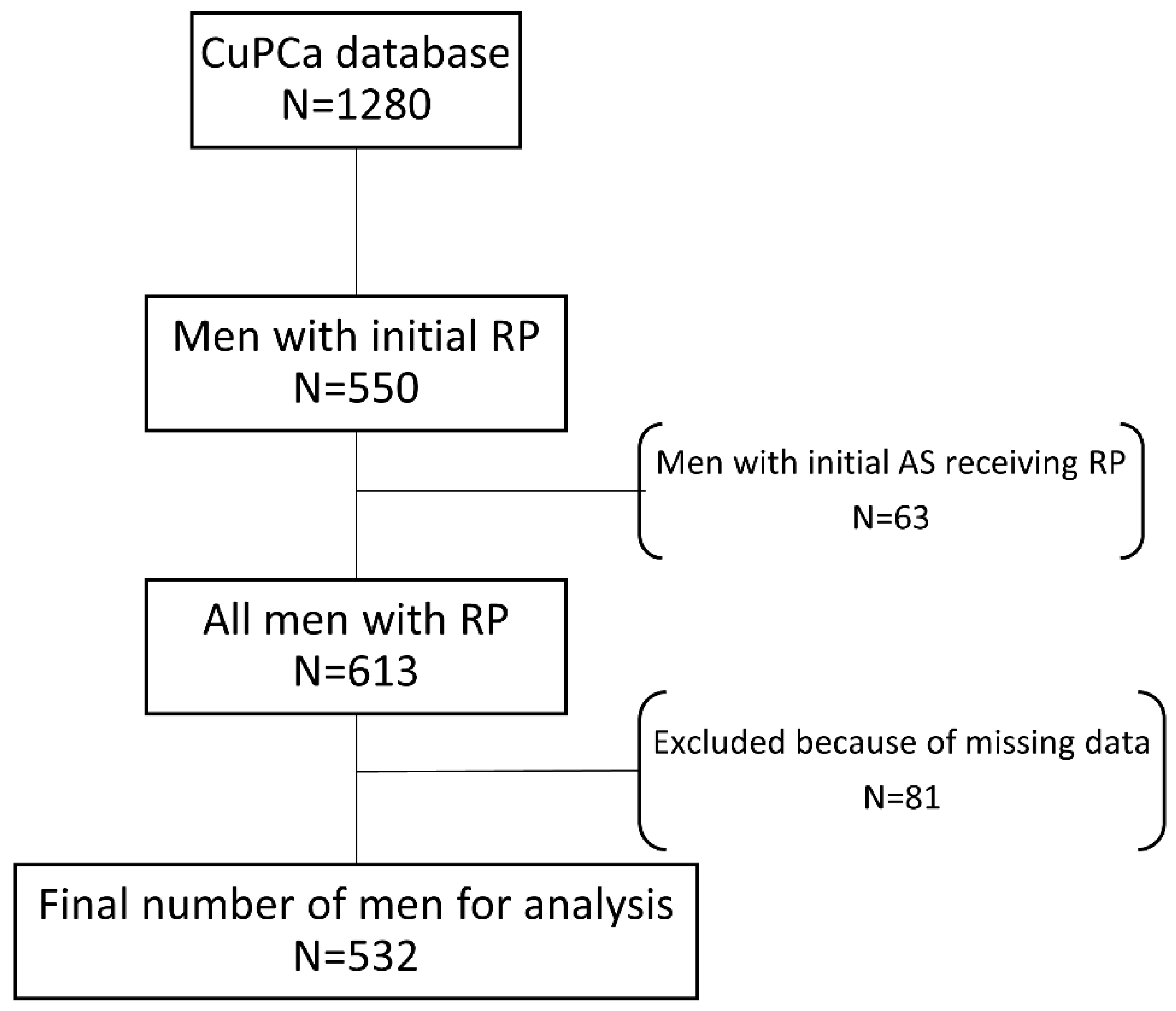
2.1. CuPCa Database
2.2. Statistics
3. Results
3.1. Overall Characteristics
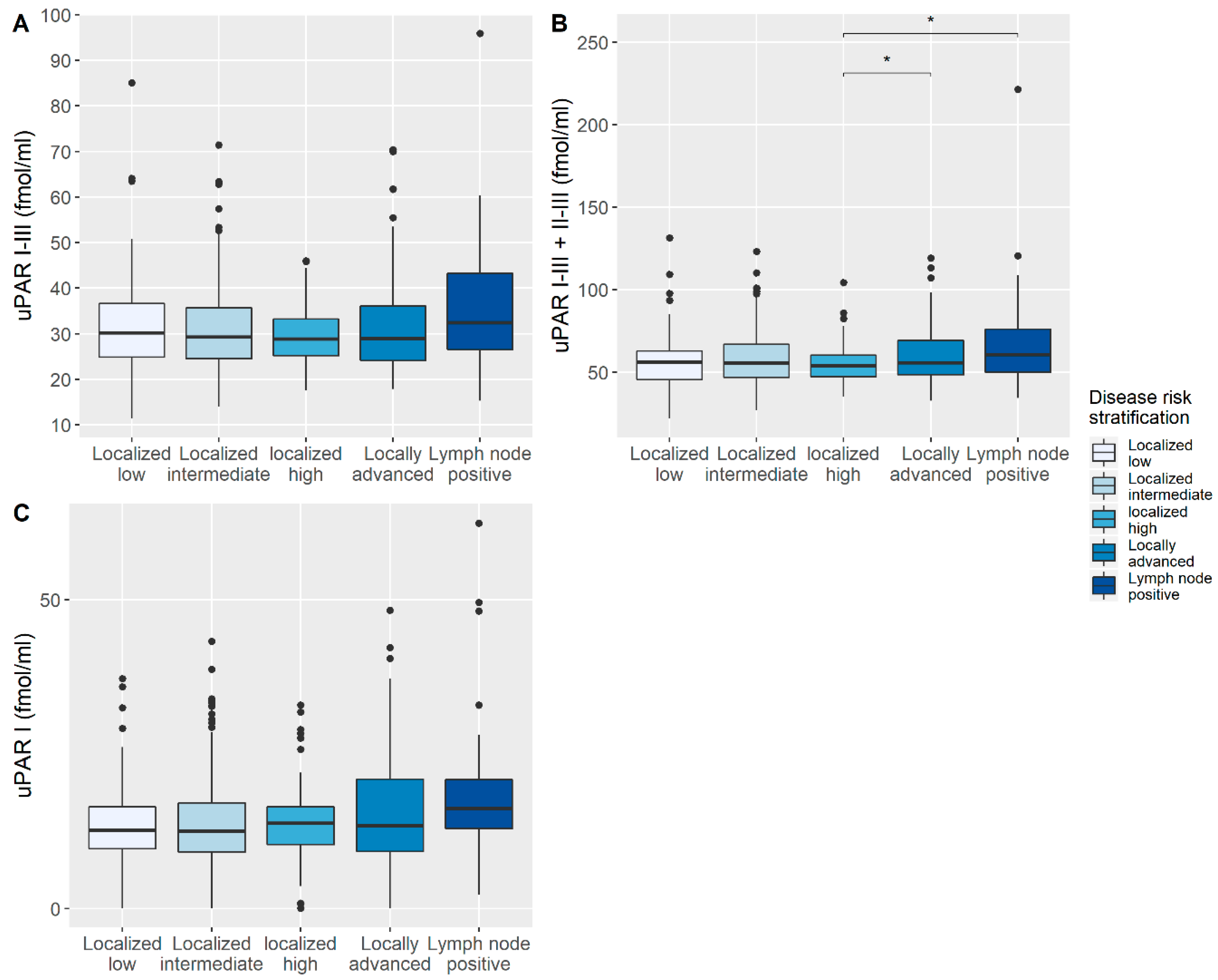
3.2. Correlation of uPAR with Disease-Specific Characteristics
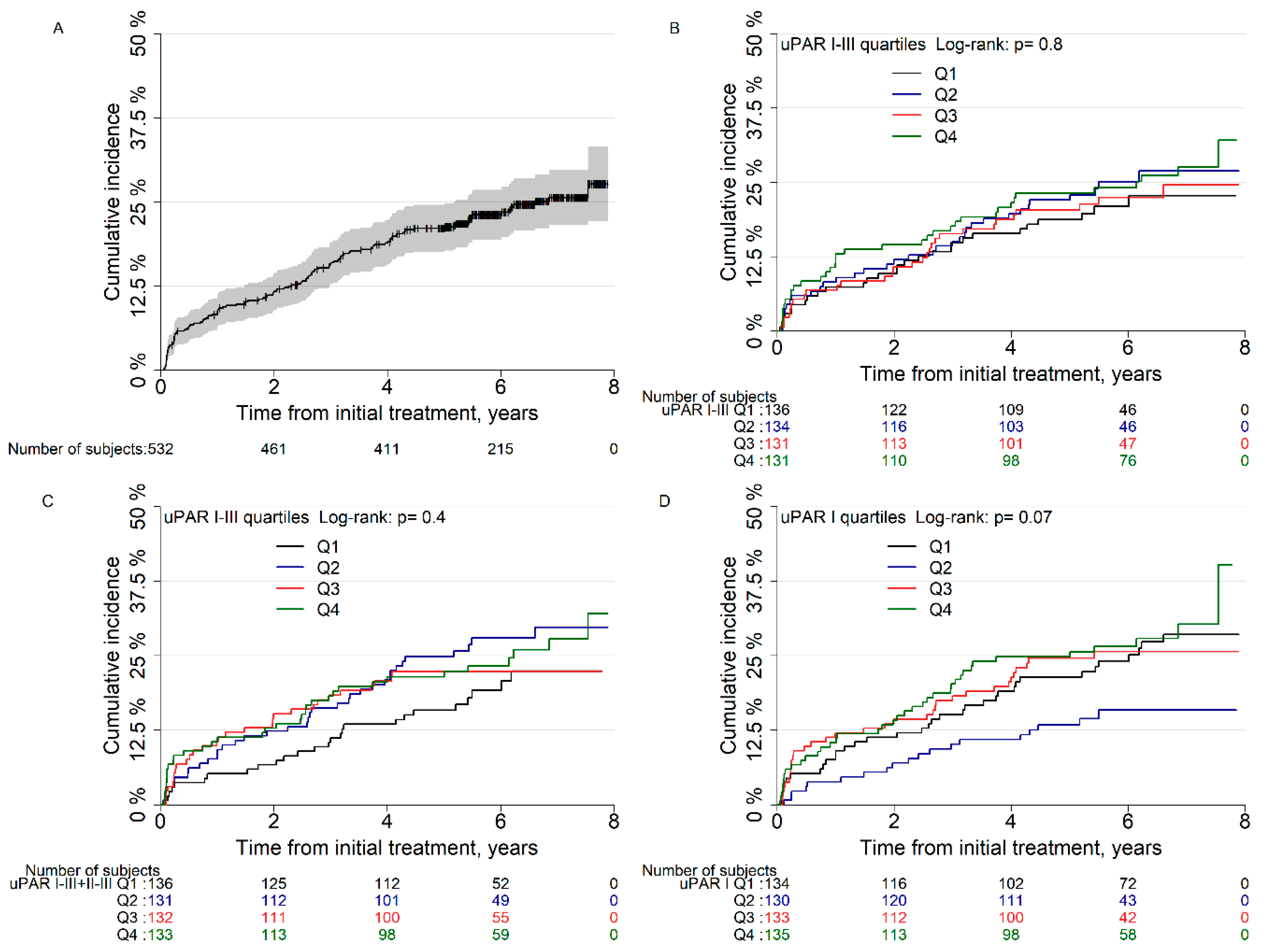
3.3. Risk of Biochemical Recurrence after Radical Prostatectomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Pound, C.R.; Partin, A.W.; Eisenberger, M.A.; Chan, D.W.; Pearson, J.D.; Walsh, P.C. Natural History of Progression After PSA Elevation Following Radical Prostatectomy. JAMA 1999, 281, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Thompson, R.H.; Tollefson, M.K.; Rangel, L.J.; Bergstralh, E.J.; Blute, M.L.; Karnes, R.J. Long-Term Risk of Clinical Progression After Biochemical Recurrence Following Radical Prostatectomy: The Impact of Time from Surgery to Recurrence. Eur. Urol. 2011, 59, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.C.; Verspaget, H.W.; Ganesh, S.; Kubben, F.J.; Vahrmeijer, A.L.; Van De Velde, C.J.; Kuppen, P.J.; Quax, P.H.; Sier, C.F. Clinical Applications of the Urokinase Receptor (uPAR) for Cancer Patients. Curr. Pharm. Des. 2011, 17, 1890–1910. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W.; Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36. [Google Scholar] [CrossRef]
- Høyer-Hansen, G.; Lund, I.K. Urokinase Receptor Variants in Tissue and Body Fluids. Int. Rev. Cytol. 2007, 44, 65–102. [Google Scholar] [CrossRef]
- Piironen, T.; Laursen, B.; Pass, J.; List, K.; Gårdsvoll, H.; Ploug, M.; Danø, K.; Høyer-Hansen, G. Specific Immunoassays for Detection of Intact and Cleaved Forms of the Urokinase Receptor. Clin. Chem. 2004, 50, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Haupt, T.; Petersen, J.; Ellekilde, G.; Klausen, H.H.; Thorball, C.; Eugen-Olsen, J.; Andersen, O. Plasma suPAR levels are associated with mortality, admission time, and Charlson Comorbidity Index in the acutely admitted medical patient: A prospective observational study. Crit. Care 2012, 16, R130. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Voigt, S.; Kruschinski, C.; Sanson, E.; Dückers, H.; Horn, A.; Yagmur, E.; Zimmermann, H.W.; Trautwein, C.; Tacke, F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit. Care 2011, 15, R63. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Hong, S.; Huang, S. Role of Urokinase Receptor in Tumor Progression and Development. Theranostics 2013, 3, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Riisbro, R.; Christensen, I.J.; Piironen, T.; Greenall, M.; Larsen, B.; Stephens, R.W.; Han, C.; Høyer-Hansen, G.; Smith, K.; Brünner, N.; et al. Prognostic significance of soluble urokinase plasminogen activator receptor in serum and cytosol of tumor tissue from patients with primary breast cancer. Clin. Cancer Res. 2002, 8, 1132–1141. [Google Scholar] [PubMed]
- Shariat, S.F.; Roehrborn, C.G.; McConnell, J.D.; Park, S.; Alam, N.; Wheeler, T.M.; Slawin, K.M. Association of the Circulating Levels of the Urokinase System of Plasminogen Activation With the Presence of Prostate Cancer and Invasion, Progression, and Metastasis. J. Clin. Oncol. 2007, 25, 349–355. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.; et al. Biochemical Outcome After Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Lippert, S.; Berg, K.D.; Høyer-Hansen, G.; Lund, I.K.; Iversen, P.; Christensen, I.J.; Brasso, K.; Røder, M.A. Copenhagen uPAR prostate cancer (CuPCa) database: Protocol and early results. Biomark. Med. 2016, 10, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, G.; Berg, K.D.; Lippert, S.; Christensen, I.J.; Brasso, K.; Høyer-Hansen, G.; Røder, M.A. Plasma levels of intact and cleaved urokinase plasminogen activator receptor (uPAR) in men with clinically localised prostate cancer. J. Clin. Pathol. 2017, 70, 1063–1068. [Google Scholar] [CrossRef]
- Gupta, A.; Lotan, Y.; Ashfaq, R.; Roehrborn, C.G.; Raj, G.V.; Aragaki, C.C.; Montorsi, F.; Shariat, S.F. Predictive Value of the Differential Expression of the Urokinase Plasminogen Activation Axis in Radical Prostatectomy Patients. Eur. Urol. 2009, 55, 1124–1134. [Google Scholar] [CrossRef]
- Almasi, C.E.; Brasso, K.; Iversen, P.; Pappot, H.; Høyer-Hansen, G.; Danø, K.; Christensen, I.J. Prognostic and predictive value of intact and cleaved forms of the urokinase plasminogen activator receptor in metastatic prostate cancer. Prostate 2010, 71, 899–907. [Google Scholar] [CrossRef]
- Røder, M.A.; Brasso, K.; Berg, K.D.; Thomsen, F.B.; Gruschy, L.; Rusch, E.; Iversen, P. Patients undergoing radical prostatectomy have a better survival than the background population. Dan. Med. J. 2013, 60, A4612. [Google Scholar]
- Grøndahl-Hansen, J.; Peters, H.A.; Van Putten, W.L.; Look, M.P.; Pappot, H.; Rønne, E.; Dano, K.; Klijn, J.G.; Brünner, N.; Foekens, J.A. Prognostic significance of the receptor for urokinase plasminogen activator in breast cancer. Clin. Cancer Res. 1995, 1, 1079–1087. [Google Scholar]
- Dohn, L.H.; Illemann, M.; Høyer-Hansen, G.; Christensen, I.J.; Hostmark, J.; Litlekalsoy, J.; Von Der Maase, H.; Pappot, H.; Laerum, O.D. Urokinase-type plasminogen activator receptor (uPAR) expression is associated with T-stage and survival in urothelial carcinoma of the bladder. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 165.e15–165.e24. [Google Scholar] [CrossRef]
- Shariat, S.F.; Monoski, M.A.; Andrews, B.; Wheeler, T.M.; Lerner, S.P.; Slawin, K.M. Association of plasma urokinase-type plasminogen activator and its receptor with clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder. Urology 2003, 61, 1053–1058. [Google Scholar] [CrossRef]
- Almasi, C.E.; Drivsholm, L.; Pappot, H.; Christensen, I.J.; Høyer-Hansen, G. The liberated domain I of urokinase plasminogen activator receptor-a new tumour marker in small cell lung cancer. APMIS 2012, 121, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Almasi, C.E.; Christensen, I.J.; Høyer-Hansen, G.; Danø, K.; Pappot, H.; Dienemann, H.; Muley, T. Urokinase receptor forms in serum from non-small cell lung cancer patients: Relation to prognosis. Lung Cancer 2011, 74, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, H.; Gao, B.; Zhang, Y.; Lin, Q.; Shi, J.; Liu, J.; Liu, J. Prognostic value of urokinase plasminogen activator system in non‑small cell lung cancer: A systematic review and meta‑analysis. Mol. Clin. Oncol. 2017, 8, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.W.; Nielsen, H.J.; Christensen, I.J.; Thorlacius-Ussing, O.; Sørensen, S.; Danø, K.; Bruünner, N. Plasma urokinase receptor levels in patients with colorectal cancer: Relationship to prognosis. J. Natl. Cancer Inst. 1999, 91, 869–874. [Google Scholar] [CrossRef]
- Illemann, M.; Laerum, O.D.; Hasselby, J.P.; Thurison, T.; Høyer-Hansen, G.; Nielsen, H.J.; Christensen, I.J. The Danish Study Group on Early Detection of Colorectal Cancer Urokinase-type plasminogen activator receptor (uPAR) on tumor-associated macrophages is a marker of poor prognosis in colorectal cancer. Cancer Med. 2014, 3, 855–864. [Google Scholar] [CrossRef]
| Variable | Radical Prostatectomy (N = 532) | |
|---|---|---|
| uPAR (I–III) fmol/mL, median [IQR] | 29.3 [24.7–35.6] | |
| uPAR (I–III + II–III) fmol/mL, median [IQR] | 55.6 [46.8–65.1] | |
| uPAR (I) fmol/mL, median [IQR] | 13.2 [9.7–17.2] | |
| Follow-up time (years), median [IQR] | 6.2 [5.3–7.1] | |
| Age (years), median [IQR] | 64.6 [59.5–67.9] | |
| PSA (ng/mL), median [IQR] | 8.0 [5.9–12.0] | |
| Gleason score (%) | ≤6 | 145 (27.3) |
| 3 + 4 | 246 (46.2) | |
| 4 + 3 | 89 (16.7) | |
| ≥8 | 52 (9.8) | |
| Margin status (%) | Negative | 416 (78.2) |
| Positive | 116 (21.8) | |
| Tumor stage (%) | T2a + b | 61 (11.5) |
| T2c | 340 (63.9) | |
| T3 | 131 (24.6) | |
| Lymph node status (%) | Negative | 502 (94.4) |
| Positive | 30 (5.6) | |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | ||
| uPAR I-III | Q1 | Reference | Reference | ||
| Q2 | 1.20 (0.73–1.99) | 0.47 | 1.16 (0.70–1.94) | 0.56 | |
| Q3 | 1.07 (0.64–1.80) | 0.80 | 1.26 (0.74–2.15) | 0.40 | |
| Q4 | 1.26 (0.76–2.07) | 0.37 | 1.32 (0.79–2.18) | 0.29 | |
| uPAR I-III + II-III | Q1 | Reference | Reference | ||
| Q2 | 1.49 (0.90–2.47) | 0.12 | 1.32 (0.79–2.33) | 0.29 | |
| Q3 | 1.19 (0.70–2.03) | 0.52 | 1.35 (0.78–2.15) | 0.28 | |
| Q4 | 1.38 (0.83–2.31) | 0.21 | 1.27 (0.75–2.15) | 0.38 | |
| uPAR I | Q1 | Reference | Reference | ||
| Q2 | 0.56 (0.32–0.98) | 0.04 * | 0.68 (0.38–1.22) | 0.20 | |
| Q3 | 1.01 (0.63–1.63) | 0.95 | 1.08 (0.66–1.75) | 0.76 | |
| Q4 | 1.14 (0.72–1.81) | 0.58 | 1.01 (0.63–1.62) | 0.96 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroomberg, H.V.; Kristensen, G.; Drimer Berg, K.; Lippert, S.; Brasso, K.; Røder, M.A. The Association between Plasma Levels of Intact and Cleaved uPAR Levels and the Risk of Biochemical Recurrence after Radical Prostatectomy for Prostate Cancer. Diagnostics 2020, 10, 877. https://doi.org/10.3390/diagnostics10110877
Stroomberg HV, Kristensen G, Drimer Berg K, Lippert S, Brasso K, Røder MA. The Association between Plasma Levels of Intact and Cleaved uPAR Levels and the Risk of Biochemical Recurrence after Radical Prostatectomy for Prostate Cancer. Diagnostics. 2020; 10(11):877. https://doi.org/10.3390/diagnostics10110877
Chicago/Turabian StyleStroomberg, Hein Vincent, Gitte Kristensen, Kasper Drimer Berg, Solvej Lippert, Klaus Brasso, and Martin Andreas Røder. 2020. "The Association between Plasma Levels of Intact and Cleaved uPAR Levels and the Risk of Biochemical Recurrence after Radical Prostatectomy for Prostate Cancer" Diagnostics 10, no. 11: 877. https://doi.org/10.3390/diagnostics10110877
APA StyleStroomberg, H. V., Kristensen, G., Drimer Berg, K., Lippert, S., Brasso, K., & Røder, M. A. (2020). The Association between Plasma Levels of Intact and Cleaved uPAR Levels and the Risk of Biochemical Recurrence after Radical Prostatectomy for Prostate Cancer. Diagnostics, 10(11), 877. https://doi.org/10.3390/diagnostics10110877





