A tRNA- and Anticodon-Centric View of the Evolution of Aminoacyl-tRNA Synthetases, tRNAomes, and the Genetic Code
Abstract
1. Introduction
2. Materials and Methods
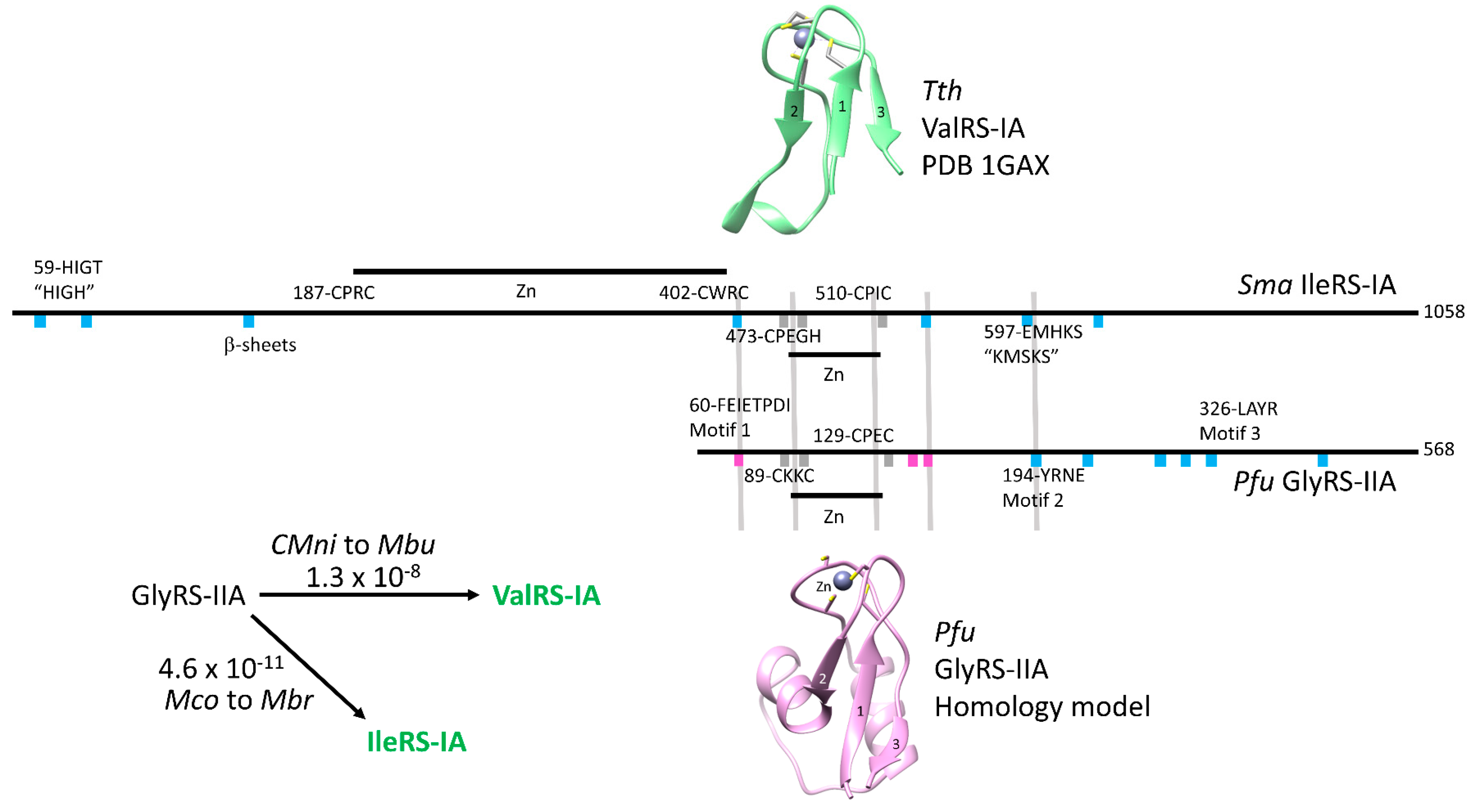
2.1. Homology of GlyRS-IIA and IleRS-IA and GlyRS-IIA and ValRS-IA
2.2. Determining Kinship among aaRS Enzymes
2.3. Molecular Graphics
3. The Hypothesis
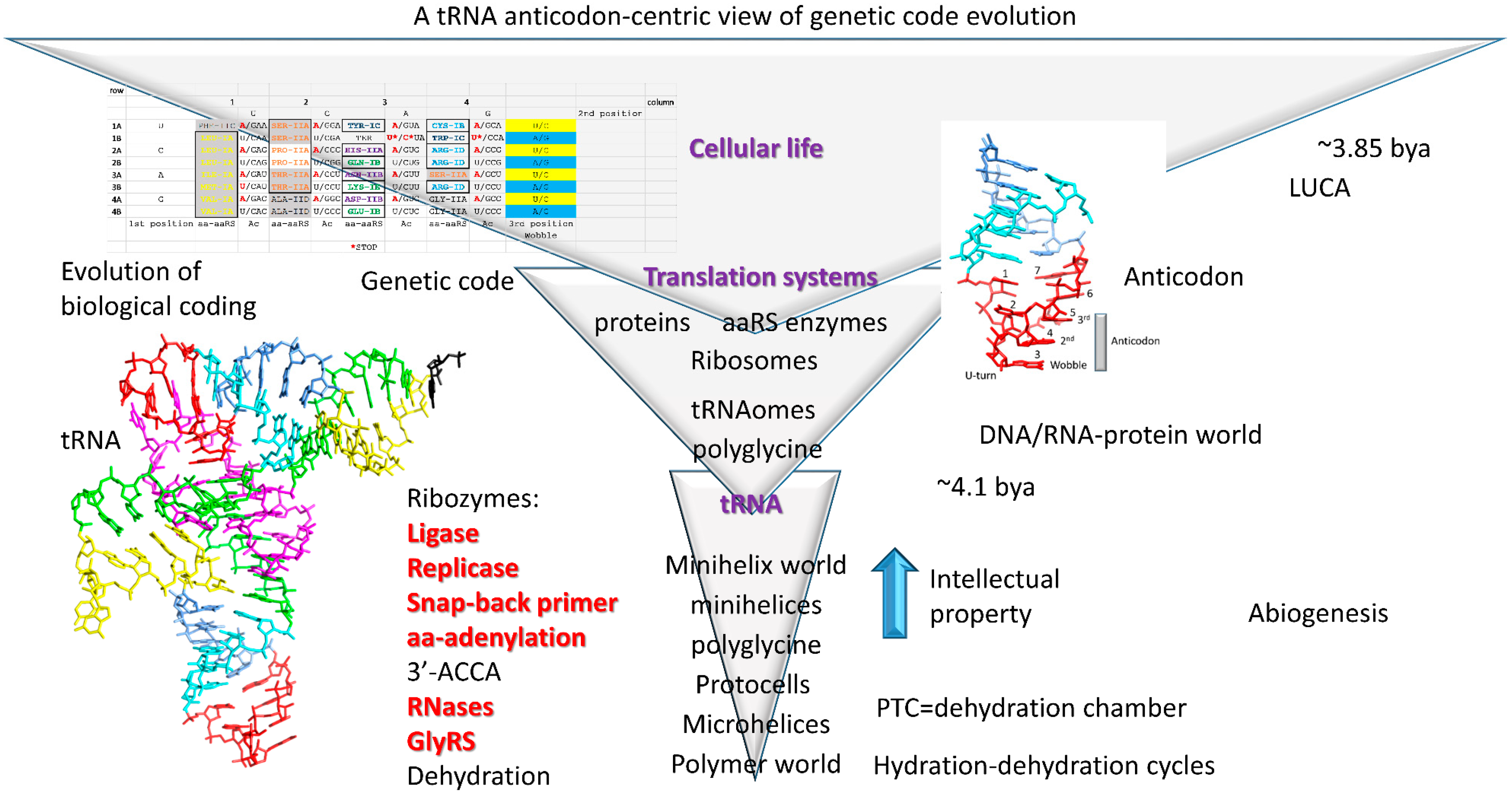
4. Evolution of tRNA
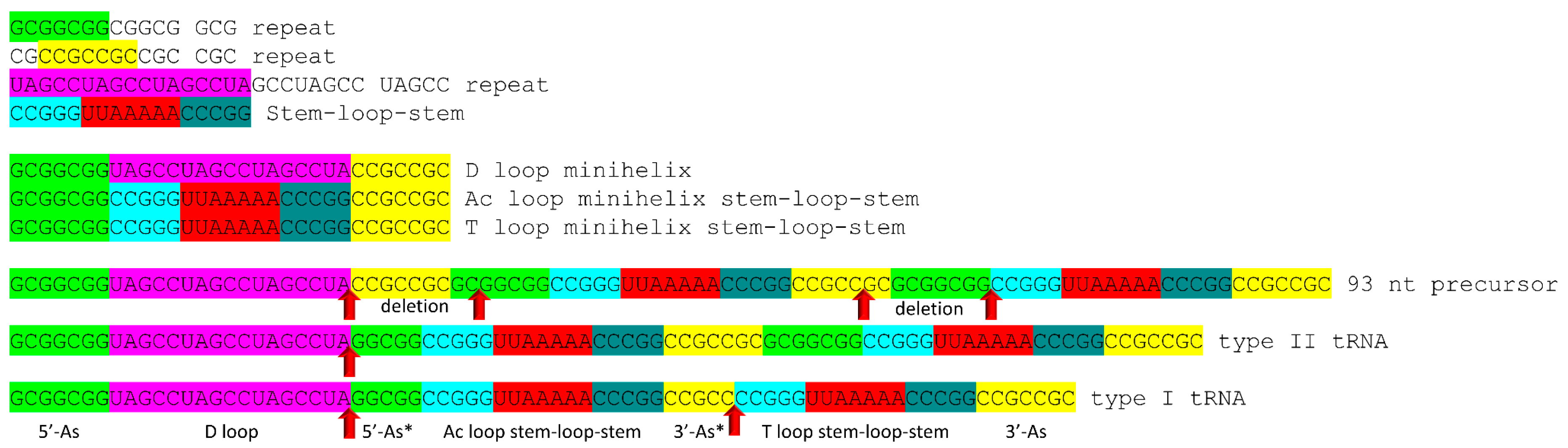
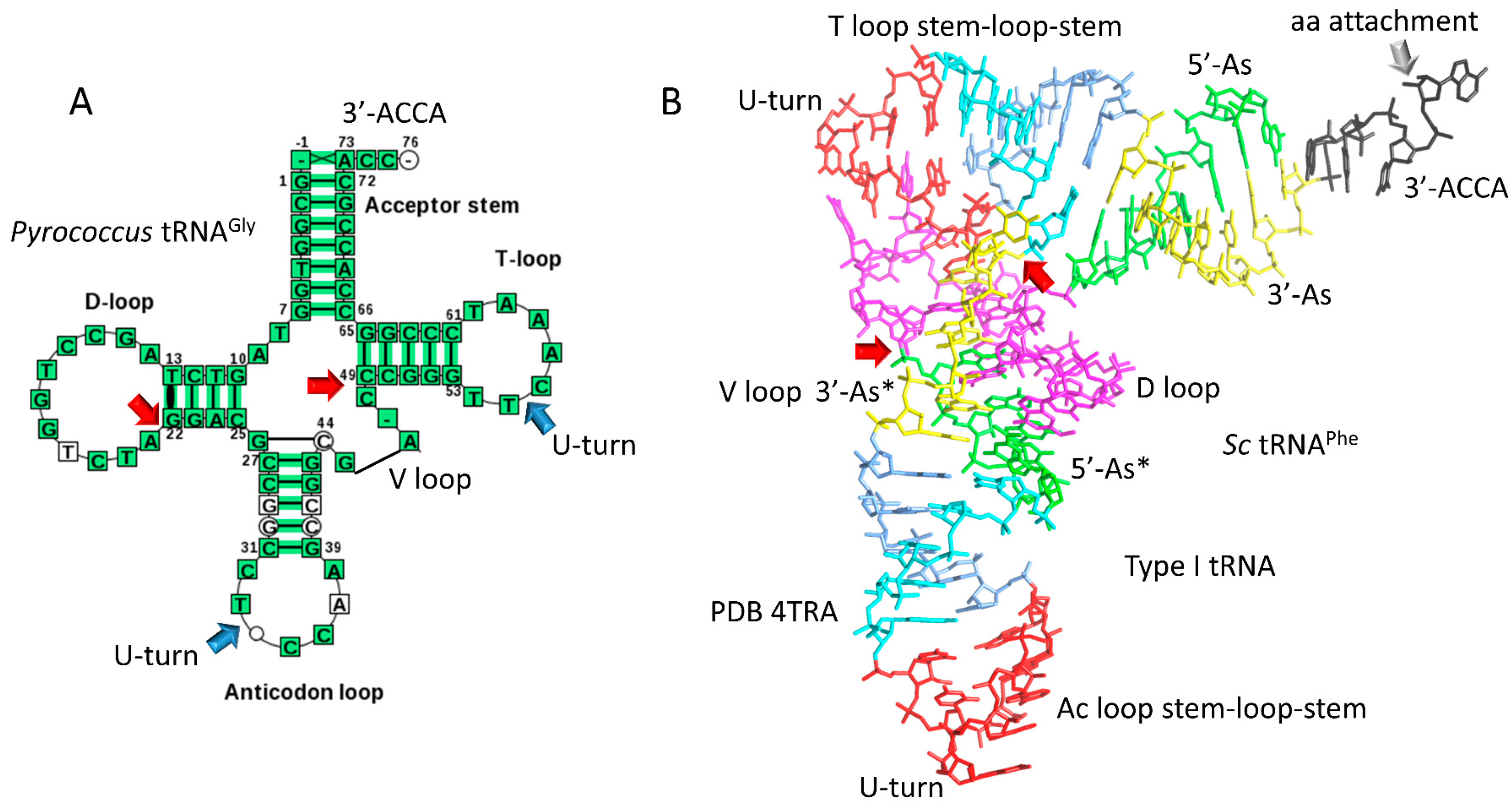
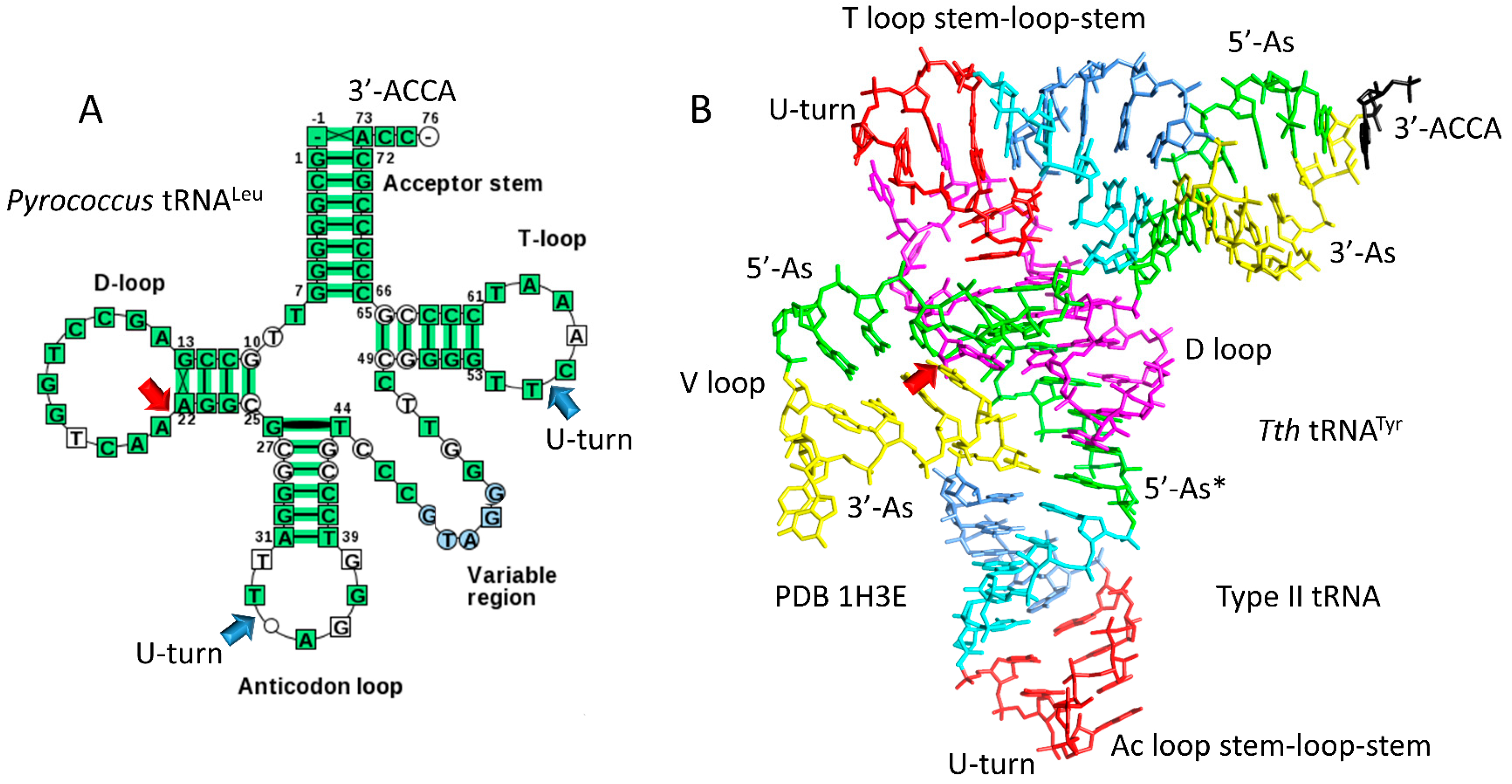
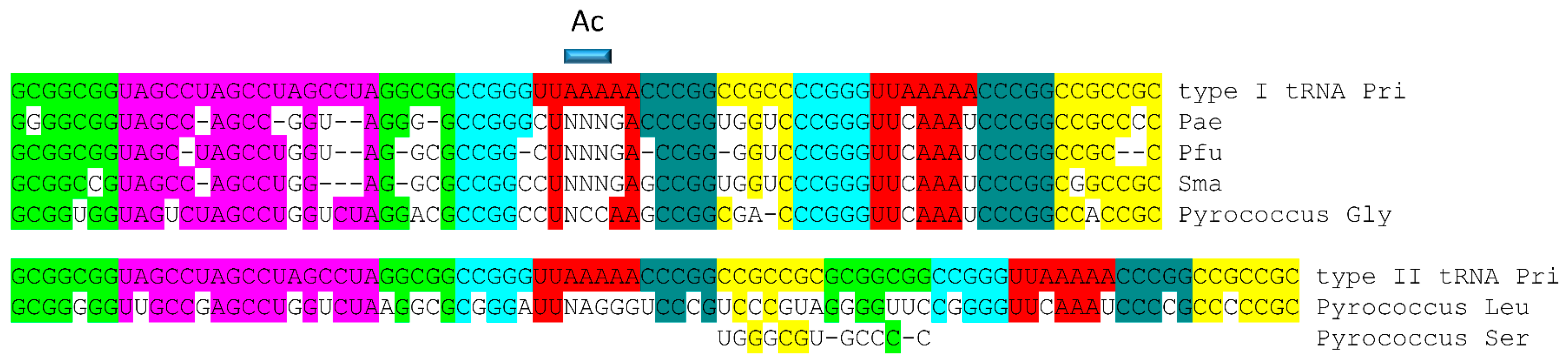
4.1. A Model for tRNA Evolution
4.2. Evolution from Order to Chaos
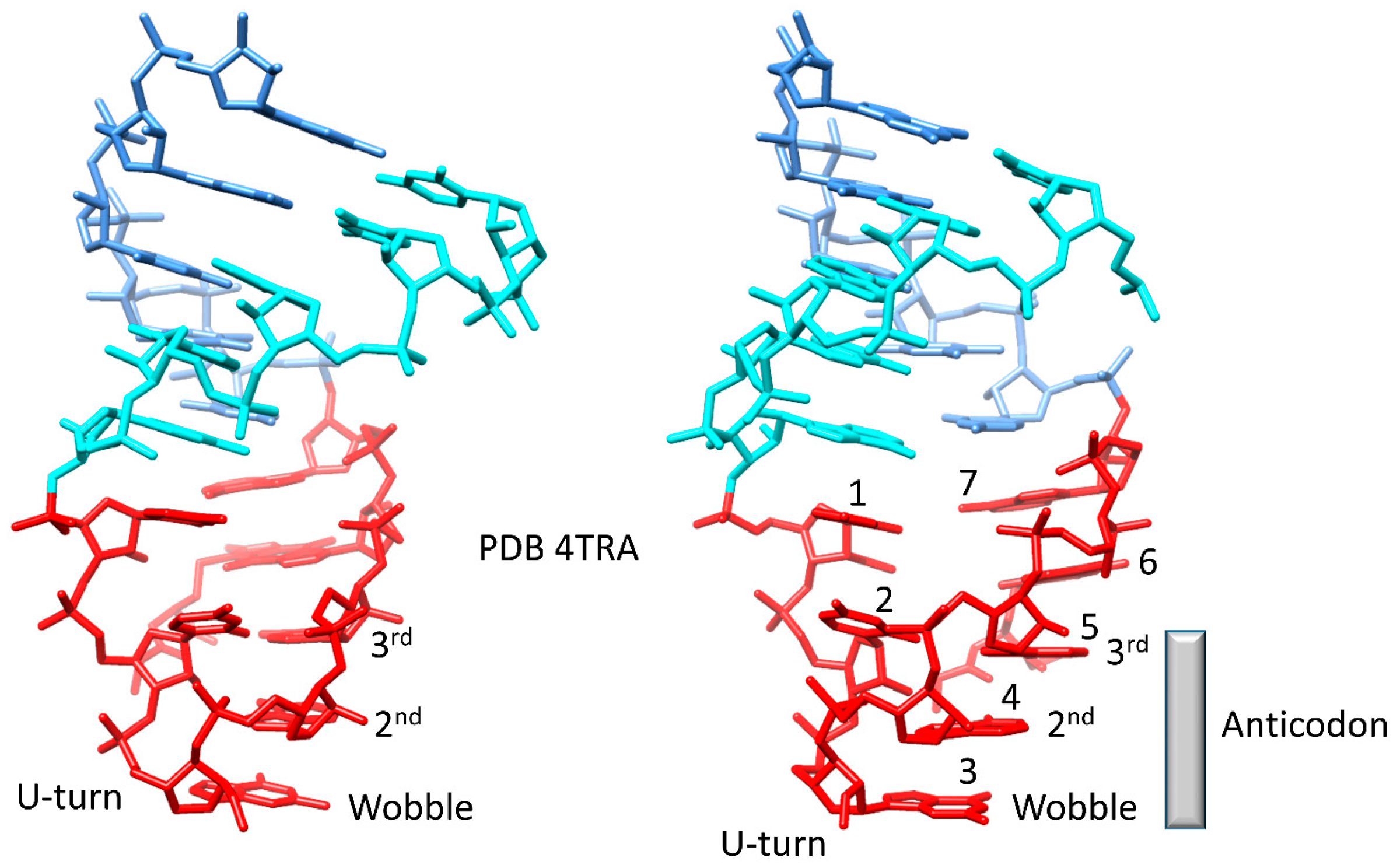
4.3. Evolution around the tRNA Anticodon
4.4. Addition of 3′-ACCA
5. Glycine as the Primordial Amino Acid
6. Coevolution of aaRS Enzymes and the Genetic Code
6.1. Evolution of Class II aaRS
6.2. Evolution of Class I aaRS
6.3. Homology of Class I and Class II aaRS
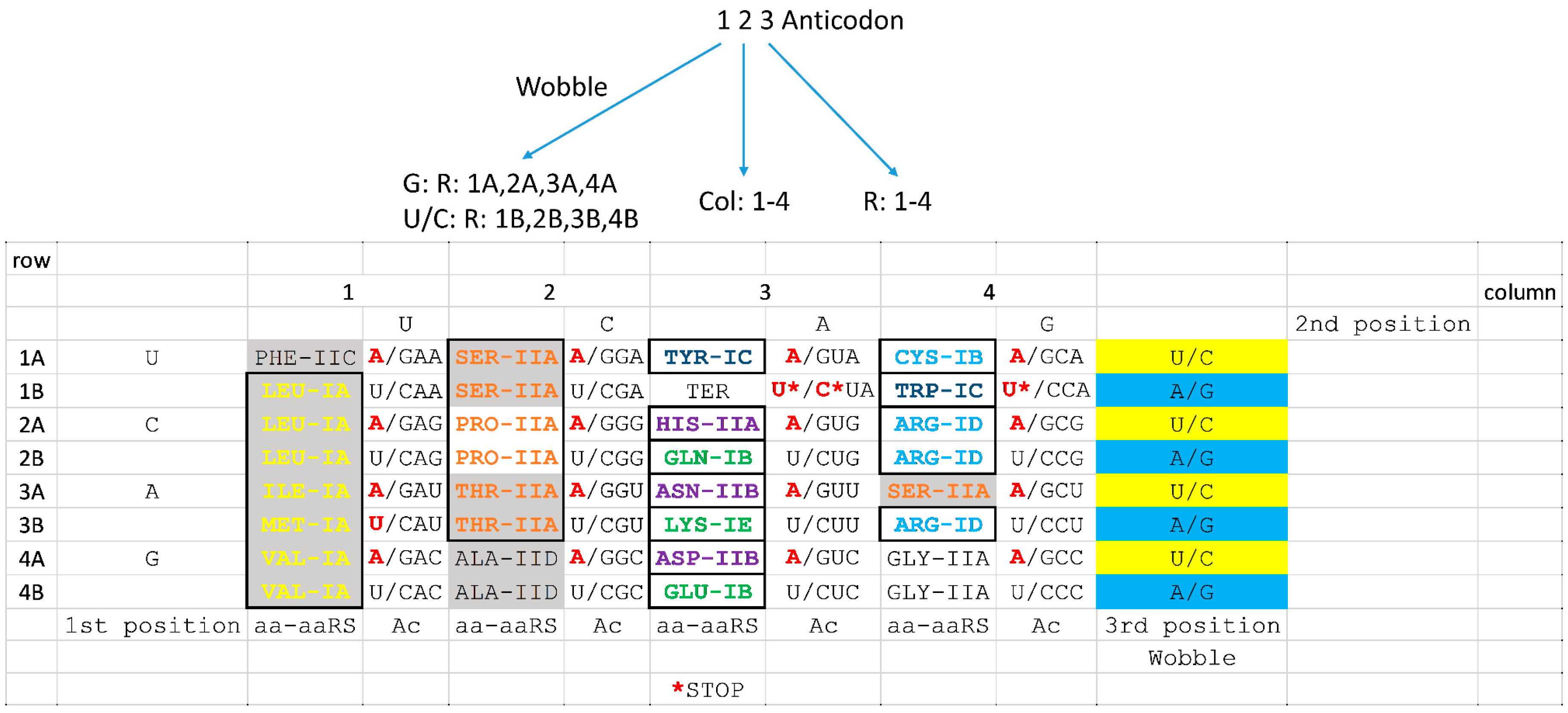
6.4. Coevolution of aaRS Clades and the Genetic Code
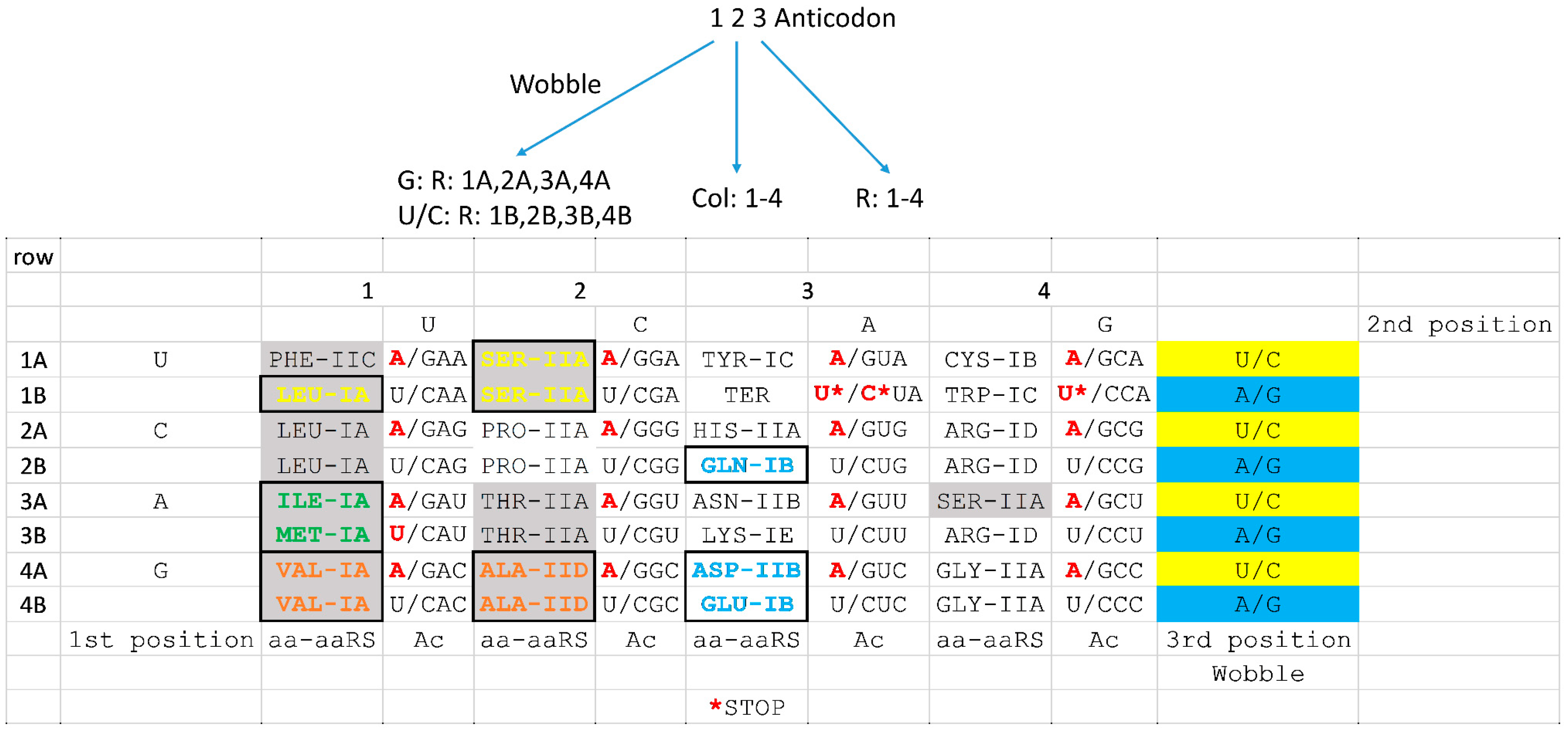
7. Coevolution of tRNAomes and the Genetic Code
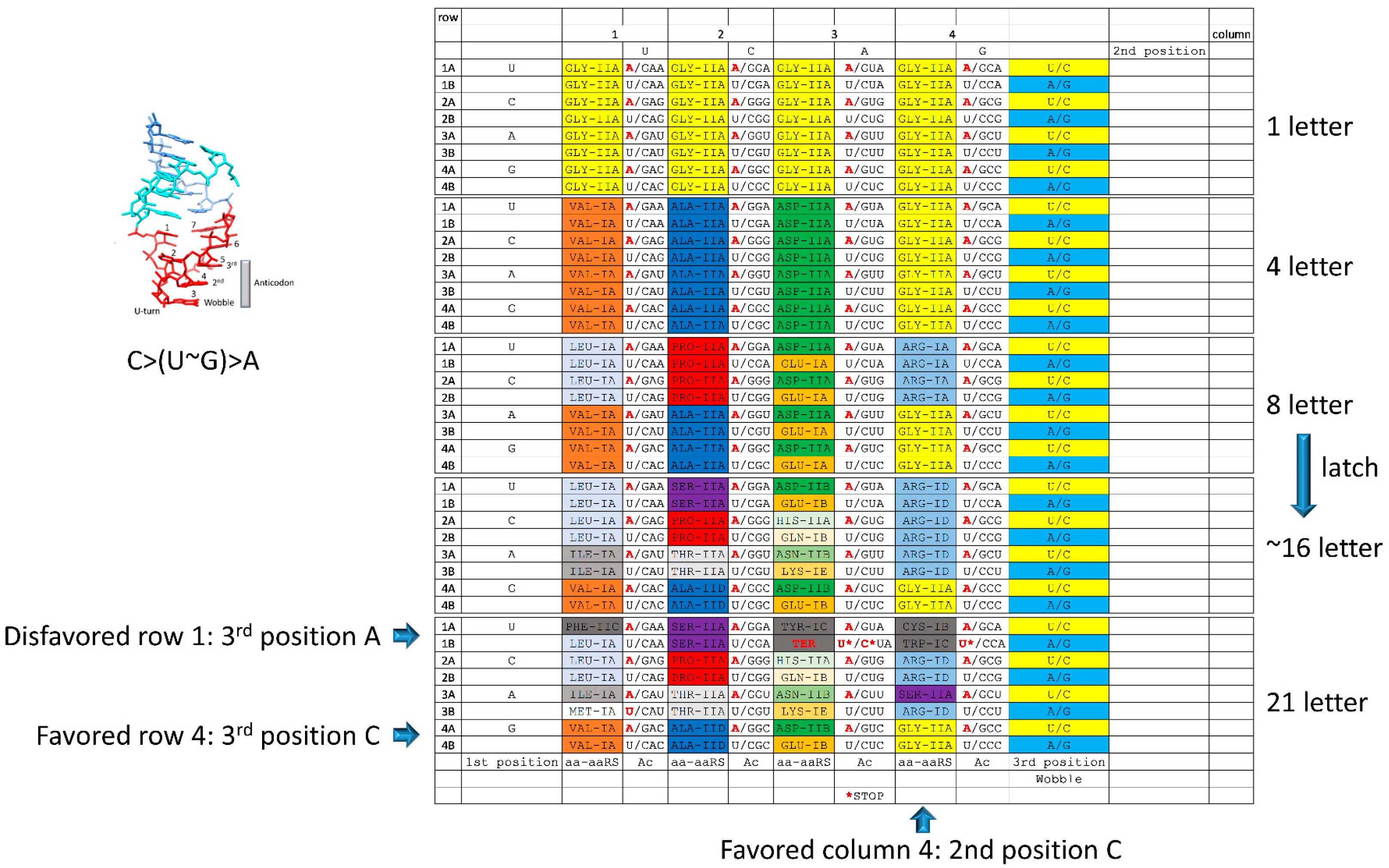
8. Evolution of the Genetic Code
9. The tRNA-Centric View
10. Simulation of Genetic Code Evolution
11. Evolution of the Genetic Code as an Artificial Intelligence Problem
12. Life on Earth
Author Contributions
Conflicts of Interest
Abbreviations
| aaRS | Aminoacyl-tRNA synthetase (i.e., GlyRS) |
| Ac loop | Anticodon loop |
| LUCA | Last universal common (cellular) ancestor |
| NCBI | National Center for Bioinformatics Information |
| PTC | Peptidyl transferase center |
| T loop | T loop or TΨC loop |
| V loop | Variable loop |
References
- Pak, D.; Du, N.; Kim, Y.; Sun, Y.; Burton, Z.F. Rooted tRNAomes and evolution of the genetic code. Transcription 2018, 9, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Pak, D.; Kim, Y.; Burton, Z.F. Aminoacyl-tRNA synthetase evolution and sectoring of the genetic code. Transcription 2018, 9, 205–224. [Google Scholar] [CrossRef]
- Pak, D.; Root-Bernstein, R.; Burton, Z.F. tRNA structure and evolution and standardization to the three nucleotide genetic code. Transcription 2017, 8, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kowiatek, B.; Opron, K.; Burton, Z.F. Type-II tRNAs and Evolution of Translation Systems and the Genetic Code. Int. J. Mol. Sci. 2018, 19, 3275. [Google Scholar] [CrossRef] [PubMed]
- Opron, K.; Burton, Z.F. Ribosome Structure, Function, and Early Evolution. Int. J. Mol. Sci. 2018, 20, 40. [Google Scholar] [CrossRef]
- Perona, J.J.; Gruic-Sovulj, I. Synthetic and editing mechanisms of aminoacyl-tRNA synthetases. Top. Curr. Chem. 2014, 344, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Kim, Y.; Sanjay, A.; Burton, Z.F. tRNA evolution from the proto-tRNA minihelix world. Transcription 2016, 7, 153–163. [Google Scholar] [CrossRef]
- Burton, Z.F.; Opron, K.; Wei, G.; Geiger, J.H. A model for genesis of transcription systems. Transcription 2016, 7, 1–13. [Google Scholar] [CrossRef][Green Version]
- Juhling, F.; Morl, M.; Hartmann, R.K.; Sprinzl, M.; Stadler, P.F.; Putz, J. tRNAdb 2009: Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009, 37, D159–D162. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. GtRNAdb 2.0: An expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 2016, 44, D184–D189. [Google Scholar] [CrossRef]
- Yarus, M. The meaning of a minuscule ribozyme. Philos. Trans. R Soc. Lond. B Biol. Sci. 2011, 366, 2902–2909. [Google Scholar] [CrossRef]
- Turk, R.M.; Chumachenko, N.V.; Yarus, M. Multiple translational products from a five-nucleotide ribozyme. Proc. Natl. Acad. Sci. USA 2010, 107, 4585–4589. [Google Scholar] [CrossRef] [PubMed]
- Chumachenko, N.V.; Novikov, Y.; Yarus, M. Rapid and simple ribozymic aminoacylation using three conserved nucleotides. J. Am. Chem. Soc. 2009, 131, 5257–5263. [Google Scholar] [CrossRef]
- Smith, T.F.; Hartman, H. The evolution of Class II Aminoacyl-tRNA synthetases and the first code. FEBS Lett. 2015, 589, 3499–3507. [Google Scholar] [CrossRef]
- O’Donoghue, P.; Luthey-Schulten, Z. On the evolution of structure in aminoacyl-tRNA synthetases. Microbiol. Mol. Biol. Rev. 2003, 67, 550–573. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cui, Z.; Gottlieb, R.L.; Zhang, B. A selected ribozyme catalyzing diverse dipeptide synthesis. Chem. Biol. 2002, 9, 619–628. [Google Scholar] [CrossRef]
- Balke, D.; Wichert, C.; Appel, B.; Muller, S. Generation and selection of ribozyme variants with potential application in protein engineering and synthetic biology. Appl. Microbiol. Biotechnol. 2014, 98, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.M.; Allison, J.R.; Hongdilokkul, N.; Gaillon, L.; Kast, P.; van Gunsteren, W.F.; Marliere, P.; Hilvert, D. Directed evolution of a model primordial enzyme provides insights into the development of the genetic code. PLoS Genet. 2013, 9, e1003187. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Yang, Z.; Lasker, K.; Schneidman-Duhovny, D.; Webb, B.; Huang, C.C.; Pettersen, E.F.; Goddard, T.D.; Meng, E.C.; Sali, A.; Ferrin, T.E. UCSF Chimera, MODELLER, and IMP: An integrated modeling system. J. Struct. Biol. 2012, 179, 269–278. [Google Scholar] [CrossRef]
- Deamer, D. The Role of Lipid Membranes in Life’s Origin. Life 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W. The Narrow Road to the Deep Past: In Search of the Chemistry of the Origin of Life. Angew. Chem. Int. Ed. Engl. 2017, 56, 11037–11043. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W. An optimal degree of physical and chemical heterogeneity for the origin of life? Philos Trans. R Soc. Lond. B Biol. Sci. 2011, 366, 2894–2901. [Google Scholar] [CrossRef]
- Schrum, J.P.; Zhu, T.F.; Szostak, J.W. The origins of cellular life. Cold Spring Harb Perspect Biol 2010, 2, a002212. [Google Scholar] [CrossRef]
- Scheffers, D.J.; Pinho, M.G. Bacterial cell wall synthesis: New insights from localization studies. Microbiol. Mol. Biol. Rev. 2005, 69, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V. Frozen Accident Pushing 50: Stereochemistry, Expansion, and Chance in the Evolution of the Genetic Code. Life 2017, 7, 22. [Google Scholar] [CrossRef]
- Koonin, E.V.; Novozhilov, A.S. Origin and evolution of the genetic code: the universal enigma. IUBMB Life 2009, 61, 99–111. [Google Scholar] [CrossRef]
- Koonin, E.V.; Novozhilov, A.S. Origin and Evolution of the Universal Genetic Code. Annu. Rev. Genet. 2017, 51, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Ribas de Pouplana, L.; Torres, A.G.; Rafels-Ybern, A. What Froze the Genetic Code? Life 2017, 7, 14. [Google Scholar] [CrossRef]
- Dodd, M.S.; Papineau, D.; Grenne, T.; Slack, J.F.; Rittner, M.; Pirajno, F.; O’Neil, J.; Little, C.T. Evidence for early life in Earth’s oldest hydrothermal vent precipitates. Nature 2017, 543, 60–64. [Google Scholar] [CrossRef]
- Schopf, J.W.; Kitajima, K.; Spicuzza, M.J.; Kudryavtsev, A.B.; Valley, J.W. SIMS analyses of the oldest known assemblage of microfossils document their taxon-correlated carbon isotope compositions. Proc. Natl. Acad. Sci. USA 2018, 115, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.A.; Boehnke, P.; Harrison, T.M.; Mao, W.L. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc. Natl. Acad. Sci. USA 2015, 112, 14518–14521. [Google Scholar] [CrossRef]
- Westhof, E.; Dumas, P.; Moras, D. Restrained refinement of two crystalline forms of yeast aspartic acid and phenylalanine transfer RNA crystals. Acta Crystallogr. A 1988, 44 Pt 2, 112–123. [Google Scholar] [CrossRef]
- Yaremchuk, A.; Kriklivyi, I.; Tukalo, M.; Cusack, S. Class I tyrosyl-tRNA synthetase has a class II mode of cognate tRNA recognition. EMBO J. 2002, 21, 3829–3840. [Google Scholar] [CrossRef]
- Saint-Leger, A.; Bello, C.; Dans, P.D.; Torres, A.G.; Novoa, E.M.; Camacho, N.; Orozco, M.; Kondrashov, F.A.; Ribas de Pouplana, L. Saturation of recognition elements blocks evolution of new tRNA identities. Sci. Adv. 2016, 2, e1501860. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. The origin of the tRNA molecule: Independent data favor a specific model of its evolution. Biochimie 2012, 94, 1464–1466. [Google Scholar] [CrossRef]
- Di Giulio, M. Formal proof that the split genes of tRNAs of Nanoarchaeum equitans are an ancestral character. J. Mol. Evol. 2009, 69, 505–511. [Google Scholar] [CrossRef]
- Di Giulio, M. A comparison among the models proposed to explain the origin of the tRNA molecule: A synthesis. J. Mol. Evol. 2009, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Widmann, J.; Di Giulio, M.; Yarus, M.; Knight, R. tRNA creation by hairpin duplication. J. Mol. Evol. 2005, 61, 524–530. [Google Scholar] [CrossRef]
- Tallmadge, R.L.; Zygelyte, E.; Van de Walle, G.R.; Kristie, T.M.; Felippe, M.J.B. Effect of a Histone Demethylase Inhibitor on Equine Herpesvirus-1 Activity In Vitro. Front. Vet. Sci. 2018, 5, 34. [Google Scholar] [CrossRef]
- Nagaswamy, U.; Fox, G.E. RNA ligation and the origin of tRNA. Orig. Life Evol. Biosph. 2003, 33, 199–209. [Google Scholar] [CrossRef]
- Samanta, B.; Joyce, G.F. A reverse transcriptase ribozyme. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Quigley, G.J.; Rich, A. Structural domains of transfer RNA molecules. Science 1976, 194, 796–806. [Google Scholar] [CrossRef]
- Rozov, A.; Wolff, P.; Grosjean, H.; Yusupov, M.; Yusupova, G.; Westhof, E. Tautomeric G*U pairs within the molecular ribosomal grip and fidelity of decoding in bacteria. Nucleic Acids Res. 2018, 46, 7425–7435. [Google Scholar] [CrossRef]
- Rozov, A.; Demeshkina, N.; Khusainov, I.; Westhof, E.; Yusupov, M.; Yusupova, G. Novel base-pairing interactions at the tRNA wobble position crucial for accurate reading of the genetic code. Nat. Commun. 2016, 7, 10457. [Google Scholar] [CrossRef]
- Rozov, A.; Demeshkina, N.; Westhof, E.; Yusupov, M.; Yusupova, G. New Structural Insights into Translational Miscoding. Trends Biochem. Sci. 2016, 41, 798–814. [Google Scholar] [CrossRef] [PubMed]
- Rozov, A.; Westhof, E.; Yusupov, M.; Yusupova, G. The ribosome prohibits the G*U wobble geometry at the first position of the codon-anticodon helix. Nucleic Acids Res. 2016, 44, 6434–6441. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, H.S. Clues to tRNA Evolution from the Distribution of Class II tRNAs and Serine Codons in the Genetic Code. Life 2016, 6, 10. [Google Scholar] [CrossRef]
- Bernhardt, H.S.; Patrick, W.M. Genetic code evolution started with the incorporation of glycine, followed by other small hydrophilic amino acids. J. Mol. Evol. 2014, 78, 307–309. [Google Scholar] [CrossRef]
- Bernhardt, H.S.; Tate, W.P. Evidence from glycine transfer RNA of a frozen accident at the dawn of the genetic code. Biol. Direct. 2008, 3, 53. [Google Scholar] [CrossRef]
- Csárdi, G.; Nepusz, T. The igraph software package for complex network research. Int. J. Complex. Syst. 2006, 1695, 1–9. [Google Scholar]
- Carter, C.W., Jr.; Li, L.; Weinreb, V.; Collier, M.; Gonzalez-Rivera, K.; Jimenez-Rodriguez, M.; Erdogan, O.; Kuhlman, B.; Ambroggio, X.; Williams, T.; et al. The Rodin-Ohno hypothesis that two enzyme superfamilies descended from one ancestral gene: An unlikely scenario for the origins of translation that will not be dismissed. Biol. Direct. 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Rodin, A.S.; Rodin, S.N.; Carter, C.W., Jr. On primordial sense-antisense coding. J. Mol. Evol. 2009, 69, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Rodin, S.N.; Ohno, S. Two types of aminoacyl-tRNA synthetases could be originally encoded by complementary strands of the same nucleic acid. Orig. Life Evol. Biosph. 1995, 25, 565–589. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. Some pungent arguments against the physico-chemical theories of the origin of the genetic code and corroborating the coevolution theory. J. Theor. Biol. 2017, 414, 1–4. [Google Scholar] [CrossRef]
- Wong, J.T.; Ng, S.K.; Mat, W.K.; Hu, T.; Xue, H. Coevolution Theory of the Genetic Code at Age Forty: Pathway to Translation and Synthetic Life. Life 2016, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F.; Eruysal, E.R.; Narendran, A.; Vare, V.Y.P.; Vangaveti, S.; Ranganathan, S.V. Celebrating wobble decoding: Half a century and still much is new. RNA Biol. 2018, 15, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Agris, P.F.; Narendran, A.; Sarachan, K.; Vare, V.Y.P.; Eruysal, E. The Importance of Being Modified: The Role of RNA Modifications in Translational Fidelity. Enzymes 2017, 41, 1–50. [Google Scholar] [CrossRef]
- Grosjean, H.; Westhof, E. An integrated, structure- and energy-based view of the genetic code. Nucleic Acids Res. 2016, 44, 8020–8040. [Google Scholar] [CrossRef]
- Wolf, Y.I.; Koonin, E.V. On the origin of the translation system and the genetic code in the RNA world by means of natural selection, exaptation, and subfunctionalization. Biol. Direct. 2007, 2, 14. [Google Scholar] [CrossRef]
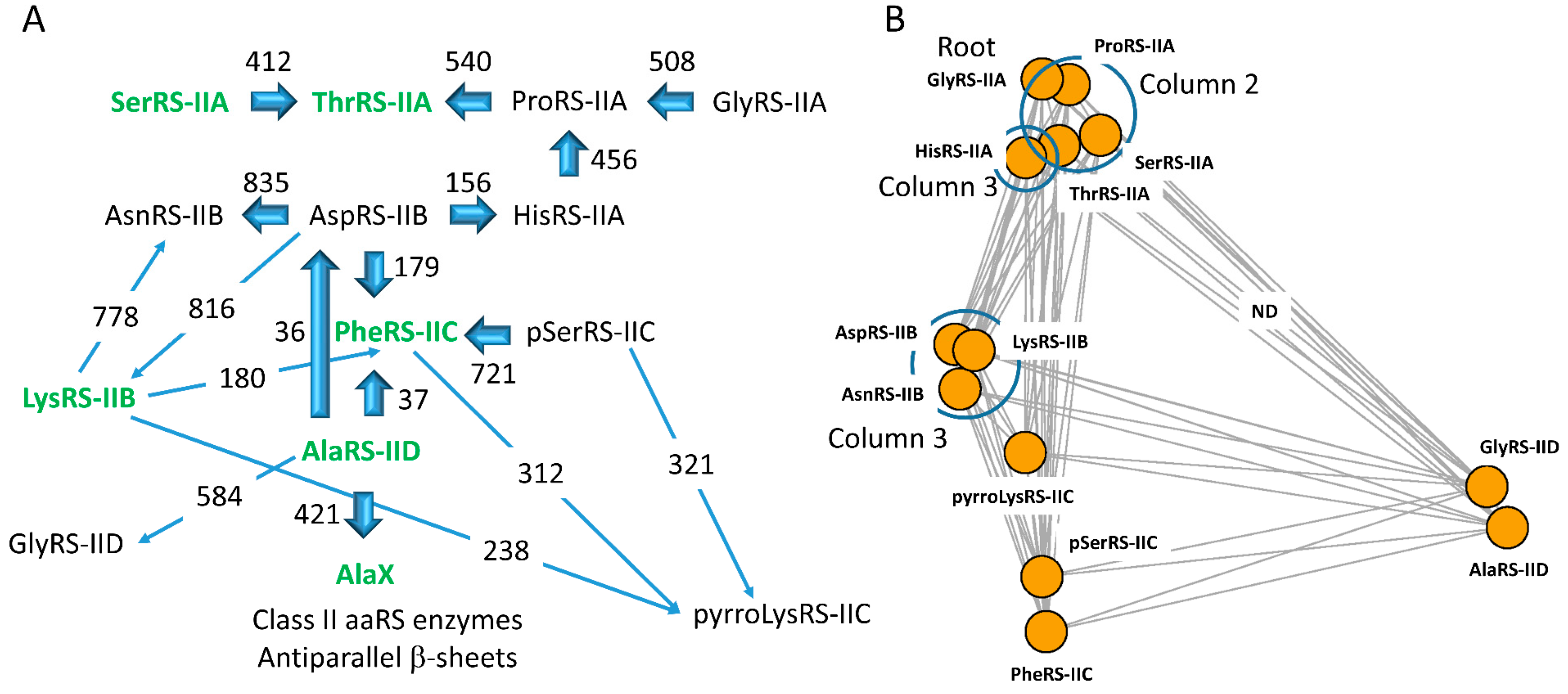

| SerRS-IIA | ThrRS-IIA | ProRS-IIA | GlyRS-IIA | HisRS-IIA | AspRS-IIB | AsnRS-IIB | LysRS-IIB | PheRS-IIC | pSerRS-IIC | AlaRS-IID | pyrroLysRS-IIC | GlyRS-IID | target | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SerRS-IIA | NR | 412 | 384 | 275 | 159 | 58 | 59 | 74 | 77 | 81 | 26 | 156 | ND | |
| ThrRS-IIA | 412 | NR | 540 | 554 | 456 | 80 | ND | 75 | 77 | 69 | ND | 129 | ND | |
| ProRS-IIA | 373 | 540 | NR | 495 | 378 | 63 | ND | 70 | 96 | 79 | ND | 127 | ND | |
| GlyRS-IIA | 307 | 554 | 608 | NR | 392 | 63 | ND | 98 | 79 | 71 | ND | 141 | ND | |
| HisRS-IIA | 149 | 456 | 339 | 393 | NR | 79 | ND | 181 | 104 | 83 | 17 | 123 | ND | |
| AspRS-IIB | 68 | 80 | 71 | 57 | 156 | NR | 807 | 816 | 179 | 98 | 37 | 224 | 36 | |
| AsnRS-IIB | 59 | ND | ND | ND | ND | 807 | NR | 778 | 98 | 81 | 33 | 216 | ND | |
| LysRS-IIB | 69 | 75 | 75 | 66 | 180 | 815 | 778 | NR | 180 | 101 | 30 | 265 | 33 | |
| PheRS-IIC | 72 | 77 | 69 | 60 | 64 | 107 | 98 | 106 | NR | 719 | 37 | 203 | 37 | |
| pSerRS-IIC | 75 | 69 | 75 | 61 | 72 | 99 | 81 | 102 | 721 | NR | 32 | 321 | 32 | |
| AlaRS-IID | ND | ND | ND | ND | ND | 36 | 33 | ND | 37 | 32 | NR | 37 | 597 | |
| pyrroLysRS-IIC | 103 | 129 | 124 | 107 | 118 | 229 | 216 | 243 | 350 | 335 | 37 | NR | 37 | |
| GlyRS-IID | ND | ND | ND | ND | ND | 36 | ND | 33 | 40 | 36 | 596 | 37 | NR | |
| query |
| IleRS-IA | LeuRS-IA | MetRS-IA | ValRS-IA | ArgRS-ID | TrpRS-IC | TyrRS-IC | CysRS-IB | GluRS-IB | GlnRS-IB | LysRS-IE | target | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IleRS-IA | NR | 1449 | 846 | 1508 | 259 | 50 | 55 | 301 | 113 | 137 | 73 | |
| LeuRS-IA | 1347 | NR | 780 | 1331 | 250 | 38 | 47 | 332 | 115 | 129 | 73 | |
| MetRS-IA | 817 | 774 | NR | 805 | 349 | 52 | 35 | 435 | 156 | 126 | 159 | |
| ValRS-IA | 1663 | 1322 | 854 | NR | 259 | 50 | 55 | 301 | 118 | 154 | 73 | |
| ArgRS-ID | 261 | 274 | 370 | 266 | NR | 56 | 58 | 348 | 151 | 100 | 196 | |
| TrpRS-IC | 34 | 38 | 68 | 34 | 52 | NR | 495 | 54 | 69 | 44 | 36 | |
| TyrRS-IC | 33 | 29 | 33 | 25 | 41 | 485 | NR | 57 | 54 | 40 | 42 | |
| CysRS-IB | 353 | 394 | 446 | 360 | 387 | 64 | 83 | NR | 258 | 293 | 220 | |
| GluRS-IB | 59 | 86 | 139 | 61 | 121 | 65 | 67 | 167 | NR | 1203 | 331 | |
| GlnRS-IB | 59 | 72 | 126 | 63 | 123 | 47 | 48 | 156 | 1538 | NR | 313 | |
| LysRS-IE | 181 | 209 | 234 | 189 | 249 | 67 | 73 | 330 | 549 | 281 | NR | |
| query |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Opron, K.; Burton, Z.F. A tRNA- and Anticodon-Centric View of the Evolution of Aminoacyl-tRNA Synthetases, tRNAomes, and the Genetic Code. Life 2019, 9, 37. https://doi.org/10.3390/life9020037
Kim Y, Opron K, Burton ZF. A tRNA- and Anticodon-Centric View of the Evolution of Aminoacyl-tRNA Synthetases, tRNAomes, and the Genetic Code. Life. 2019; 9(2):37. https://doi.org/10.3390/life9020037
Chicago/Turabian StyleKim, Yunsoo, Kristopher Opron, and Zachary F. Burton. 2019. "A tRNA- and Anticodon-Centric View of the Evolution of Aminoacyl-tRNA Synthetases, tRNAomes, and the Genetic Code" Life 9, no. 2: 37. https://doi.org/10.3390/life9020037
APA StyleKim, Y., Opron, K., & Burton, Z. F. (2019). A tRNA- and Anticodon-Centric View of the Evolution of Aminoacyl-tRNA Synthetases, tRNAomes, and the Genetic Code. Life, 9(2), 37. https://doi.org/10.3390/life9020037






