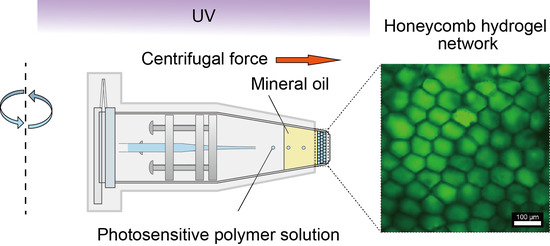
Controlled Construction of Stable Network Structure Composed of Honeycomb-Shaped Microhydrogels
Abstract
1. Introduction
2. Materials and Methods
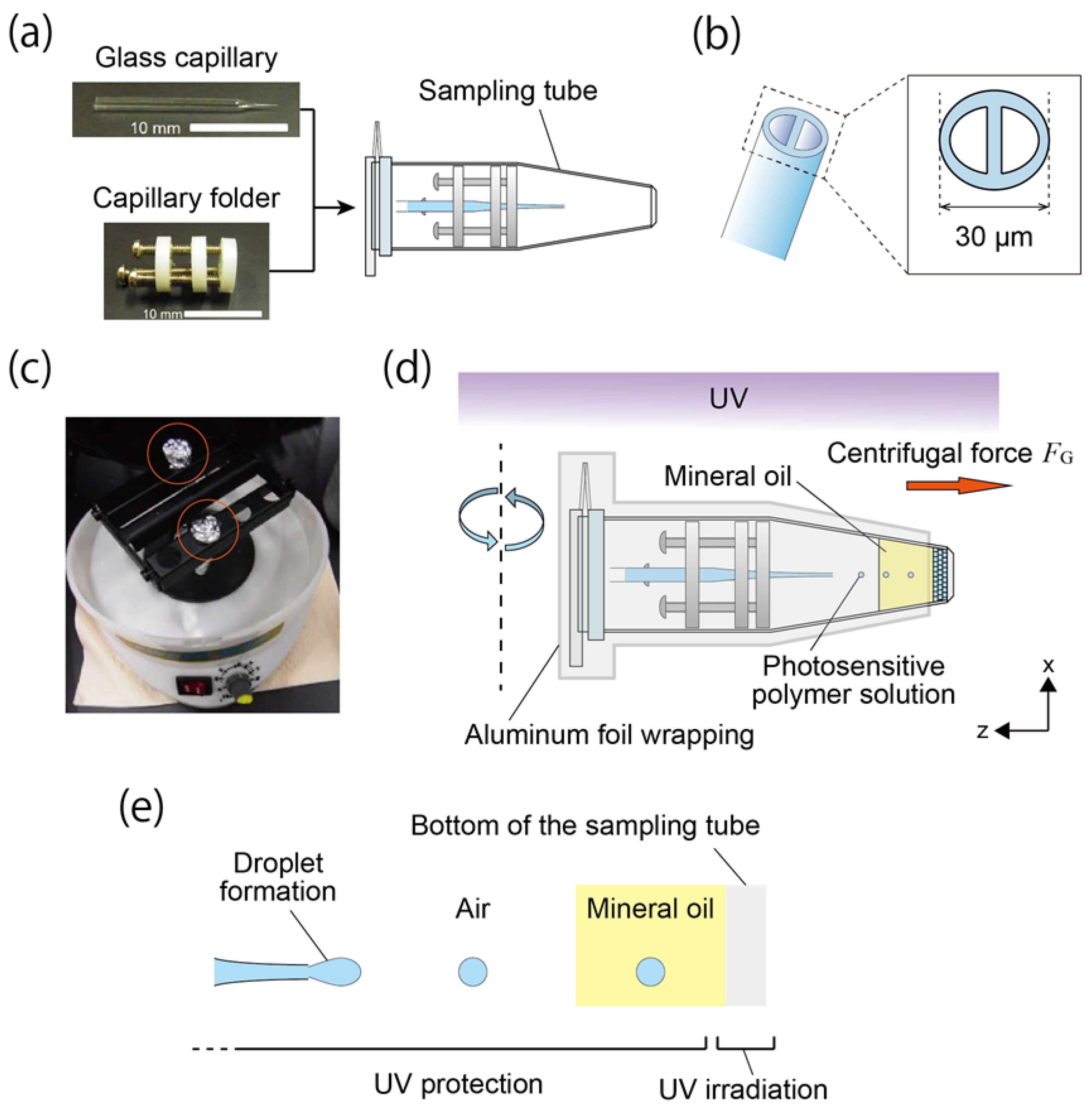
2.1. Materials and Experimental Setup
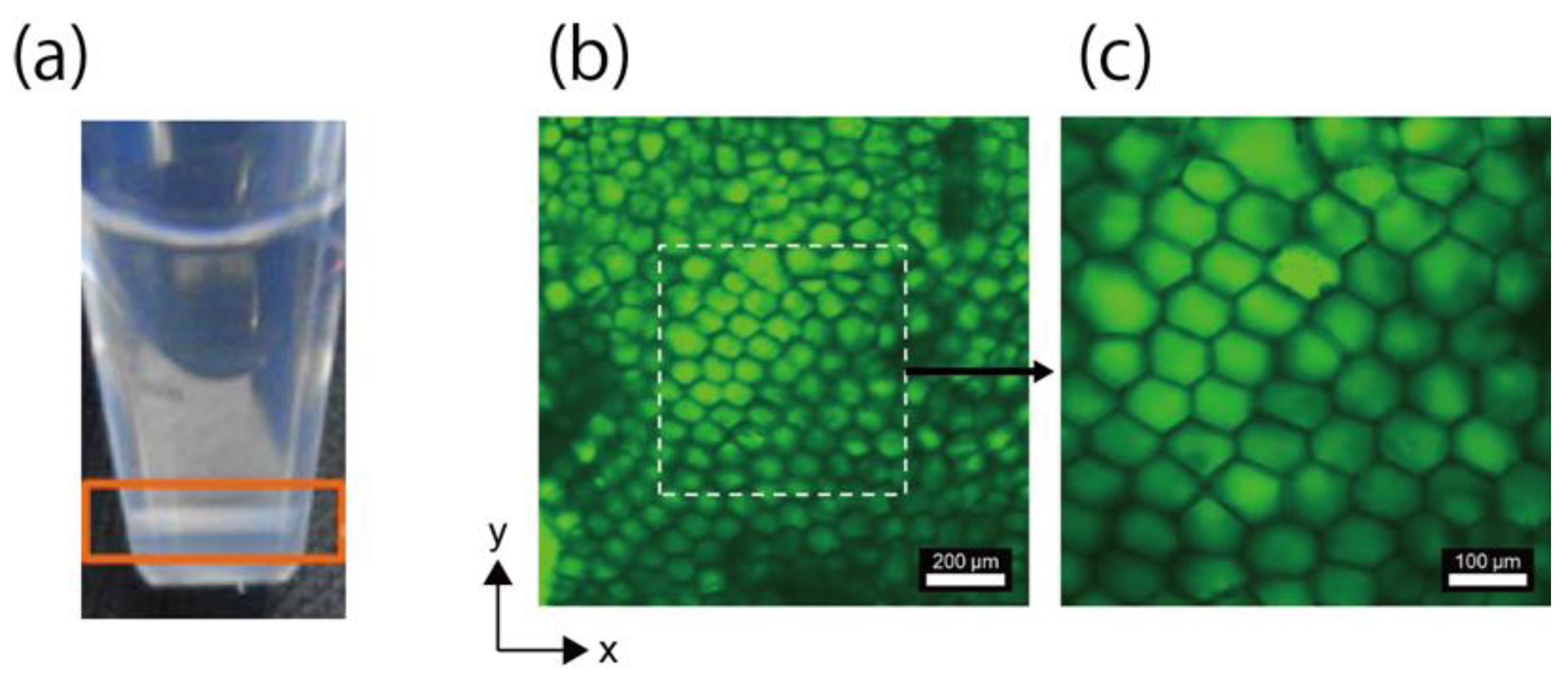
2.2. Fabrication and Analysis of Honeycomb Hydrogel Network
2.3. Experiment and Analysis of Fluorescence Recovery after Photobleaching (FRAP)
3. Results and Discussion
3.1. Fabrication of Network Structure
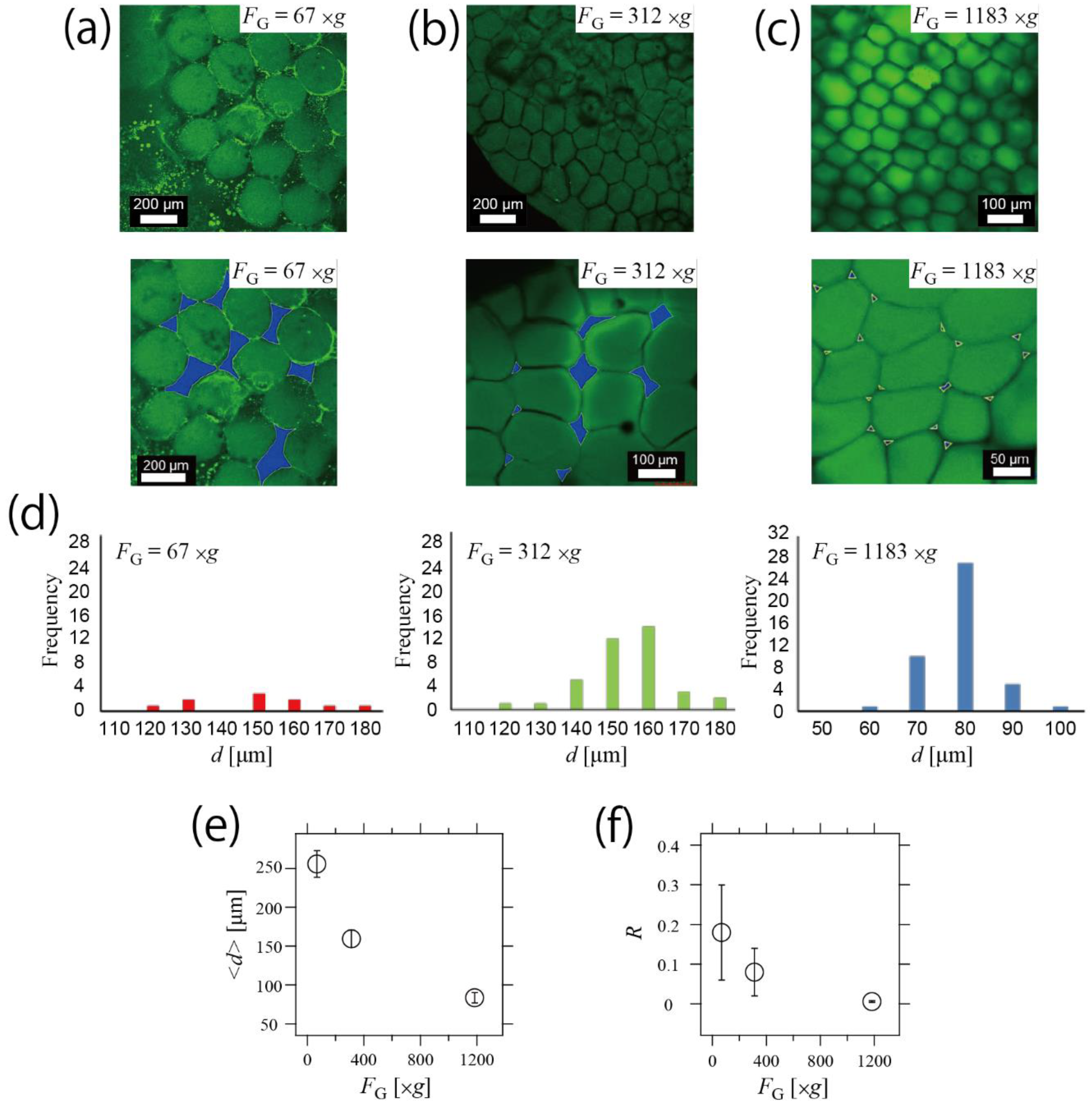
3.2. Control of Structure in Hydrogel Network by Changing Centrifugal Force
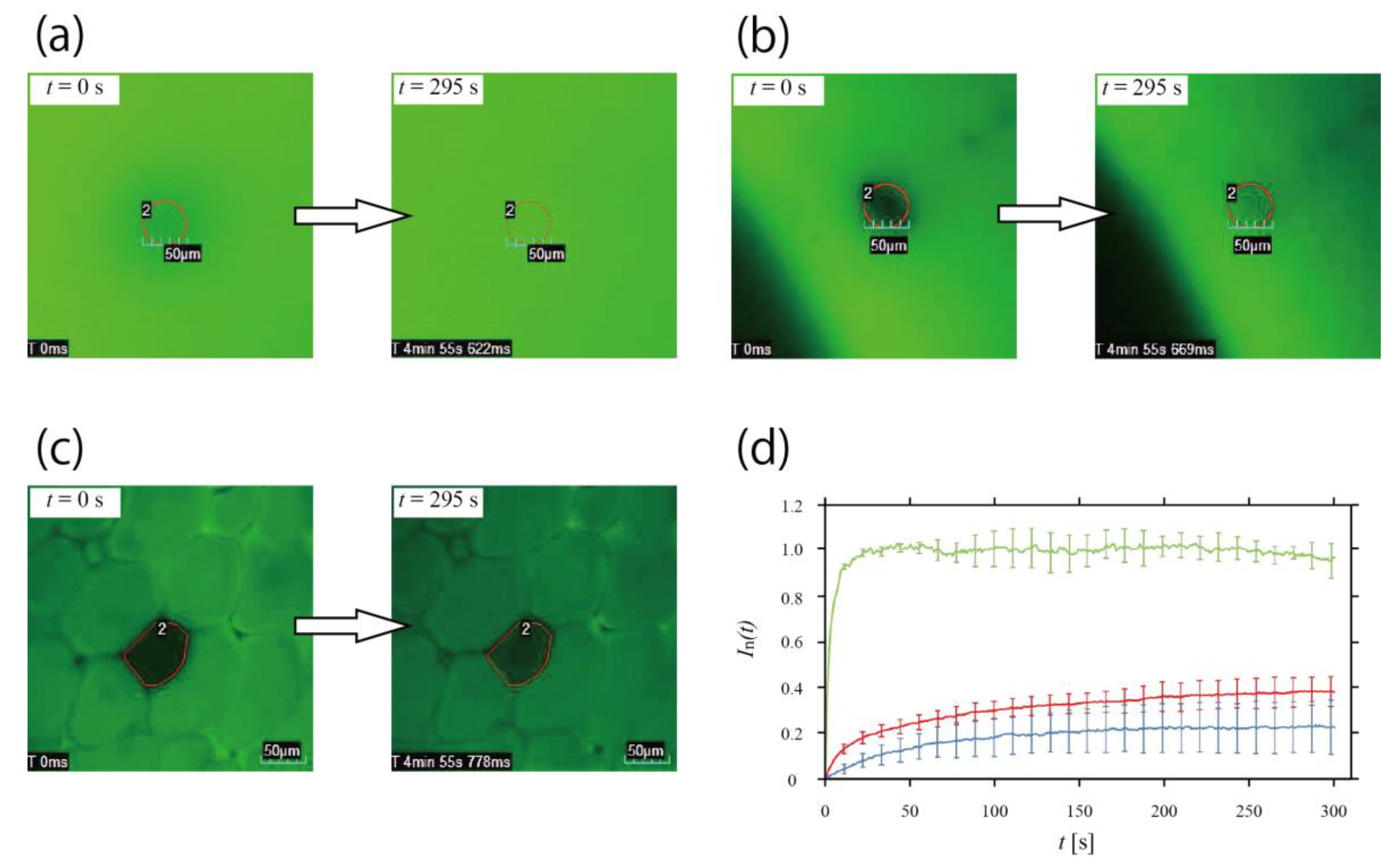
3.3. Inhibition of Molecular Diffusion in Honeycomb Hydrogel Network
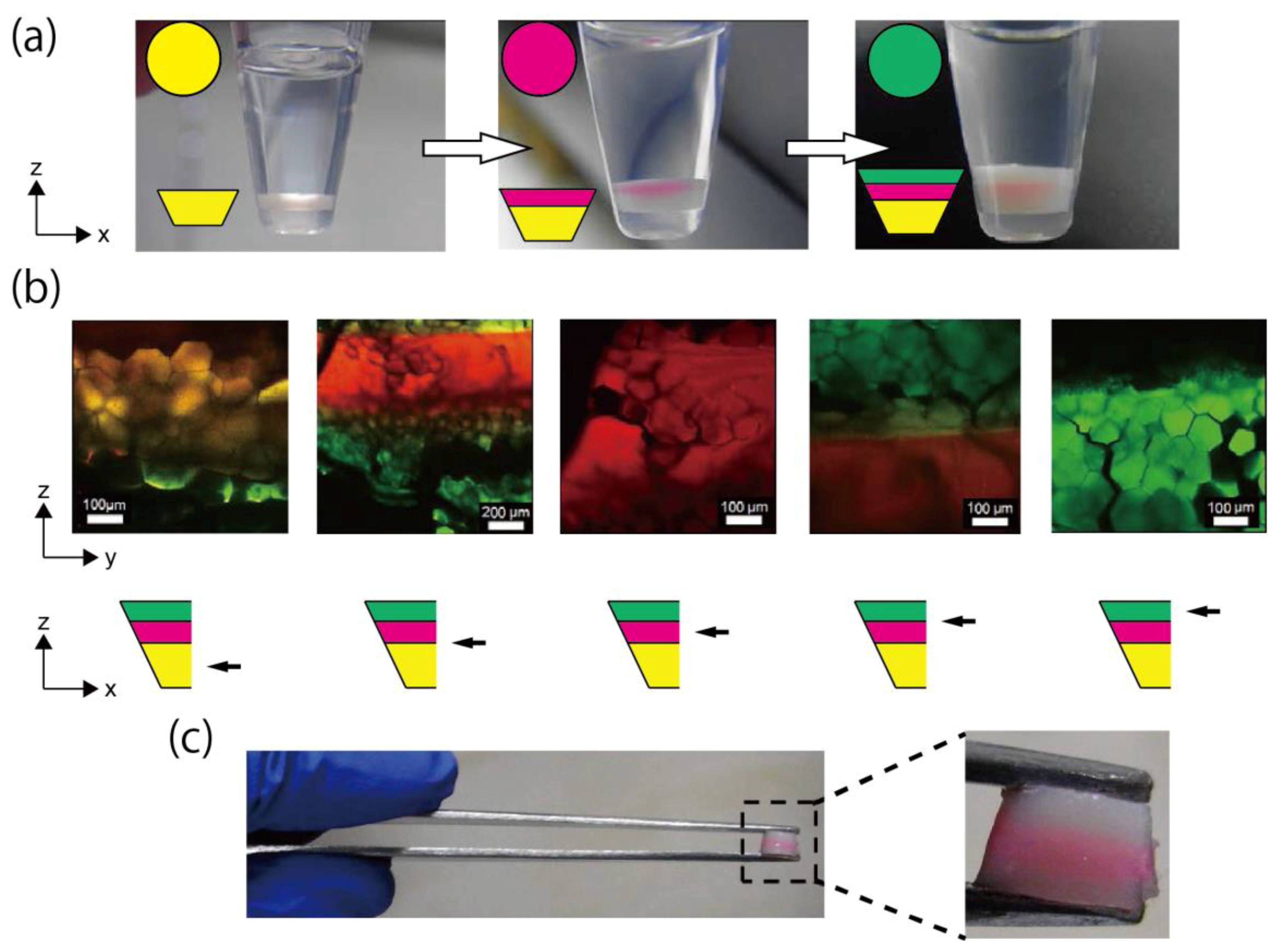
3.4. Fabrication of Triple-Layered Honeycomb Hydrogel Network
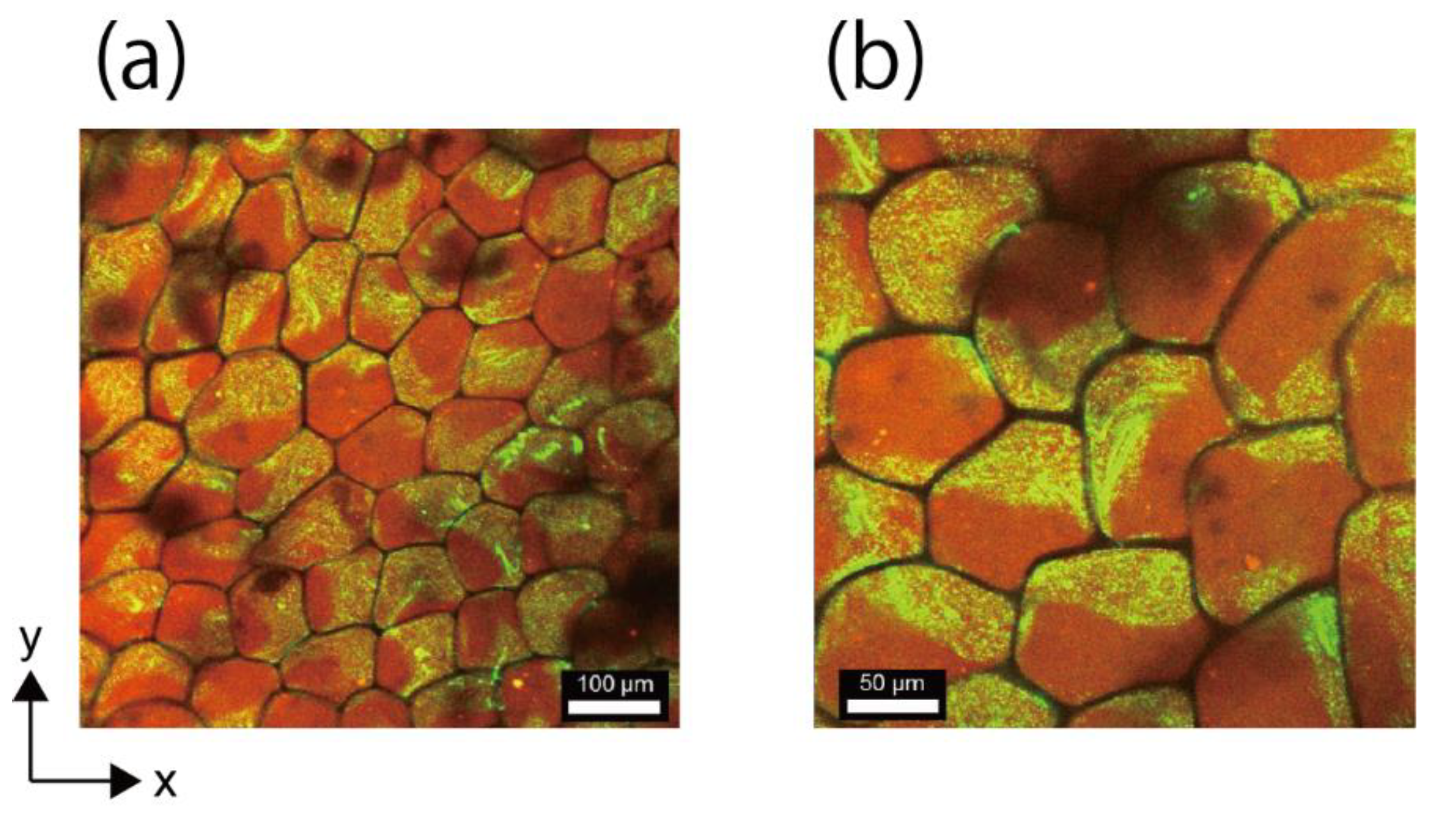
3.5. Fabrication of Network Structure Composed of Double-Faced Honeycomb Hydrogels
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef]
- Hervé, J.C.; Derangeon, M. Gap-junction-mediated cell-to-cell communication. Cell Tissue Res. 2013, 352, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Asai, R. A reaction–diffusion wave on the skin of the marine angelfish Pomacanthus. Nature 1995, 376, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Miura, T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 2010, 329, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Toiya, M.; González-Ochoa, H.O.; Vanag, V.K.; Fraden, S.; Epstein, I.R. Synchronization of chemical micro-oscillators. J. Phys. Chem. Lett. 2010, 1, 1241–1246. [Google Scholar] [CrossRef]
- Tompkins, N.; Li, N.; Girabawe, C.; Heymann, M.; Ermentrout, G.B.; Epstein, I.R.; Fraden, S. Testing Turing’s theory of morphogenesis in chemical cells. Proc. Natl. Acad. Sci. USA 2014, 111, 4397–4402. [Google Scholar] [CrossRef] [PubMed]
- Lentini, R.; Martín, N.Y.; Forlin, M.; Belmonte, L.; Fontana, J.; Cornella, M.; Martini, L.; Tamburini, S.; Bentley, W.E.; Jousson, O.; et al. Two-Way Chemical Communication between Artificial and Natural Cells. ACS Cent. Sci. 2017, 3, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Schilling, M.; Aufinger, L.; Mückla, A.; Simmel, F.C. Chemical communication between bacteria and cell-free gene expression systems within linear chains of emulsion droplets. Integr. Biol. 2016, 8, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Thutupalli, S.; Herminghaus, S.; Seemann, R. Bilayer membranes in micro-fluidics: From gel emulsions to soft functional devices. Soft Matter 2011, 7, 1312–1320. [Google Scholar] [CrossRef]
- Holden, M.A.; Needham, D.; Bayley, H. Functional bionetworks from nanoliter water droplets. J. Am. Chem. Soc. 2007, 129, 8650–8655. [Google Scholar] [CrossRef] [PubMed]
- Baxani, D.K.; Morgan, A.J.; Jamieson, W.D.; Allender, C.J.; Barrow, D.A.; Castell, O.K. Bilayer Networks within a Hydrogel Shell: A Robust Chassis for Artificial Cells and a Platform for Membrane Studies. Angew. Chem. Int. Ed. 2016, 55, 14240. [Google Scholar] [CrossRef] [PubMed]
- Villar, G.; Graham, A.D.; Bayley, H. A tissue-like printed material. Science 2013, 340, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Elani, Y.; Law, R.V.; Ces, O. Vesicle-based artificial cells as chemical microreactors with spatially segregated reaction pathways. Nat. Commun. 2014, 5, 5305. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, G.; Friddin, M.S.; Salehi-Reyhani, A.; Barlow, N.E.; Brooks, N.J.; Ces, O.; Elani, Y. Sculpting and fusing biomimetic vesicle networks using optical tweezers. Nat. Commun. 2018, 9, 1882. [Google Scholar] [CrossRef] [PubMed]
- Challita, E.J.; Najem, J.S.; Monroe, R.; Leo, D.J.; Freeman, E.C. Encapsulating Networks of Droplet Interface Bilayers in a Thermoreversible Organogel. Sci. Rep. 2018, 8, 6494. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, M.; Bayley, H.; Maglia, G.; Sapra, K.T. Multi-compartment encapsulation of communicating droplets and droplet networks in hydrogel as a model for artificial cells. Sci. Rep. 2017, 7, 45167. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Onoe, H.; Takinoue, M.; Takeuchi, S. Controlled Synthesis of 3D Multi-Compartmental Particles with Centrifuge-Based Microdroplet Formation from a Multi-Barrelled Capillary. Adv. Mater. 2012, 24, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Onoe, H.; Nagai, K.H.; Takinoue, M. Complex-shaped three-dimensional multi-compartmental microparticles generated by diffusional and Marangoni microflows in centrifugally discharged droplets. Sci. Rep. 2016, 6, 20793. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Nomura, F.; Honda, M.; Takeda, S.; Inaba, T.; Takiguchi, K.; Itoh, T.J.; Ishijima, A.; Umeda, T.; Hotani, H. Morphological and topological transformation of membrane vesicles. J. Biol. Phys. 2002, 28, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Imai, M.; Taniguchi, T. Shape deformation of ternary vesicles coupled with phase separation. Phys. Rev. Lett. 2008, 100, 148102. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayakawa, M.; Umeyama, S.; Nagai, K.H.; Onoe, H.; Takinoue, M. Controlled Construction of Stable Network Structure Composed of Honeycomb-Shaped Microhydrogels. Life 2018, 8, 38. https://doi.org/10.3390/life8040038
Hayakawa M, Umeyama S, Nagai KH, Onoe H, Takinoue M. Controlled Construction of Stable Network Structure Composed of Honeycomb-Shaped Microhydrogels. Life. 2018; 8(4):38. https://doi.org/10.3390/life8040038
Chicago/Turabian StyleHayakawa, Masayuki, Satoshi Umeyama, Ken H. Nagai, Hiroaki Onoe, and Masahiro Takinoue. 2018. "Controlled Construction of Stable Network Structure Composed of Honeycomb-Shaped Microhydrogels" Life 8, no. 4: 38. https://doi.org/10.3390/life8040038
APA StyleHayakawa, M., Umeyama, S., Nagai, K. H., Onoe, H., & Takinoue, M. (2018). Controlled Construction of Stable Network Structure Composed of Honeycomb-Shaped Microhydrogels. Life, 8(4), 38. https://doi.org/10.3390/life8040038