Applications of Diquafosol Sodium in Ophthalmology: A Comprehensive Review of Therapeutic Utility
Abstract
1. Introduction
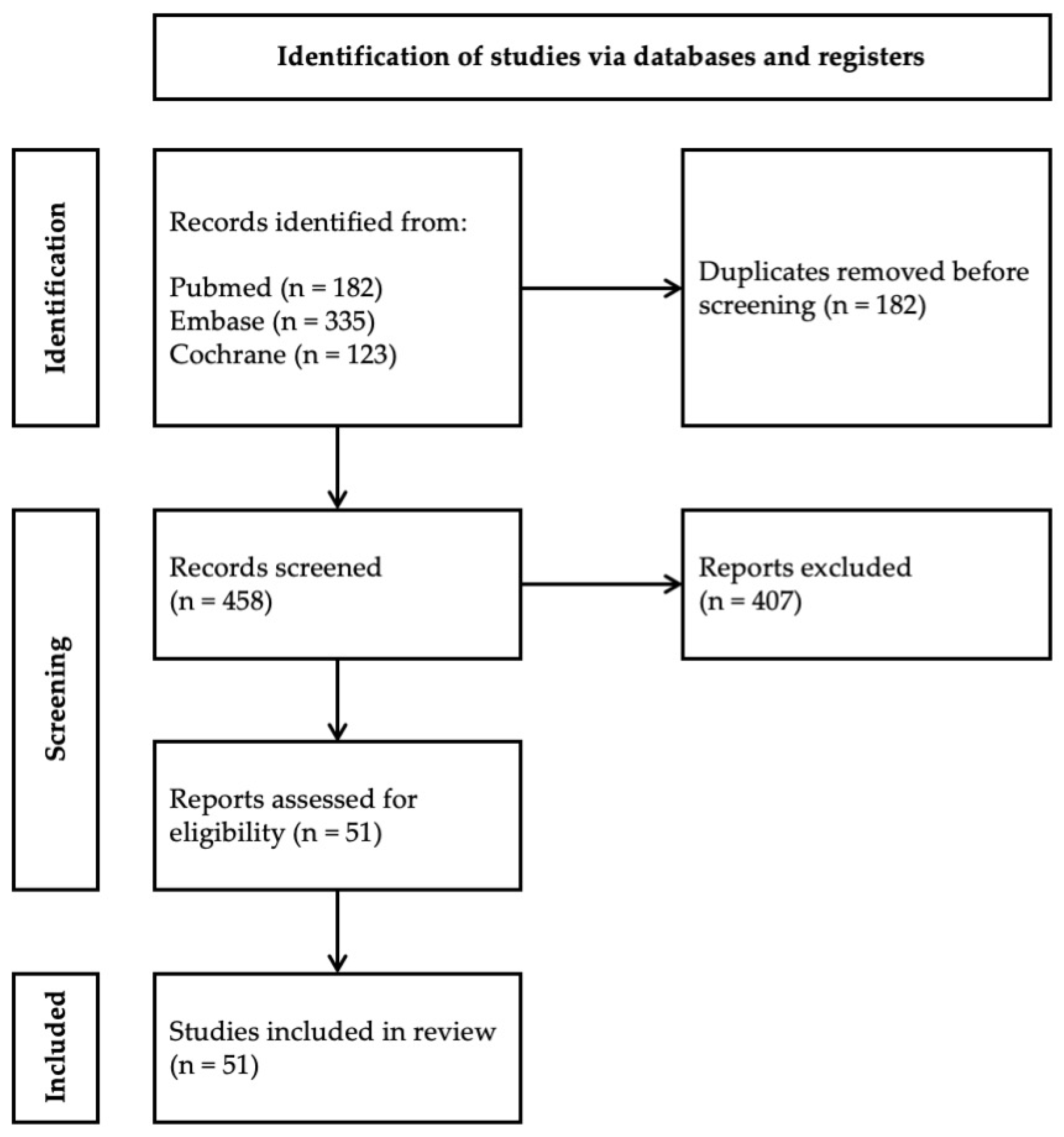
2. Methods
3. Pharmacologic Properties
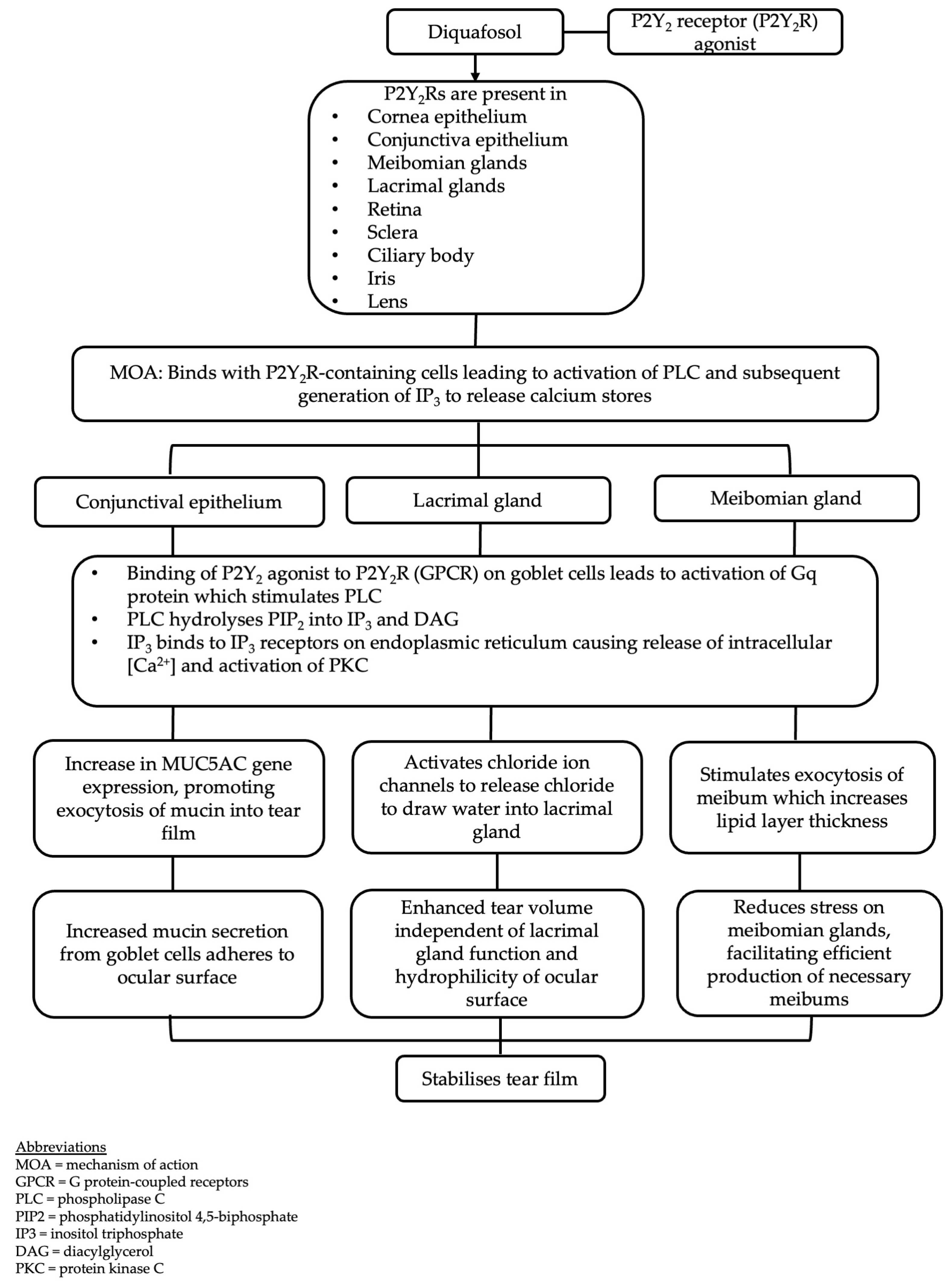
3.1. Mechanism of Action
3.2. Pharmacokinetics
3.3. Commercially Available Formulations
3.4. Adverse Effects
3.5. Effects on Tear Stimulation
3.6. Effects on Lipid Secretion
3.7. Effects on Mucin Secretion
4. Therapeutic Efficacy
4.1. Dry Eye Disease
4.2. Meibomian Gland Dysfunction
4.3. Aqueous-Deficient Dry Eye Disease
4.4. Ocular Graft-Versus-Host Disease (oGVHD)
4.5. Glaucoma Medication- and Preservative-Related Ocular Surface Disease
4.6. Cataract Surgery
4.7. Contact Lens Wear
4.8. Keratorefractive Surgery
4.9. Long-Acting Diquafosol (DQS-LX) Formulation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, D.; Tong, L.; Prasath, A.; Lim, B.X.H.; Lim, D.K.; Lim, C.H.L. Novel therapeutics for dry eye disease. Ann. Med. 2023, 55, 1211–1212. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wei, J.; Zhou, J.; Zou, W. Prevalence and Incidence of Dry Eye Disease in Asia: A Systematic Review and Meta-Analysis. Ophthalmic Res. 2022, 65, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xia, W.; Wang, M.; Chang, X.; Wang, J.; Jin, S.; Wang, J.; Wei, W.; Rudan, I. Variations of dry eye disease prevalence by age, sex and geographic characteristics in China: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 020503. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef]
- Buchholz, P.; Steeds, C.S.; Stern, L.S.; Wiederkehr, D.P.; Doyle, J.J.; Katz, L.M.; Figueiredo, F.C. Utility assessment to measure the impact of dry eye disease. Ocul. Surf. 2006, 4, 155–161. [Google Scholar] [CrossRef]
- Yang, W.; Luo, Y.; Wu, S.; Niu, X.; Yan, Y.; Qiao, C.; Ming, W.; Zhang, Y.; Wang, H.; Chen, D.; et al. Estimated Annual Economic Burden of Dry Eye Disease Based on a Multi-Center Analysis in China: A Retrospective Study. Front. Med. 2021, 8, 771352. [Google Scholar] [CrossRef]
- Yu, J.; Asche, C.V.; Fairchild, C.J. The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 2011, 30, 379–387. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. A New Perspective on Dry Eye Classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 2020, 46 (Suppl. S1), S2–S13. [Google Scholar] [CrossRef]
- Yokoi, N.; Georgiev, G.A. Tear Film-Oriented Diagnosis and Tear Film-Oriented Therapy for Dry Eye Based on Tear Film Dynamics. Investig. Ophthalmol. Vis. Sci. 2018, 59, Des13–Des22. [Google Scholar] [CrossRef]
- Barbosa Ribeiro, B.; Marta, A.; Ponces Ramalhão, J.; Marques, J.H.; Barbosa, I. Pulsed Light Therapy in the Management of Dry Eye Disease: Current Perspectives. Clin. Ophthalmol. 2022, 16, 3883–3893. [Google Scholar] [CrossRef] [PubMed]
- Blades, K.J.; Patel, S.; Aidoo, K.E. Oral antioxidant therapy for marginal dry eye. Eur. J. Clin. Nutr. 2001, 55, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.E.; Lau, B.S.R.; Lim, B.X.H.; Du, R.; Giannaccare, G.; Tong, L.; Stapleton, F.; Lim, C.H.L. Low-level light therapy and intense pulse light therapy in meibomian gland dysfunction. A systematic review and meta-analysis. Contact Lens Anterior Eye 2024, 48, 102344. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Imanaka, T.; Sakamoto, A. Diquafosol ophthalmic solution for dry eye treatment. Adv. Ther. 2012, 29, 579–589. [Google Scholar] [CrossRef]
- Kojima, T.; Nagata, T.; Kudo, H.; Müller-Lierheim, W.G.K.; van Setten, G.B.; Dogru, M.; Tsubota, K. The Effects of High Molecular Weight Hyaluronic Acid Eye Drop Application in Environmental Dry Eye Stress Model Mice. Int. J. Mol. Sci. 2020, 21, 3516. [Google Scholar] [CrossRef]
- Sun, X.; Liu, L.; Liu, C. Topical diquafosol versus hyaluronic acid for the treatment of dry eye disease: A meta-analysis of randomized controlled trials. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3355–3367. [Google Scholar] [CrossRef]
- Cowlen, M.S.; Zhang, V.Z.; Warnock, L.; Moyer, C.F.; Peterson, W.M.; Yerxa, B.R. Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp. Eye Res. 2003, 77, 77–84. [Google Scholar] [CrossRef]
- Murakami, T.; Fujihara, T.; Horibe, Y.; Nakamura, M. Diquafosol elicits increases in net Cl- transport through P2Y2 receptor stimulation in rabbit conjunctiva. Ophthalmic Res. 2004, 36, 89–93. [Google Scholar] [CrossRef]
- Lee, H.J.; Yang, S.; Cheon, E.J.; Shin, S.; Byun, Y.S.; Kim, H.S.; Chung, S.H. Diquafosol ophthalmic solution enhances mucin expression via ERK activation in human conjunctival epithelial cells with hyperosmotic stress. Mol. Vis. 2022, 28, 114–123. [Google Scholar]
- Report on the Deliberation Results. 2010. Available online: https://www.pmda.go.jp/files/000153954.pdf (accessed on 9 February 2025).
- Jun, I.; Choi, S.; Lee, G.Y.; Choi, Y.J.; Lee, H.K.; Kim, E.K.; Seo, K.Y.; Kim, T.I. Effects of Preservative-free 3% Diquafosol in Patients with Pre-existing Dry Eye Disease after Cataract Surgery: A Randomized Clinical Trial. Sci. Rep. 2019, 9, 12659. [Google Scholar] [CrossRef]
- Inspire Announces Results of Phase 3 PROLACRIA Trial for Dry Eye. Fierce Biotech. Available online: https://www.fiercebiotech.com/biotech/inspire-announces-results-of-phase-3-prolacria-trial-for-dry-eye (accessed on 12 February 2025).
- Matsumoto, Y.; Ohashi, Y.; Watanabe, H.; Tsubota, K. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: A Japanese phase 2 clinical trial. Ophthalmology 2012, 119, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Takamura, E.; Tsubota, K.; Watanabe, H.; Ohashi, Y. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br. J. Ophthalmol. 2012, 96, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Tauber, J.; Davitt, W.F.; Bokosky, J.E.; Nichols, K.K.; Yerxa, B.R.; Schaberg, A.E.; LaVange, L.M.; Mills-Wilson, M.C.; Kellerman, D.J. Double-masked, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea 2004, 23, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Sun, X.; Ma, Z.; Wang, Q.; Xu, X.; Chen, X.; Shao, Y.; Yao, K.; Tang, L.; Gu, Y.; et al. A randomised, parallel-group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and Singapore. Br. J. Ophthalmol. 2015, 99, 903–908. [Google Scholar] [CrossRef]
- Kamiya, K.; Nakanishi, M.; Ishii, R.; Kobashi, H.; Igarashi, A.; Sato, N.; Shimizu, K. Clinical evaluation of the additive effect of diquafosol tetrasodium on sodium hyaluronate monotherapy in patients with dry eye syndrome: A prospective, randomized, multicenter study. Eye 2012, 26, 1363–1368. [Google Scholar] [CrossRef]
- Hwang, H.S.; Sung, Y.M.; Lee, W.S.; Kim, E.C. Additive Effect of preservative-free sodium hyaluronate 0.1% in treatment of dry eye syndrome with diquafosol 3% eye drops. Cornea 2014, 33, 935–941. [Google Scholar] [CrossRef]
- Shimazaki-Den, S.; Iseda, H.; Dogru, M.; Shimazaki, J. Effects of diquafosol sodium eye drops on tear film stability in short BUT type of dry eye. Cornea 2013, 32, 1120–1125. [Google Scholar] [CrossRef]
- Ohashi, Y.; Munesue, M.; Shimazaki, J.; Takamura, E.; Yokoi, N.; Watanabe, H.; Nomura, A.; Shimada, F. Long-Term Safety and Effectiveness of Diquafosol for the Treatment of Dry Eye in a Real-World Setting: A Prospective Observational Study. Adv. Ther. 2020, 37, 707–717. [Google Scholar] [CrossRef]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef]
- Peterson, T.S.; Camden, J.M.; Wang, Y.; Seye, C.I.; Wood, W.G.; Sun, G.Y.; Erb, L.; Petris, M.J.; Weisman, G.A. P2Y2 nucleotide receptor-mediated responses in brain cells. Mol. Neurobiol. 2010, 41, 356–366. [Google Scholar] [CrossRef]
- Kargarpour, Z.; Cicko, S.; Köhler, T.C.; Zech, A.; Stoshikj, S.; Bal, C.; Renner, A.; Idzko, M.; El-Gazzar, A. Blocking P2Y2 purinergic receptor prevents the development of lipopolysaccharide-induced acute respiratory distress syndrome. Front. Immunol. 2023, 14, 1310098. [Google Scholar] [CrossRef] [PubMed]
- Bellefeuille, S.D.; Molle, C.M.; Gendron, F.P. Reviewing the role of P2Y receptors in specific gastrointestinal cancers. Purinergic Signal. 2019, 15, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bremond-Gignac, D.; Gicquel, J.J.; Chiambaretta, F. Pharmacokinetic evaluation of diquafosol tetrasodium for the treatment of Sjögren’s syndrome. Expert Opin. Drug Metab. Toxicol. 2014, 10, 905–913. [Google Scholar] [CrossRef]
- Dota, A.; Sakamoto, A.; Nagano, T.; Murakami, T.; Matsugi, T. Effect of Diquafosol Ophthalmic Solution on Airflow-Induced Ocular Surface Disorder in Diabetic Rats. Clin. Ophthalmol. 2020, 14, 1019–1024. [Google Scholar] [CrossRef]
- Byun, Y.S.; Yoo, Y.S.; Kwon, J.Y.; Joo, J.S.; Lim, S.A.; Whang, W.J.; Mok, J.W.; Choi, J.S.; Joo, C.K. Diquafosol promotes corneal epithelial healing via intracellular calcium-mediated ERK activation. Exp. Eye Res. 2016, 143, 89–97. [Google Scholar] [CrossRef]
- Yokoi, N.; Kato, H.; Kinoshita, S. Facilitation of tear fluid secretion by 3% diquafosol ophthalmic solution in normal human eyes. Am. J. Ophthalmol. 2014, 157, 85–92.e1. [Google Scholar] [CrossRef]
- Oguz, H.; Yokoi, N.; Kinoshita, S. The height and radius of the tear meniscus and methods for examining these parameters. Cornea 2000, 19, 497–500. [Google Scholar] [CrossRef]
- Yokoi, N.; Bron, A.J.; Tiffany, J.M.; Kinoshita, S. Reflective meniscometry: A new field of dry eye assessment. Cornea 2000, 19, S37–S43. [Google Scholar] [CrossRef]
- Yokoi, N.; Kato, H.; Kinoshita, S. The increase of aqueous tear volume by diquafosol sodium in dry-eye patients with Sjögren’s syndrome: A pilot study. Eye 2016, 30, 857–864. [Google Scholar] [CrossRef]
- Ikeda, K.; Simsek, C.; Kojima, T.; Higa, K.; Kawashima, M.; Dogru, M.; Shimizu, T.; Tsubota, K.; Shimazaki, J. The effects of 3% diquafosol sodium eye drop application on meibomian gland and ocular surface alterations in the Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.I.; Sakamoto, A.; Fujisawa, K. Diquafosol tetrasodium elicits total cholesterol release from rabbit meibomian gland cells via P2Y(2) purinergic receptor signalling. Sci. Rep. 2021, 11, 6989. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Arita, R. Increase in tear film lipid layer thickness after instillation of 3% diquafosol ophthalmic solution in healthy human eyes. Ocul. Surf. 2017, 15, 730–735. [Google Scholar] [CrossRef]
- Fukuoka, S.; Arita, R. Tear film lipid layer increase after diquafosol instillation in dry eye patients with meibomian gland dysfunction: A randomized clinical study. Sci. Rep. 2019, 9, 9091. [Google Scholar] [CrossRef]
- Inatomi, T.; Spurr-Michaud, S.; Tisdale, A.S.; Zhan, Q.; Feldman, S.T.; Gipson, I.K. Expression of secretory mucin genes by human conjunctival epithelia. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1684–1692. [Google Scholar]
- Gipson, I.K. Distribution of mucins at the ocular surface. Exp. Eye Res. 2004, 78, 379–388. [Google Scholar] [CrossRef]
- Mantelli, F.; Argüeso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef]
- Jin, Y.; Seo, K.Y.; Kim, S.W. Comparing two mucin secretagogues for the treatment of dry eye disease: A prospective randomized crossover trial. Sci. Rep. 2024, 14, 13306. [Google Scholar] [CrossRef]
- Hori, Y.; Kageyama, T.; Sakamoto, A.; Shiba, T.; Nakamura, M.; Maeno, T. Comparison of Short-Term Effects of Diquafosol and Rebamipide on Mucin 5AC Level on the Rabbit Ocular Surface. J. Ocul. Pharmacol. Ther. 2017, 33, 493–497. [Google Scholar] [CrossRef]
- Terakado, K.; Yogo, T.; Kohara, Y.; Soeta, S.; Nezu, Y.; Harada, Y.; Hara, Y.; Amasaki, H.; Tagawa, M. Conjunctival expression of the P2Y2 receptor and the effects of 3% diquafosol ophthalmic solution in dogs. Vet. J. 2014, 202, 48–52. [Google Scholar] [CrossRef]
- Liu, S.; Yang, G.; Li, Q.; Tang, S. Safety and efficacy of topical diquafosol for the treatment of dry eye disease: An updated meta-analysis of randomized controlled trials. Indian J. Ophthalmol. 2023, 71, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.T.; Ahn, S.M.; Eom, Y.; Kim, H.M.; Song, J.S. Immediate Effects of 3% Diquafosol and 0.1% Hyaluronic Acid Ophthalmic Solution on Tear Break-Up Time in Normal Human Eyes. J. Ocul. Pharmacol. Ther. 2015, 31, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.T.; Kim, M.; Song, J.S.; Kim, T.I.; Chung, T.Y.; Choi, C.Y.; Kim, H.S.; An, W.J.; Jeong, S.J.; Lee, H.S.; et al. Proteomic analysis of tears in dry eye disease: A prospective, double-blind multicenter study. Ocul. Surf. 2023, 29, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Song, J.S.; Kim, H.M. Effectiveness of Topical Cyclosporin A 0.1%, Diquafosol Tetrasodium 3%, and Their Combination, in Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2022, 38, 682–694. [Google Scholar] [CrossRef]
- Arita, R.; Suehiro, J.; Haraguchi, T.; Maeda, S.; Maeda, K.; Tokoro, H.; Amano, S. Topical diquafosol for patients with obstructive meibomian gland dysfunction. Br. J. Ophthalmol. 2013, 97, 725–729. [Google Scholar] [CrossRef]
- Kang, D.H.; Lee, Y.W.; Hwang, K.Y.; Koh, K.M.; Kwon, Y.A.; Kim, B.Y.; Song, S.W.; Kim, K.Y. Changes of tear film lipid layer thickness by 3% diquafosol ophthalmic solutions in patients with dry eye syndrome. Int. J. Ophthalmol. 2019, 12, 1555–1560. [Google Scholar] [CrossRef]
- Koh, S.; Ikeda, C.; Takai, Y.; Watanabe, H.; Maeda, N.; Nishida, K. Long-term results of treatment with diquafosol ophthalmic solution for aqueous-deficient dry eye. Jpn. J. Ophthalmol. 2013, 57, 440–446. [Google Scholar] [CrossRef]
- Koh, S.; Maeda, N.; Ikeda, C.; Oie, Y.; Soma, T.; Tsujikawa, M.; Watanabe, H.; Nishida, K. Effect of diquafosol ophthalmic solution on the optical quality of the eyes in patients with aqueous-deficient dry eye. Acta Ophthalmol. 2014, 92, e671–e675. [Google Scholar] [CrossRef]
- Yokoi, N.; Sonomura, Y.; Kato, H.; Komuro, A.; Kinoshita, S. Three percent diquafosol ophthalmic solution as an additional therapy to existing artificial tears with steroids for dry-eye patients with Sjögren’s syndrome. Eye 2015, 29, 1204–1212. [Google Scholar] [CrossRef]
- Yamane, M.; Ogawa, Y.; Fukui, M.; Kamoi, M.; Uchino, M.; Saijo-Ban, Y.; Kozuki, N.; Mukai, S.; Mori, T.; Okamoto, S.; et al. Long-Term Topical Diquafosol Tetrasodium Treatment of Dry Eye Disease Caused by Chronic Graft-Versus-Host Disease: A Retrospective Study. Eye Contact Lens 2018, 44 (Suppl. S2), S215–S220. [Google Scholar] [CrossRef]
- Yamane, M.; Ogawa, Y.; Fukui, M.; Kamoi, M.; Saijo-Ban, Y.; Yaguchi, S.; Mukai, S.; Kawakita, T.; Simmura, S.; Tsubota, K. Long-term rebamipide and diquafosol in two cases of immune-mediated dry eye. Optom. Vis. Sci. 2015, 92, S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.W.; Min, J.S. Clinical evaluation of the effect of diquafosol ophthalmic solution in glaucoma patients with dry eye syndrome. Jpn. J. Ophthalmol. 2016, 60, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ha, J.Y.; Piao, H.L.; Sung, M.S.; Park, S.W. The protective effect of 3% diquafosol on meibomian gland morphology in glaucoma patients treated with prostaglandin analogs: A 12-month follow-up study. BMC Ophthalmol. 2020, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cheng, W.; Liu, C.; Jin, X.; Ming, S.; Zhao, D.; Feng, X. Evaluation of effects of 3% diquafosol ophthalmic solution on preocular tear film stability after trabeculectomy. Int. Ophthalmol. 2023, 43, 1903–1910. [Google Scholar] [CrossRef]
- Park, D.H.; Chung, J.K.; Seo, D.R.; Lee, S.J. Clinical Effects and Safety of 3% Diquafosol Ophthalmic Solution for Patients with Dry Eye After Cataract Surgery: A Randomized Controlled Trial. Am. J. Ophthalmol. 2016, 163, 122–131.e2. [Google Scholar] [CrossRef]
- Teshigawara, T.; Akaishi, M.; Mizuki, Y.; Takeuchi, M.; Hata, S.; Meguro, A.; Mizuki, N. Effect of Long-Acting Diquafosol Sodium on Astigmatism Measurement Repeatability in Preoperative Cataract Cases with Dry Eyes: A Multicenter Prospective Study. Ophthalmol. Ther. 2024, 13, 1743–1755. [Google Scholar] [CrossRef]
- Kobashi, H.; Kamiya, K.; Igarashi, A.; Miyake, T.; Shimizu, K. Intraocular Scattering after Instillation of Diquafosol Ophthalmic Solution. Optom. Vis. Sci. 2015, 92, e303–e309. [Google Scholar] [CrossRef]
- Shigeyasu, C.; Yamada, M.; Akune, Y.; Fukui, M. Diquafosol for Soft Contact Lens Dryness: Clinical Evaluation and Tear Analysis. Optom. Vis. Sci. 2016, 93, 973–978. [Google Scholar] [CrossRef]
- Ogami, T.; Asano, H.; Hiraoka, T.; Yamada, Y.; Oshika, T. The Effect of Diquafosol Ophthalmic Solution on Clinical Parameters and Visual Function in Soft Contact Lens-Related Dry Eye. Adv. Ther. 2021, 38, 5534–5547. [Google Scholar] [CrossRef]
- Xie, C.; Wei, R. Long-term changes in the ocular surface during orthokeratology lens wear and their correlations with ocular discomfort symptoms. Contact Lens Anterior Eye 2023, 46, 101757. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Tang, Y.; Wu, H.; Luo, Z.; Gao, W.; Hu, Z.; Hou, L.; Wang, M.; Yang, Z.; et al. Short-term application of diquafosol ophthalmic solution benefits children with dry eye wearing orthokeratology lens. Front. Med. 2023, 10, 1130117. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, Y.; Li, M.; Shi, Y.; Liu, L.; Sun, L.; Ye, H.; Zou, J. Combination Therapy with Diquafosol Sodium and Sodium Hyaluronate in Eyes with Dry Eye Disease After Small Incision Lenticule Extraction. In Vivo 2023, 37, 2829–2834. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nejima, R.; Masuda, A.; Maruyama, Y.; Minami, K.; Miyata, K.; Amano, S. Effect of diquafosol tetrasodium eye drop for persistent dry eye after laser in situ keratomileusis. Cornea 2014, 33, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Di, Y.; Li, Y. Combination therapy with 3% diquafosol tetrasodium ophthalmic solution and sodium hyaluronate: An effective therapy for patients with dry eye after femtosecond laser-assisted in situ keratomileusis. Front. Med. 2023, 10, 1160499. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ibrahim, O.M.A.; Kojima, T.; Dogru, M.; Shimazaki, J.; Tsubota, K. Corneal In Vivo Laser-Scanning Confocal Microscopy Findings in Dry Eye Patients with Sjögren’s Syndrome. Diagnostics 2020, 10, 497. [Google Scholar] [CrossRef]
- Hori, Y.; Oka, K.; Inai, M. Efficacy and Safety of the Long-Acting Diquafosol Ophthalmic Solution DE-089C in Patients with Dry Eye: A Randomized, Double-Masked, Placebo-Controlled Phase 3 Study. Adv. Ther. 2022, 39, 3654–3667. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sasaki, T.; Maruyama, T.; Murayama, K.; Shinoda, K. Effectiveness and Adherence of Dry Eye Patients Who Switched from Short- to Long-Acting Diquafosol Ophthalmic Solution. J. Clin. Med. 2023, 12, 4495. [Google Scholar] [CrossRef]
- Arita, R.; Fukuoka, S.; Kaido, M. Tolerability of Diquas LX on tear film and meibomian glands findings in a real clinical scenario. PLoS ONE 2024, 19, e0305020. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yang, I.J.; Nguyen, L.T.H.; Gum, S.I.; Yu, S.; Lee, G.J.; Kim, B.A.; Jung, J.C.; Park, Y.J. Effect of Diquafosol on Hyperosmotic Stress-induced Tumor Necrosis Factor-α and Interleukin-6 Expression in Human Corneal Epithelial Cells. Korean J. Ophthalmol. 2020, 34, 1–10. [Google Scholar] [CrossRef]
- Ozdemir, S.; Yeo, S.W.J.; Lee, J.J.; Bhaskar, A.; Finkelstein, E.; Tong, L. Patient Medication Preferences for Managing Dry Eye Disease: The Importance of Medication Side Effects. Patient 2022, 15, 679–690. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Nichols, K.K. Dry Eye Disease Associated with Meibomian Gland Dysfunction: Focus on Tear Film Characteristics and the Therapeutic Landscape. Ophthalmol. Ther. 2023, 12, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Donthineni, P.R.; Kammari, P.; Shanbhag, S.S.; Singh, V.; Das, A.V.; Basu, S. Incidence, demographics, types and risk factors of dry eye disease in India: Electronic medical records driven big data analytics report I. Ocul. Surf. 2019, 17, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Donthineni, P.R.; Doctor, M.B.; Shanbhag, S.; Kate, A.; Galor, A.; Djalilian, A.R.; Singh, S.; Basu, S. Aqueous-deficient dry eye disease: Preferred practice pattern guidelines on clinical approach, diagnosis, and management. Indian J. Ophthalmol. 2023, 71, 1332–1347. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Holler, E.; Sandmaier, B.M.; Huang, H.; Mohty, M. Acute graft-versus-host disease. Nat. Rev. Dis. Primers 2023, 9, 27. [Google Scholar] [CrossRef]
- Sakoda, Y.; Hashimoto, D.; Asakura, S.; Takeuchi, K.; Harada, M.; Tanimoto, M.; Teshima, T. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood 2007, 109, 1756–1764. [Google Scholar] [CrossRef]
- Surico, P.L.; Luo, Z.K. Understanding Ocular Graft-versus-Host Disease to Facilitate an Integrated Multidisciplinary Approach. Transplant. Cell. Ther. 2024, 30, S570–S584. [Google Scholar] [CrossRef]
- Yang, F.; Hayashi, I.; Sato, S.; Saijo-Ban, Y.; Yamane, M.; Fukui, M.; Shimizu, E.; He, J.; Shibata, S.; Mukai, S.; et al. Eyelid blood vessel and meibomian gland changes in a sclerodermatous chronic GVHD mouse model. Ocul. Surf. 2022, 26, 328–341. [Google Scholar] [CrossRef]
- Perez, V.L.; Mousa, H.M.; Soifer, M.; Beatty, C.; Sarantopoulos, S.; Saban, D.R.; Levy, R.B. Meibomian Gland Dysfunction: A Route of Ocular Graft-Versus-Host Disease Progression That Drives a Vicious Cycle of Ocular Surface Inflammatory Damage. Am. J. Ophthalmol. 2023, 247, 42–60. [Google Scholar] [CrossRef]
- Ogawa, Y.; Shimmura, S.; Kawakita, T.; Yoshida, S.; Kawakami, Y.; Tsubota, K. Epithelial mesenchymal transition in human ocular chronic graft-versus-host disease. Am. J. Pathol. 2009, 175, 2372–2381. [Google Scholar] [CrossRef]
- Shamloo, K.; Barbarino, A.; Alfuraih, S.; Sharma, A. Graft Versus Host Disease-Associated Dry Eye: Role of Ocular Surface Mucins and the Effect of Rebamipide, a Mucin Secretagogue. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4511–4519. [Google Scholar] [CrossRef]
- Kolko, M.; Gazzard, G.; Baudouin, C.; Beier, S.; Brignole-Baudouin, F.; Cvenkel, B.; Fineide, F.; Hedengran, A.; Hommer, A.; Jespersen, E.; et al. Impact of glaucoma medications on the ocular surface and how ocular surface disease can influence glaucoma treatment. Ocul. Surf. 2023, 29, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Vadoothker, S.; Munir, W.M.; Saeedi, O. Ocular Surface Disease and Glaucoma Medications: A Clinical Approach. Eye Contact Lens 2019, 45, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Coakes, R.L.; Mackie, I.A.; Seal, D.V. Effects of long-term treatment with timolol on lacrimal gland function. Br. J. Ophthalmol. 1981, 65, 603–605. [Google Scholar] [CrossRef]
- Kuppens, E.V.; de Jong, C.A.; Stolwijk, T.R.; de Keizer, R.J.; van Best, J.A. Effect of timolol with and without preservative on the basal tear turnover in glaucoma. Br. J. Ophthalmol. 1995, 79, 339–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Kam, W.R.; Liu, Y.; Chen, X.; Sullivan, D.A. Influence of Pilocarpine and Timolol on Human Meibomian Gland Epithelial Cells. Cornea 2017, 36, 719–724. [Google Scholar] [CrossRef]
- Aydin Kurna, S.; Acikgoz, S.; Altun, A.; Ozbay, N.; Sengor, T.; Olcaysu, O.O. The effects of topical antiglaucoma drugs as monotherapy on the ocular surface: A prospective study. J. Ophthalmol. 2014, 2014, 460483. [Google Scholar] [CrossRef]
- Nijm, L.M.; De Benito-Llopis, L.; Rossi, G.C.; Vajaranant, T.S.; Coroneo, M.T. Understanding the Dual Dilemma of Dry Eye and Glaucoma: An International Review. Asia Pac. J. Ophthalmol. 2020, 9, 481–490. [Google Scholar] [CrossRef]
- Mocan, M.C.; Uzunosmanoglu, E.; Kocabeyoglu, S.; Karakaya, J.; Irkec, M. The Association of Chronic Topical Prostaglandin Analog Use with Meibomian Gland Dysfunction. J. Glaucoma 2016, 25, 770–774. [Google Scholar] [CrossRef]
- Rabinowitz, M.P.; Katz, L.J.; Moster, M.R.; Myers, J.S.; Pro, M.J.; Spaeth, G.L.; Sharma, P.; Stefanyszyn, M.A. Unilateral Prostaglandin-Associated Periorbitopathy: A Syndrome Involving Upper Eyelid Retraction Distinguishable from the Aging Sunken Eyelid. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 373–378. [Google Scholar] [CrossRef]
- Vitoux, M.A.; Kessal, K.; Melik Parsadaniantz, S.; Claret, M.; Guerin, C.; Baudouin, C.; Brignole-Baudouin, F.; Réaux-Le Goazigo, A. Benzalkonium chloride-induced direct and indirect toxicity on corneal epithelial and trigeminal neuronal cells: Proinflammatory and apoptotic responses in vitro. Toxicol. Lett. 2020, 319, 74–84. [Google Scholar] [CrossRef]
- Ivakhnitskaia, E.; Souboch, V.; Dallacasagrande, V.; Mizerska, K.; Souboch, E.; Sarkar, J.; Guaiquil, V.H.; Tseng, K.Y.; Hirata, H.; Rosenblatt, M.I. Benzalkonium chloride, a common ophthalmic preservative, compromises rat corneal cold sensitive nerve activity. Ocul. Surf. 2022, 26, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Van Went, C.; Alalwani, H.; Brasnu, E.; Pham, J.; Hamard, P.; Baudouin, C.; Labbé, A. Corneal sensitivity in patients treated medically for glaucoma or ocular hypertension. J. Fr. Ophtalmol. 2011, 34, 684–690. (In French) [Google Scholar] [CrossRef] [PubMed]
- Martone, G.; Frezzotti, P.; Tosi, G.M.; Traversi, C.; Mittica, V.; Malandrini, A.; Pichierri, P.; Balestrazzi, A.; Motolese, P.A.; Motolese, I.; et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am. J. Ophthalmol. 2009, 147, 725–735.e1. [Google Scholar] [CrossRef] [PubMed]
- Ammar, D.A.; Noecker, R.J.; Kahook, M.Y. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv. Ther. 2010, 27, 837–845. [Google Scholar] [CrossRef]
- Liang, H.; Brignole-Baudouin, F.; Riancho, L.; Baudouin, C. Reduced in vivo ocular surface toxicity with polyquad-preserved travoprost versus benzalkonium-preserved travoprost or latanoprost ophthalmic solutions. Ophthalmic Res. 2012, 48, 89–101. [Google Scholar] [CrossRef]
- Kahook, M.Y.; Rapuano, C.J.; Messmer, E.M.; Radcliffe, N.M.; Galor, A.; Baudouin, C. Preservatives and ocular surface disease: A review. Ocul. Surf. 2024, 34, 213–224. [Google Scholar] [CrossRef]
- Herreras, J.M.; Pastor, J.C.; Calonge, M.; Asensio, V.M. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology 1992, 99, 1082–1088. [Google Scholar] [CrossRef]
- Chung, S.H.; Lee, S.K.; Cristol, S.M.; Lee, E.S.; Lee, D.W.; Seo, K.Y.; Kim, E.K. Impact of short-term exposure of commercial eyedrops preserved with benzalkonium chloride on precorneal mucin. Mol. Vis. 2006, 12, 415–421. [Google Scholar]
- Martinez-de-la-Casa, J.M.; Perez-Bartolome, F.; Urcelay, E.; Santiago, J.L.; Moreno-Montañes, J.; Arriola-Villalobos, P.; Benitez-Del-Castillo, J.M.; Garcia-Feijoo, J. Tear cytokine profile of glaucoma patients treated with preservative-free or preserved latanoprost. Ocul. Surf. 2017, 15, 723–729. [Google Scholar] [CrossRef]
- Boimer, C.; Birt, C.M. Preservative exposure and surgical outcomes in glaucoma patients: The PESO study. J. Glaucoma 2013, 22, 730–735. [Google Scholar] [CrossRef]
- Tomić, M.; Kaštelan, S.; Soldo, K.M.; Salopek-Rabatić, J. Influence of BAK-preserved prostaglandin analog treatment on the ocular surface health in patients with newly diagnosed primary open-angle glaucoma. BioMed Res. Int. 2013, 2013, 603782. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, H.; Pan, J.; Zhou, D.; Chen, W.; Li, W.; Chen, Y.; Liu, Z. Benzalkonium chloride induces subconjunctival fibrosis through the COX-2-modulated activation of a TGF-β1/Smad3 signaling pathway. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8111–8122. [Google Scholar] [CrossRef] [PubMed]
- Broadway, D.C.; Grierson, I.; O’Brien, C.; Hitchings, R.A. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch. Ophthalmol. 1994, 112, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Merani, R.; McPherson, Z.E.; Luckie, A.P.; Gilhotra, J.S.; Runciman, J.; Durkin, S.; Muecke, J.; Donaldson, M.; Aralar, A.; Rao, A.; et al. Aqueous Chlorhexidine for Intravitreal Injection Antisepsis: A Case Series and Review of the Literature. Ophthalmology 2016, 123, 2588–2594. [Google Scholar] [CrossRef]
- Epstein, N.E. Review: Perspective on ocular toxicity of presurgical skin preparations utilizing Chlorhexidine Gluconate/Hibiclens/Chloraprep. Surg. Neurol. Int. 2021, 12, 335. [Google Scholar] [CrossRef]
- Green, K.; Livingston, V.; Bowman, K.; Hull, D.S. Chlorhexidine Effects on Corneal Epithelium and Endothelium. Arch. Ophthalmol. 1980, 98, 1273–1278. [Google Scholar] [CrossRef]
- Hamill, M.B.; Osato, M.S.; Wilhelmus, K.R. Experimental evaluation of chlorhexidine gluconate for ocular antisepsis. Antimicrob. Agents Chemother. 1984, 26, 793–796. [Google Scholar] [CrossRef]
- Dasgupta, S. The course of dry eye following phacoemulsification and manual—SICS: A prospective study based on Indian scenario. Int. Eye Sci. 2016, 16, 1789–1794. [Google Scholar] [CrossRef]
- Miyake, K.; Yokoi, N. Influence on ocular surface after cataract surgery and effect of topical diquafosol on postoperative dry eye: A multicenter prospective randomized study. Clin. Ophthalmol. 2017, 11, 529–540. [Google Scholar] [CrossRef]
- Kato, K.; Miyake, K.; Kondo, N.; Asano, S.; Takeda, J.; Takahashi, A.; Takashima, Y.; Kondo, M. Conjunctival Goblet Cell Density Following Cataract Surgery with Diclofenac Versus Diclofenac and Rebamipide: A Randomized Trial. Am. J. Ophthalmol. 2017, 181, 26–36. [Google Scholar] [CrossRef]
- Oh, T.; Jung, Y.; Chang, D.; Kim, J.; Kim, H. Changes in the tear film and ocular surface after cataract surgery. Jpn. J. Ophthalmol. 2012, 56, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Yanai, R.; Yamada, N.; Ueda, K.; Tajiri, M.; Matsumoto, T.; Kido, K.; Nakamura, S.; Saito, F.; Nishida, T. Evaluation of povidone-iodine as a disinfectant solution for contact lenses: Antimicrobial activity and cytotoxicity for corneal epithelial cells. Contact Lens Anterior Eye 2006, 29, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.P.; Ahdoot, M.; Marcus, E.; Asbell, P.A. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 2009, 25, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.B.; Kim, H.S. Phototoxic effects of an operating microscope on the ocular surface and tear film. Cornea 2014, 33, 82–90. [Google Scholar] [CrossRef]
- Ipek, T.; Hanga, M.P.; Hartwig, A.; Wolffsohn, J.; O’Donnell, C. Dry eye following cataract surgery: The effect of light exposure using an in-vitro model. Contact Lens Anterior Eye 2018, 41, 128–131. [Google Scholar] [CrossRef]
- Ram, J.; Gupta, A.; Brar, G.; Kaushik, S.; Gupta, A. Outcomes of phacoemulsification in patients with dry eye. J. Cataract. Refract. Surg. 2002, 28, 1386–1389. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.K.; Ha, Y.; Paik, H.J.; Kim, D.H. Improved accuracy of intraocular lens power calculation by preoperative management of dry eye disease. BMC Ophthalmol. 2021, 21, 364. [Google Scholar] [CrossRef]
- Trattler, W.B.; Majmudar, P.A.; Donnenfeld, E.D.; McDonald, M.B.; Stonecipher, K.G.; Goldberg, D.F. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: The effect of dry eye. Clin. Ophthalmol. 2017, 11, 1423–1430. [Google Scholar] [CrossRef]
- Epitropoulos, A.T.; Matossian, C.; Berdy, G.J.; Malhotra, R.P.; Potvin, R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J. Cataract. Refract. Surg. 2015, 41, 1672–1677. [Google Scholar] [CrossRef]
- Yang, F.; Yang, L.; Ning, X.; Liu, J.; Wang, J. Effect of dry eye on the reliability of keratometry for cataract surgery planning. J. Fr. Ophtalmol. 2024, 47, 103999. [Google Scholar] [CrossRef]
- Hiraoka, T.; Asano, H.; Ogami, T.; Nakano, S.; Okamoto, Y.; Yamada, Y.; Oshika, T. Influence of Dry Eye Disease on the Measurement Repeatability of Corneal Curvature Radius and Axial Length in Patients with Cataract. J. Clin. Med. 2022, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T. Contact Lens-Associated Dry Eye Disease: Recent Advances Worldwide and in Japan. Investig. Ophthalmol. Vis. Sci. 2018, 59, Des102–Des108. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Inoue, K.; Kuchiba, A.; Yamaguchi, T.; Amano, S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology 2009, 116, 379–384. [Google Scholar] [CrossRef]
- Nagahara, Y.; Koh, S.; Oshita, Y.; Nagano, T.; Mano, H.; Nishida, K.; Watanabe, H. Diquafosol Ophthalmic Solution Increases Pre- and Postlens Tear Film During Contact Lens Wear in Rabbit Eyes. Eye Contact Lens 2017, 43, 378–382. [Google Scholar] [CrossRef]
- Yang, B.; Ma, X.; Liu, L.; Cho, P. Vision-related quality of life of Chinese children undergoing orthokeratology treatment compared to single vision spectacles. Contact Lens Anterior Eye 2021, 44, 101350. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Miao, C.X.; Xu, X.Y.; Zhang, H. Analysis of corneal complications in children wearing orthokeratology lenses at night. Zhonghua Yan Ke Za Zhi 2017, 53, 198–202. (In Chinese) [Google Scholar] [CrossRef]
- De Paiva, C.S.; Chen, Z.; Koch, D.D.; Hamill, M.B.; Manuel, F.K.; Hassan, S.S.; Wilhelmus, K.R.; Pflugfelder, S.C. The incidence and risk factors for developing dry eye after myopic LASIK. Am. J. Ophthalmol. 2006, 141, 438–445. [Google Scholar] [CrossRef]
- Nair, S.; Kaur, M.; Sharma, N.; Titiyal, J.S. Refractive surgery and dry eye—An update. Indian J. Ophthalmol. 2023, 71, 1105–1114. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yam, G.H.; Lin, M.T.; Teo, E.; Koh, S.K.; Deng, L.; Zhou, L.; Tong, L.; Mehta, J.S. Comparison of tear proteomic and neuromediator profiles changes between small incision lenticule extraction (SMILE) and femtosecond laser-assisted in-situ keratomileusis (LASIK). J. Adv. Res. 2021, 29, 67–81. [Google Scholar] [CrossRef]
- Konomi, K.; Chen, L.L.; Tarko, R.S.; Scally, A.; Schaumberg, D.A.; Azar, D.; Dartt, D.A. Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Investig. Ophthalmol. Vis. Sci. 2008, 49, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Spierer, O. Dry Eye Post-Laser-Assisted In Situ Keratomileusis: Major Review and Latest Updates. J. Ophthalmol. 2018, 2018, 4903831. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Kim, J.Y.; Chin, H.S.; Suh, Y.J.; Kim, T.I.; Seo, K.Y. Assessment of meibomian glands and tear film in post-refractive surgery patients. Clin. Exp. Ophthalmol. 2017, 45, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Yokoi, N.; Shimazaki, J.; Hori, Y.; Tsubota, K.; on behalf of the Japan Dry Eye Society. Adherence to Eye Drops Usage in Dry Eye Patients and Reasons for Non-Compliance: A Web-Based Survey. J. Clin. Med. 2022, 11, 367. [Google Scholar] [CrossRef]
- Teo, C.H.Y.; Ong, H.S.; Liu, Y.C.; Tong, L. Meibomian gland dysfunction is the primary determinant of dry eye symptoms: Analysis of 2346 patients. Ocul. Surf. 2020, 18, 604–612. [Google Scholar] [CrossRef]
- Yang, B.; Wen, K.; Li, J.; Zhang, S.; Fan, Z.; Liang, X.; Liang, L. Quantitative evaluation of lipid layer thickness and blinking in children with allergic conjunctivitis. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2795–2805. [Google Scholar] [CrossRef]
| Diquas® | Diquas®-S | Diquas®-LX |
|---|---|---|
| Diquafosol sodium 3% Chlorhexidine gluconate (preservative) Dibasic sodium phosphate hydrate Disodium edetate hydrate Sodium chloride Potassium chloride Sodium hydroxide Dilute hydrochloric acid | Diquafosol sodium 3% Dibasic sodium phosphate hydrate Disodium edetate hydrate Sodium chloride Potassium chloride Hydrochloric acid Sodium hydroxide | Diquafosol sodium 3% Dibasic sodium phosphate hydrate Disodium edetate hydrate Sodium chloride Polyvinylpyrrolidone Silver nitrate pH adjuster |
| Ophthalmic Adverse Effects |
|---|
| Ocular irritation |
| Ocular discharge |
| Foreign body sensation |
| Conjunctival hyperaemia |
| Ocular pain and discomfort |
| Ocular pruritus |
| Condition | Study Type | Summary of Study Conclusions | References |
|---|---|---|---|
| Dry Eye Disease | Diquafosol 3% vs. artificial tears | Diquafosol improved the following:
| [16,24,25,26,30,53,54] |
| Diquafosol 3% vs. cyclosporine | Diquafosol 3% vs. cyclosporine 0.05% vs. cyclosporine 0.1% showed the following:
| [55,56] | |
| Meibomian Gland Dysfunction | Pre- vs. post-diquafosol 3% instillation | Diquafosol improved the following:
| [45,57] |
| Diquafosol 3% vs. artificial tears vs. gatifloxacin 0.3% | Only diquafosol increased lipid layer thickness | [58] | |
| Aqueous-Deficient Dry Eye Disease | Pre- vs. post-diquafosol 3% instillation | Diquafosol improved the following:
| [42,59,60,61] |
| Ocular Graft-Versus-Host Disease | Pre- vs. post-diquafosol 3% instillation | Diquafosol improved the following:
| [62,63] |
| Glaucoma Medication- and Preservative-Related Ocular Surface Disease | Pre- vs. post-diquafosol 3% instillation | Diquafosol improved:
| [64,65,66] |
| Cataract Surgery | Diquafosol 3% vs. artificial tears | Postoperatively, diquafosol improved the following:
| [21,67] |
| Pre- vs. post-diquafosol 3% instillation | Pre-keratometry administration of diquafosol improved the following:
| [68,69] | |
| Contact Lens Wear | Pre- vs. post-diquafosol 3% instillation Diquafosol 3% vs. artificial tears | Diquafosol improved the following:
| [70,71,72,73] |
| Keratorefractive Surgery | Diquafosol 3% vs. artificial tears | Among patients who underwent small-incision lenticule extraction (SMILE), diquafosol improved the following:
| [74,75,76,77] |
| Long-Acting Diquafosol | Long-acting diquafosol 3% vs. vehicle | Long-acting diquafosol improved the following:
| [78] |
| Long-acting diquafosol 3% vs. conventional diquafosol 3% | Long-acting formulation, compared to conventional diquafosol, provided better patient compliance | [79,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.Q.L.; Wu, D.; Toh, X.Y.; Lim, B.X.; Shih, K.C.; Tong, L.; Lim, C.H.L. Applications of Diquafosol Sodium in Ophthalmology: A Comprehensive Review of Therapeutic Utility. Life 2025, 15, 484. https://doi.org/10.3390/life15030484
Tan CQL, Wu D, Toh XY, Lim BX, Shih KC, Tong L, Lim CHL. Applications of Diquafosol Sodium in Ophthalmology: A Comprehensive Review of Therapeutic Utility. Life. 2025; 15(3):484. https://doi.org/10.3390/life15030484
Chicago/Turabian StyleTan, Chelsea Qiu Lin, Duoduo Wu, Xin Yun Toh, Blanche Xiaohong Lim, Kendrick Co Shih, Louis Tong, and Chris Hong Long Lim. 2025. "Applications of Diquafosol Sodium in Ophthalmology: A Comprehensive Review of Therapeutic Utility" Life 15, no. 3: 484. https://doi.org/10.3390/life15030484
APA StyleTan, C. Q. L., Wu, D., Toh, X. Y., Lim, B. X., Shih, K. C., Tong, L., & Lim, C. H. L. (2025). Applications of Diquafosol Sodium in Ophthalmology: A Comprehensive Review of Therapeutic Utility. Life, 15(3), 484. https://doi.org/10.3390/life15030484








