Factors Associated with Post-COVID Cardiac Conditions and Potential Prognostic Factors: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Post-COVID Cardiac Condition
2.3. Data Extraction and Methodological Assessment
2.4. Analysis of Results
3. Results
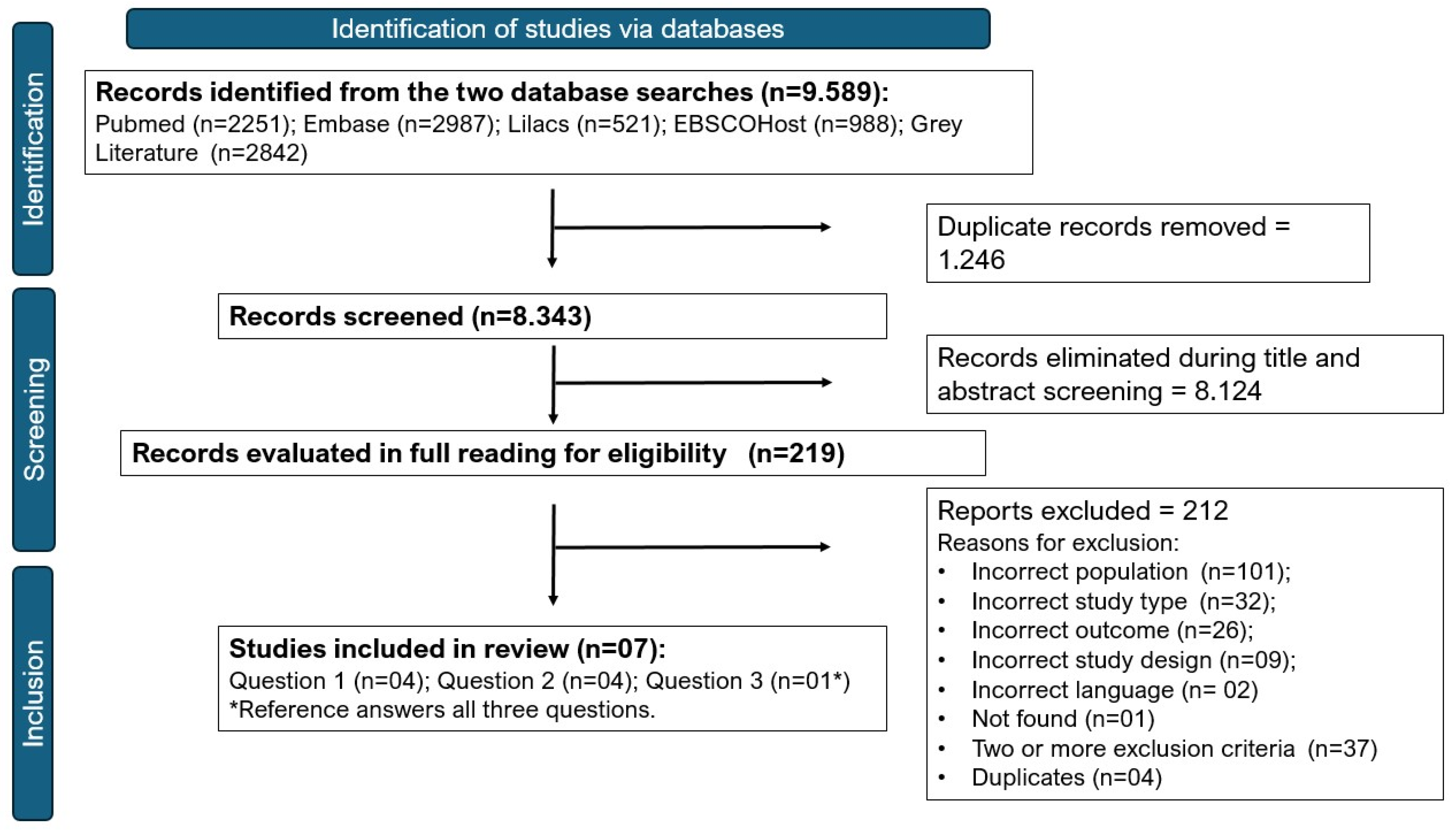
3.1. Study Selection and Characteristics
3.2. Post-COVID Cardiac Condition
3.3. Factors Associated with the Occurrence of Post-COVID Cardiac Conditions (Question 1)
3.4. Main Outcomes of Patients with Post-COVID Cardiac Conditions (Question 2)
3.5. Potential Prognostic Factors for Patients with Post-COVID Cardiac Conditions (Question 3) and Treatment Strategy
3.6. Assessment of the Methodological Quality of the Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willi, S.; Lüthold, R.; Hunt, A.; Hänggi, N.V.; Sejdiu, D.; Scaff, C.; Bender, N.; Staub, K.; Schlagenhauf, P. COVID-19 sequelae in adults aged less than 50 years: A systematic review. Travel Med. Infect. Dis. 2021, 40, 101995. [Google Scholar] [CrossRef] [PubMed]
- Organização Pan-Americana da Saúde, (OPAS). OMS Afirma Que COVID-19 é Agora Caracterizada Como Pandemia—OPAS/OMS|Organização Pan-Americana da Saúde. 2020. Available online: https://www.paho.org/pt/news/11-3-2020-who-characterizes-covid-19-pandemic (accessed on 27 February 2023).
- BRASIL M da SaúdeSE de E à C 19. NOTA TÉCNICA No 60/2021-SECOVID/GAB/SECOVID/MS. 2021; pp. 1–10. Available online: http://sei.saude.gov.br/sei/controlador_externo.php?acao=documento_conferir&id_orgao_acesso_externo=0 (accessed on 1 July 2022).
- World Health Organization (WHO). Post COVID-19 Condition (Long COVID). 2022. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition#:~:text=Definition,months%20with%20no%20other%20explanation (accessed on 30 January 2025).
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long COVID among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, 2–7. [Google Scholar] [CrossRef]
- Mitrani, R.D.; Dabas, N.; Goldberger, J.J. COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors. Heart Rhythm 2020, 17, 1984–1990. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Maria, L.S.; Batista, J.E.T.; Wachira, V.K.; Júnior, W.B.C.; Soares, A.A.; de Carvalho, I.P.S.F.; Peixoto, H. PROSPERO International Prospective Register of Systematic Reviews Factors Associated with the Development of Cardiac Sequelae, in Post-Acute COVID-19 Syndrome, and Potential Prognostic Factors in Patients with This Condition: A Systematic Review. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022336460 (accessed on 22 October 2024).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 27 July 2024).
- Hayden, J.A.; Van Der Windt, D.A.; Cartwright, J.L.; Cô, P.; Bombardier, C. Assessing Bias in Studies of Prognostic Factors. 2013. Available online: www.annals.org (accessed on 23 October 2024).
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Lensen, S. When to pool data in a meta-analysis (and when not to)? Fertil. Steril. 2023, 119, 902–903. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Zisis, S.N.; Durieux, J.C.; Mouchati, C.; Perez, J.A.; McComsey, G.A. The Protective Effect of Coronavirus Disease 2019 (COVID-19) Vaccination on Postacute Sequelae of COVID-19: A Multicenter Study From a Large National Health Research Network. Open Forum Infect. Dis. 2022, 9, ofac228. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 2022, 28, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E.; Dendy, J.M.; Li, D.L.; Crum, K.; Dixon, D.; George-Durrett, K.; Parikh, A.P.; Wassenaar, J.W.; Hughes, S.G. Cardiovascular magnetic resonance evaluation of soldiers after recovery from symptomatic SARS-CoV-2 infection: A case-control study of cardiovascular post-acute sequelae of SARS-CoV-2 infection (CV PASC). J. Cardiovasc. Magn. Reson. 2021, 23, 106. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Mok, A.H.Y.; Yan, V.K.C.; Chan, C.I.Y.; Wang, B.; Lai, F.T.T.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Yiu, K.H.; et al. Association between BNT162b2 and CoronaVac vaccination and risk of CVD and mortality after COVID-19 infection: A population-based cohort study. Cell Rep. Med. 2023, 4, 101195. [Google Scholar] [CrossRef]
- Shah, S.S.; Naidu, P.K.K.; Selvam, S.; Shetty, R.; Bhat, C.S.; Maheshwari, S. Cardiac findings in multisystem inflammatory syndrome in children: Short term follow up in a large Indian series. Ann. Pediatr. Cardiol. 2023, 16, 94–101. [Google Scholar] [CrossRef]
- Shechter, A.; Yelin, D.; Margalit, I.; Abitbol, M.; Morelli, O.; Hamdan, A.; Vaturi, M.; Eisen, A.; Sagie, A.; Kornowski, R.; et al. Assessment of Adult Patients with Long COVID Manifestations Suspected as Cardiovascular: A Single-Center Experience. J. Clin. Med. 2022, 11, 6123. [Google Scholar] [CrossRef]
- Cassar, M.P.; Tunnicliffe, E.M.; Petousi, N.; Lewandowski, A.J.; Xie, C.; Mahmod, M.; Samat, A.H.A.; Evans, R.A.; Brightling, C.E.; Ho, L.P.; et al. Symptom Persistence Despite Improvement in Cardiopulmonary Health—Insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine 2021, 41, 101159. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 132. [Google Scholar] [CrossRef]
- Gray, G.E.; Garrett, N.; Goga, A.; Fairall, L.; Reddy, T.; Yende-Zuma, N.; Collie, S.; Sanne, I.; Moultrie, H.; Mayat, F.; et al. Safety and Effectiveness of the Ad26.COV2.S VACCINE in South Africa. Top. Antivir. Med. 2022, 30 (Suppl. S1), 18. Available online: https://www.embase.com/search/results?subaction=viewrecord&id=L638149121&from=export (accessed on 4 July 2022).
- Parodi, J.B.; Indavere, A.; Jacob, P.B.; Toledo, G.C.; Micali, R.G.; Waisman, G.; Masson, W.; Epstein, E.D.; Huerin, M.S. Impact of COVID-19 vaccination in post-COVID cardiac complications. Vaccine 2023, 41, 1524–1528. [Google Scholar] [CrossRef]
- Yendewa, G.A.; Perez, J.A.; Patil, N.; McComsey, G.A. Associations between post-acute sequelae of SARS-CoV-2, COVID-19 vaccination and HIV infection: A United States cohort study. Front. Immunol. 2024, 15, 1297195. [Google Scholar] [CrossRef]
- Aziz, O.A.; Sadiq, M.; Qureshi, A.U.; Hyder, N.; Kazmi, U.; Batool, A.; Naz, S.; Mushtaq, A.; Bari, A.; Rashid, J. Short to midterm follow-up of multi-system inflammatory syndrome in children with special reference to cardiac involvement. Cardiol. Young 2023, 33, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Kunal, S.; Bagarhatta, P.; Palleda, G.M.; Bansal, A.; Batra, V.; Daga, M.K.; Tyagi, S.; Sharma, A.; Bansal, K.; Agarwal, R.; et al. Role of cardiovascular magnetic resonance imaging in COVID-19 recovered patients: A short-term follow-up study. Echocardiography 2022, 39, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Zaoui, N.; Bachir, N.; Terki, A.; Boukabous, A. COVID-19 myocarditis: “About a monocentric series of 33 cases”. Ann. Cardiol. Angeiol. 2022, 71, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article—Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682–689. [Google Scholar] [CrossRef]
| Author (Year) Country | No of Measurements of the Outcome and Follow-Up Time | Study Population and Subgroups | Characterisation of the Population/Groups | Association Factors and Measures Under Analysis | Cardiac Outcomes Analysed/Results | Results/Conclusions |
|---|---|---|---|---|---|---|
| Bowe et al. (2022) USA [18] | 7 observations: T0: positive test T1: 90 days After T1: 30 to 60 days 60 to 90 days 90 to 120 days 120 to 150 days 150 to 180 days | Population: Soldiers using the US VHA (Veterans Health Administration of United States) with at least one positive SARS-CoV-2 test between 1 March 2020 and 6 April 2022 who were alive 90 days after the first test. Exposed: 40,947 soldiers who experienced SARS-CoV-2 reinfection. Unexposed: 443,588 soldiers who were not reinfected with SARS-CoV-2. | Without reinfection: White race/colour (69.54%), male sex (87.22%), never smoked (45.55%), BMI (±): 31.04 kg/m2 (±6.05), age (±): 59.84 (±15.74). No vaccination (64.14%), 1 dose (7.99%), 2 doses (20.01%), 3 or more doses (7.86%). Type 2 diabetes (31.98%), hospitalisation during the acute phase of the 1st infection (9.54%). With reinfection: White race/colour (69.12%), male sex (86.4%), never smoked (46.5%), BMI (±): 30.97 kg/m2 (±6.1), age (±): 59.97 (±15.76). No vaccination (62.78%), 1 dose (7.52%), 2 doses (22.88%), 3 or more doses (6.82%). Type 2 diabetes (32.52%), hospitalisation during the acute phase of the 1st infection (11.83%). | Vaccination status and reinfection Measures: p-value, relative risk (95% CI), and excess risk per 1000 people in 30 days (AR) (95% CI). | Cardiovascular diseases: Dysrhythmias (tachycardia, bradycardia, ventricular arrhythmias, atrial fibrillation, and atrial flutter) Inflammatory heart disease (pericarditis and myocarditis) Ischemic heart disease (acute coronary disease, angina, myocardial infarction, ischemic cardiomyopathy) Other cardiac disorders (heart failure, non-ischemic cardiomyopathy, cardiac arrest, and cardiogenic shock) | Reinfection (regardless of vaccination status) increases the risk of post-COVID cardiovascular conditions: Reinfection x no reinfection 30 to 60 days reinfection AR: 15.37 (95% CI: 12.78; 18.21) RR: 2.19 (95% CI: 1.99, 2.41) 90 to 120 days after reinfection AR: 8.93 (6.91; 11.15) RR: 1.66 (1.51, 1.82) |
| Wan et al. (2023) Hong Kong [20] | 2 observations: acute phase: <28 days and post-acute phase: >28 days | Population: Patients aged ≥18 years with COVID-19 infection between 23 February 2021 and 31 October 2022, according to electronic health records, vaccination records, and data sets of COVID-19 infection records from the Hong Kong Special Administrative Region Groups after weighting ; Exposed: 902,601 Vaccinated for COVID-19; Unexposed: 999,740 unvaccinated | No vaccine Males: 438,139 (43.8%); Diabetes: 95,750 (9.6%); Severe COVID-19: 1209 (0.1%); Age (s±): 49.2 (±20.2) 1 dose Males: 439,332 (44.0%); Diabetes: 97,334 (9.7%); Severe COVID-19: 1196 (0.1%); Age (s±): 49.5 (±19.2) 2 doses Males: 429,225 (43.4%); Diabetes: 97,677 (9.9%); Severe COVID-19: 1222 (0.1%); Age (s±): 50.6 (±17.3) 3 doses Males: 430,408 (43.5%); Diabetes: 99,217 (10.0%); Severe COVID-19: 1426 (0.1%); Age (s±): 50.6 (±16.1) | Vaccine doses, age, sex, Charlson comorbidity index (CCI), disease severity, and type of vaccine; Measures: Incidence rate, relative risk, and their respective (95% CI) | Coronary heart disease RR (95% CI) ≥ 65 years 1 dose: 0.75 (0.60; 0.94) More than 90 days 2 doses: 0.73 (0.61; 0.88) Heart failure More than 90 days 1 dose: 0.67 (0.53; 0.85) CCI < 4 2 doses: 0.41 (0.29; 0.57) CCI ≥ 4 2 doses: 0.74 (0.59; 0.91) | Coronary artery disease: 1 vaccine dose reduces the risk in individuals aged 65 years or older, while 2 vaccine doses reduce the risk of occurrence regardless of age, sex, CCI, or vaccine type, even after 90 days post-infection. Heart failure: 1 vaccine dose reduces the risk of occurrence regardless of age, sex, CCI, or vaccine type, even after 90 days post-infection. On the other hand, 2 vaccine doses confer greater protection to individuals with CCI < 4 compared to those with CCI ≥ 4 |
| Shah et al. (2023) India [21] | 7 observations: 1, 2, 4, 7, and 8 weeks and 3 and 6 months (number and times varied between study participants with only two children followed at 6 months) | Population: 144 sequential patients diagnosed with MIS-C treated by the authors at cardiology Exposed: 85 patients with cardiac abnormalities Unexposed: 59 patients without cardiac abnormalities | Population (144 children with MIS-C) Males: n = 93 (64.6%); Weight: : (s±) = 20.5 kg (±14.7); Symptom: fever n = 137 (95.1%); Intensive Care: n = 76 (52.8%). Age groups (%): <12 months: 17 (11.8%); 12–60 months: 60 (41.7%); 60–120 months: 44 (30.6%); >120 months: 23 (16.0%) Children with cardiac abnormalities (85) Males: n = 56 (65.9%); Weight: (s±)’ 22.5 (±14.3); Intensive Care: n = 49 (57.6%) Age groups (%): <12 months: 6 (7.1%); 12–60 months: 39 (45.9%); 60–120 months: 25 (29.4%); >120 months: 15 (17.6%) | Sex, age, symptom presentations, laboratory results, duration between COVID-19 infection and current disease, echocardiographic findings, electrocardiographic findings, and management. Measures: Incidence and p-value | Ventricular dysfunction, coronary dilatation, pericarditis, atrioventricular valve regurgitation, and conduction abnormalities | No association was found between the factors analysed and the outcomes studied a (NT Pro-BNP elevated showed cardiac abnormality p-value = 0.09) |
| Zisis et al. (2022) USA [17] | 3 observations: baseline (diagnosis) 28 days 3 months | Population: Adult patients aged ≥ 18 years with SARS-CoV-2 infection (confirmed by PCR) who sought care in the United States with medical records on the TriNetX Research Network platform and # of patients diagnosed with COVID-19 at least one week after administration of the full vaccine were included in the vaccinated cohort. Exposed: 25,225 vaccinated for COVID Unexposed: 25,225 unvaccinated | Vaccinated: Females n: 15,094 (59.84%) ; White race/colour n: 17,266 (68.45%) ; Hypertension n: 11,974 (47.36%); Diabetes n: 5774 (22.89%); BMI (s±): 30.20 kg/m2 (±7.33); Unvaccinated: Females n:15,129 (59.98%); White race/colour n: 17,381 (68.90%); Hypertension n: 11,963 (47.43%); Diabetes n: 5698 (22.59%); BMI (s±): 30.68 kg/m2 (±7.40) | Vaccination Measures: incidence, relative risk (RR), and attributable risk (AR) in 28 days and 90 days | Grouped heart diseases: ICD-10 (I30–52, I21) | Full vaccination protects against the occurrence of post-COVID cardiac conditions at 28 and 90 days after infection: 28 days: RR (95% CI): 0.49 (0.43, 0.57) AR (95% CI): −15.76 (−18.96; −12.57) 90 days: RR (95% CI): 0.35 (0.29; 0.44) AR (95% CI): −13.07 (−15.55; −10.60) |
| Author Year Country | No of Outcome Measurements in the Post-COVID Period | Study Population and Subgroups | Validation Criterion of Cardiac Condition Post-COVID | Cardiac Outcomes Analysed (Measures) | Main Outcomes | Conclusions |
|---|---|---|---|---|---|---|
| Clark et al. (2021) USA [19] | Group 1 (patients with cardiopulmonary symptoms in the late convalescence phase) 3 measurements (in days): 1st ranged from 33 to 124 2nd ranged from 82 to 271 3rd ranged from 119 to 245 | Soldiers in active service or recent military retirement (previous year), who routinely exceed 6 h of strenuous activity per week Group 1: 50 soldiers who had COVID-19 and remained with cardiopulmonary symptoms in the late convalescence phase of recovery | Physical examination, ECG, and CMR | Myocarditis Pericarditis Takotsubo cardiomyopathy Biventricular systolic dysfunction (incidence and time to resolution of cardiac sequelae) | Patients with post-COVID cardiac condition: Myocarditis—4 cases (8%): 1 acute myocarditis, 3 with healing myocarditis, 1 of which (2%) had concomitant pericarditis; Takotsubo cardiomyopathy—1 case (2%); new biventricular systolic dysfunction without myocarditis—1 case (2%) Follow-up of myocarditis patients: Case 1: LGE1: 8.8% (33 d from COVID diagnosis): LGE2: 2.8% (82 d); LGE3: 0 (119d); Case 2: LGE1: 3.5% (124 d); LGE2: 3.5% (271 d); Case 3: LGE1: 5.1% (38 d); LGE2: 5.1% (122 d); LGE3: 5.1% (213 d); Case 4: LGE1: 7.4% (97 d); LGE2: 2.1% (192 d); LGE3: 0 (245 d). | Follow-up CMR among cases of myocarditis showed two patterns: recovery with gradual resolution of LGE (2 cases) and persistence (2 cases), with one soldier presenting with continuous active myocarditis with T2 elevation more than 7 months after COVID-19 diagnosis. |
| Shechter, A et al. (2022) Israel [22] | 2 measurements 60 days from negative PCR Median = 142 (IQR: 111; 197) days after COVID-19 diagnosis. | 87 patients (≥18 years) who had PCR+ for COVID-19, with persistent symptoms after 60 days of negative PCR (formal recovery), have a written referral from their attending physicians stating the exact manifestation(s) believed to be CV disease, attended between June 2020 and June 2021. Post-COVID cardiac conditions: 9 patients (10 events) | Cardiac provocation test, Holter ECG, cardiopulmonary exercise test (CPET), CCT, CMR, PFT, and chest HRCT | New CV diagnoses potentially related to COVID-19, major adverse cardiovascular events (MACE), defined as acute coronary syndrome, acute stroke, and CV death. (Incidence = absolute number per follow-up visit.) | Initial diagnoses: 10 cases; Myocarditis: 3 cases; Myopericarditis: 2 cases; Chronotropic incompetence: 3 cases; Unperturbed sinus tachycardia: 1 case; Atrial tachycardia: 1 case. At 1-year follow-up (142 days = median): Complete resolution of cases = Recovery of 100% of cases at a median of 3 months. | Recovery of 100% of cases at a median of 3 months. No MACE or death recorded. |
| Shah et al. (2023) India [21] | 7 observations: 1 week 2 weeks 4 weeks 7 weeks 8 weeks 3 months 6 months (number and times varied between study participants with only two children followed at 6 months) | Population: 144 sequential patients diagnosed with MIS-C seen by the authors at cardiology Patients with cardiac abnormalities: 85 | Echocardiogram: confirmation of one of the five abnormalities at any of the visits | Ventricular dysfunction, coronary dilatation, pericarditis, atrioventricular valve regurgitation, and conduction abnormalities. Other outcomes: coronary aneurysm. Incidence = absolute number per follow-up visit; average duration of recovery (in days). | Abnormal electrocardiogram: 39 patients; 1 week: 39; 2 weeks: 3; 4 weeks: 3; 7 and 8 weeks: 0 Pericardial effusion: 25 patients; 1 week: 22; 2 weeks: 6; 4 weeks: 1; 7 and 8 weeks: 1 Coronary artery dilatation: 37 patients; 1 week: 31; 2 weeks: 24; 4 weeks: 11; 7 weeks: 4; 8 weeks: 3 left ventricular dysfunction: 20 patients; 1 week: 20; 2 weeks: 3; 4 weeks: 1; 7 and 8 weeks:0 Atrioventricular artery regurgitation: 33 patients; 1 week: 33; 2 weeks: 2; 4 weeks: 3; 7 weeks: 2; 8 weeks: 0 | 51 children completed the follow-up (Loss = 40%). Recovery: 92% (47/51) of children; median of 30 (range: 22 to 37 days) (Kaplan–Meier analysis). Ventricular dysfunction: resolved and no child presented cardiac dysfunction at the time of hospital discharge. Pericardial effusion: completely resolved, except one, before discharge. Atrioventricular valve regurgitation correlated with ventricular dysfunction and disappeared completely when LV function improved. Three children: coronary artery dilatation persisting at the end of 8 weeks: 1 moderate left coronary artery aneurysm persisting at 182 days; 2 children: small left coronary artery aneurysm, persisting after 168 and 201 days each. |
| Cassar et al. (2021) UK [23] | 2 measurements after infection: 2/3 months; 6 months | 58 patients with confirmed moderate-to-severe COVID-19 who were admitted for at least 48 h to the Oxford University Hospitals National Health Service Foundation Trust (14 March and 25 May 2020) | ECG for each participant and interpreted according to the Minnesota Code Manual of Electrocardiographic Findings; Cardiopulmonary MRI | Coronary artery disease; atrial fibrillation, myocardial infarction, myocarditis, and pericardial effusion. (CVD incidence at 2/3 months and 6 months) | Results at 2/3 months and at 6 months: coronary artery donation: 2/58 and 1/46; atrial fibrillation: 1/58 and 1/46 (same patient); myocarditis pattern: 6/52 (11.5%) and 5/43 (11.6%); myocardial infarction: 1/52 (1.9%) and 0/43 (0.0%); pericardial effusion: 1/52 (1.9%) and 0/43 (0.0%). | Partial resolution of events. None of the patients met Lake Louise’s updated criteria for active myocarditis. |
| Studies—Question 1 | Items | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Score | ||||||
| S1 | S2 | S3 | S4 | C | D1 | D2 | D3 | ||
| Shah et al. (2023) [21] | 0 | * | * | * | 0 | * | * | * | 6 of 9 |
| Wan et al. (2023) [20] | * | * | * | * | ** | * | * | * | 9 of 9 |
| Zisis et al. (2022) [17] | * | * | * | 0 | ** | * | * | * | 8 of 9 |
| Bowe et al. (2022) [18] | 0 | * | * | * | ** | * | * | * | 8 of 9 |
| Studies—Question 2 | S1 | — | — | S4 | — | D1 | D2 | D3 | Score |
| Clark et al. (2021) [19] | 0 | — | — | 0 | — | * | * | * | 3 of 5 |
| Shah et al. (2023) [21] | 0 | — | — | * | — | * | * | * | 4 of 5 |
| Shechter et al. (2022) [22] | 0 | — | — | * | — | * | * | * | 4 of 5 |
| Cassar et al. (2021) [23] | 0 | — | — | 0 | — | * | * | * | 3 of 5 |
| Item | Assessment | Observations |
|---|---|---|
| 1 Participation | Moderate risk | Lack of clarity on the representativeness of the population |
| 2 Attrition | High risk | Loss of 40% described with important differences in PFs and outcomes measured between adherent and non-adherent groups |
| 3 PF measurement | Low risk | Data collected from medical records and standardised measurements |
| 4 Outcome measurement | Low risk | |
| 5 Confusion | High risk | Method for controlling bias in the study not informed |
| 6 Statistical analysis | High risk | High probability of error due to lack of representativeness of the compared groups (PF analysis). |
| Final analysis | High risk | High risk (3 important items in the PF analysis were classified as high risk) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maria, L.F.B.S.; Batista, J.E.T.; Wachira, V.K.; Junior, W.B.C.; Soares, A.A.d.S.M.; Carvalho, I.P.S.F.d.; Peixoto, H.M. Factors Associated with Post-COVID Cardiac Conditions and Potential Prognostic Factors: A Systematic Review. Life 2025, 15, 388. https://doi.org/10.3390/life15030388
Maria LFBS, Batista JET, Wachira VK, Junior WBC, Soares AAdSM, Carvalho IPSFd, Peixoto HM. Factors Associated with Post-COVID Cardiac Conditions and Potential Prognostic Factors: A Systematic Review. Life. 2025; 15(3):388. https://doi.org/10.3390/life15030388
Chicago/Turabian StyleMaria, Lidian Franci Batalha Santa, Josicélia Estrela Tuy Batista, Virginia Kagure Wachira, Wenderval Borges Carvalho Junior, Alexandre Anderson de Sousa Munhoz Soares, Isis Polianna Silva Ferreira de Carvalho, and Henry Maia Peixoto. 2025. "Factors Associated with Post-COVID Cardiac Conditions and Potential Prognostic Factors: A Systematic Review" Life 15, no. 3: 388. https://doi.org/10.3390/life15030388
APA StyleMaria, L. F. B. S., Batista, J. E. T., Wachira, V. K., Junior, W. B. C., Soares, A. A. d. S. M., Carvalho, I. P. S. F. d., & Peixoto, H. M. (2025). Factors Associated with Post-COVID Cardiac Conditions and Potential Prognostic Factors: A Systematic Review. Life, 15(3), 388. https://doi.org/10.3390/life15030388






