Technical and Performance Characteristics Between Different Anti-Mullerian Hormone (AMH) Assay Methods and Antral Follicle Count (AFC) in Malaysian Women with Infertility: A University-Based Centre Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Method Comparison
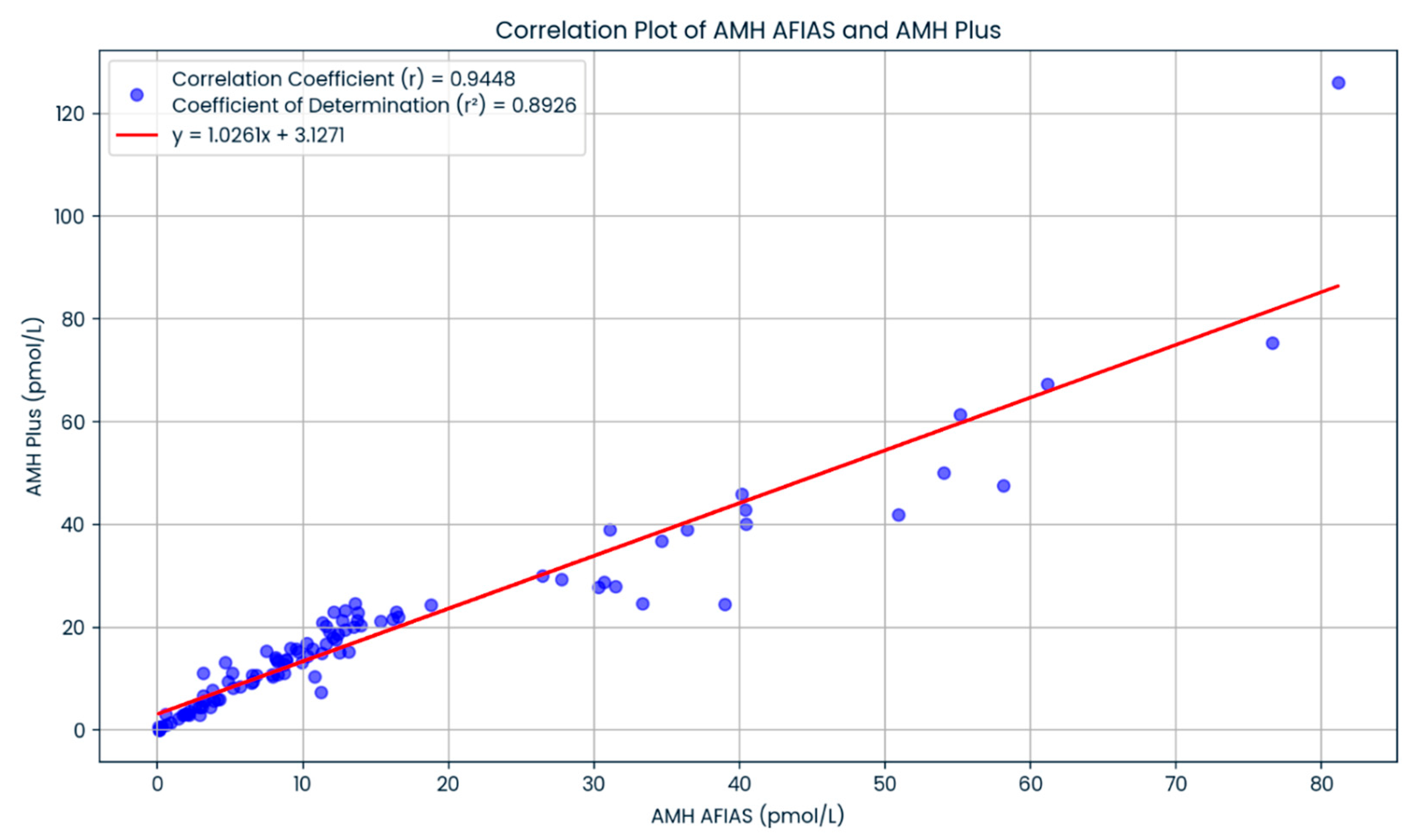
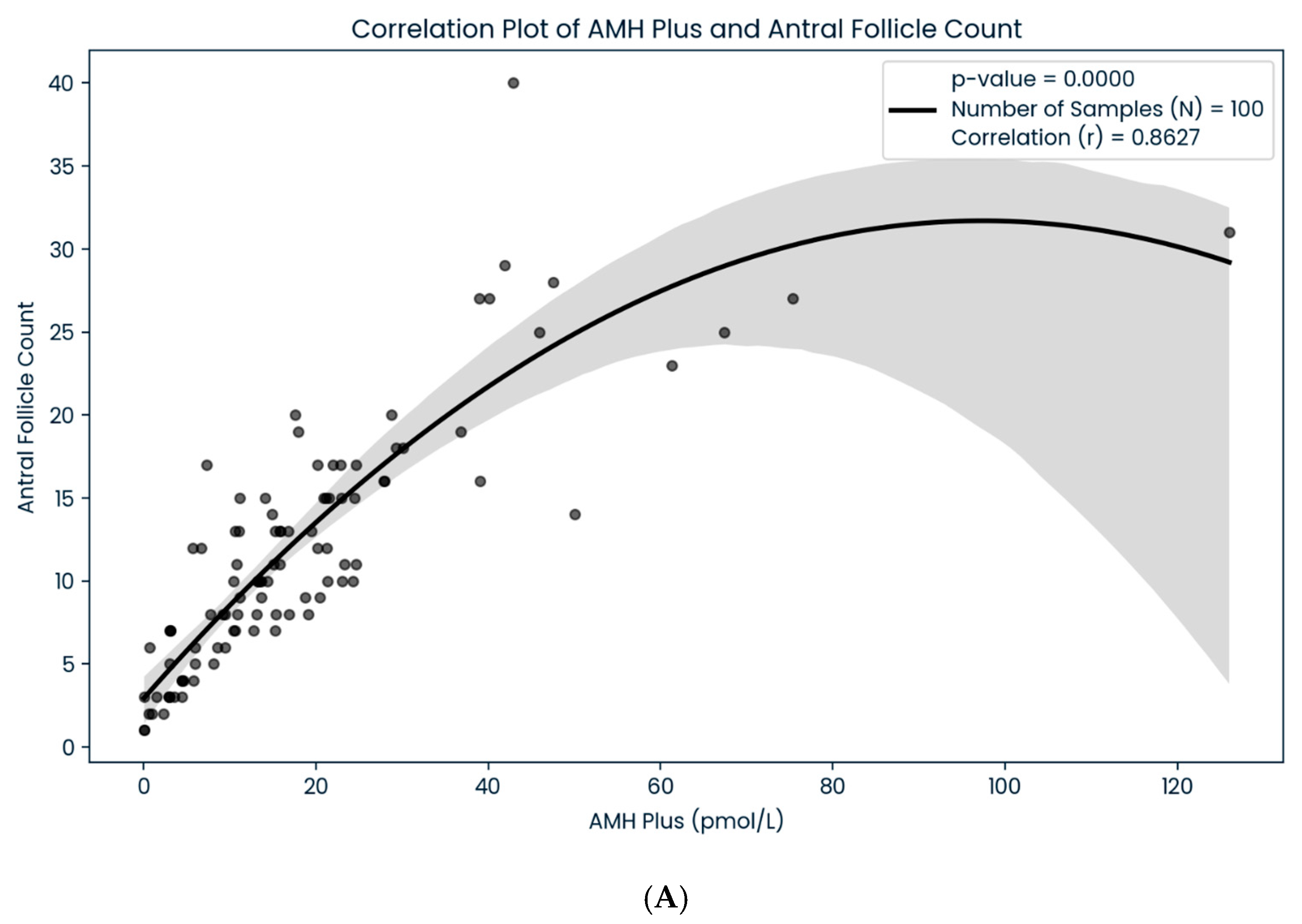
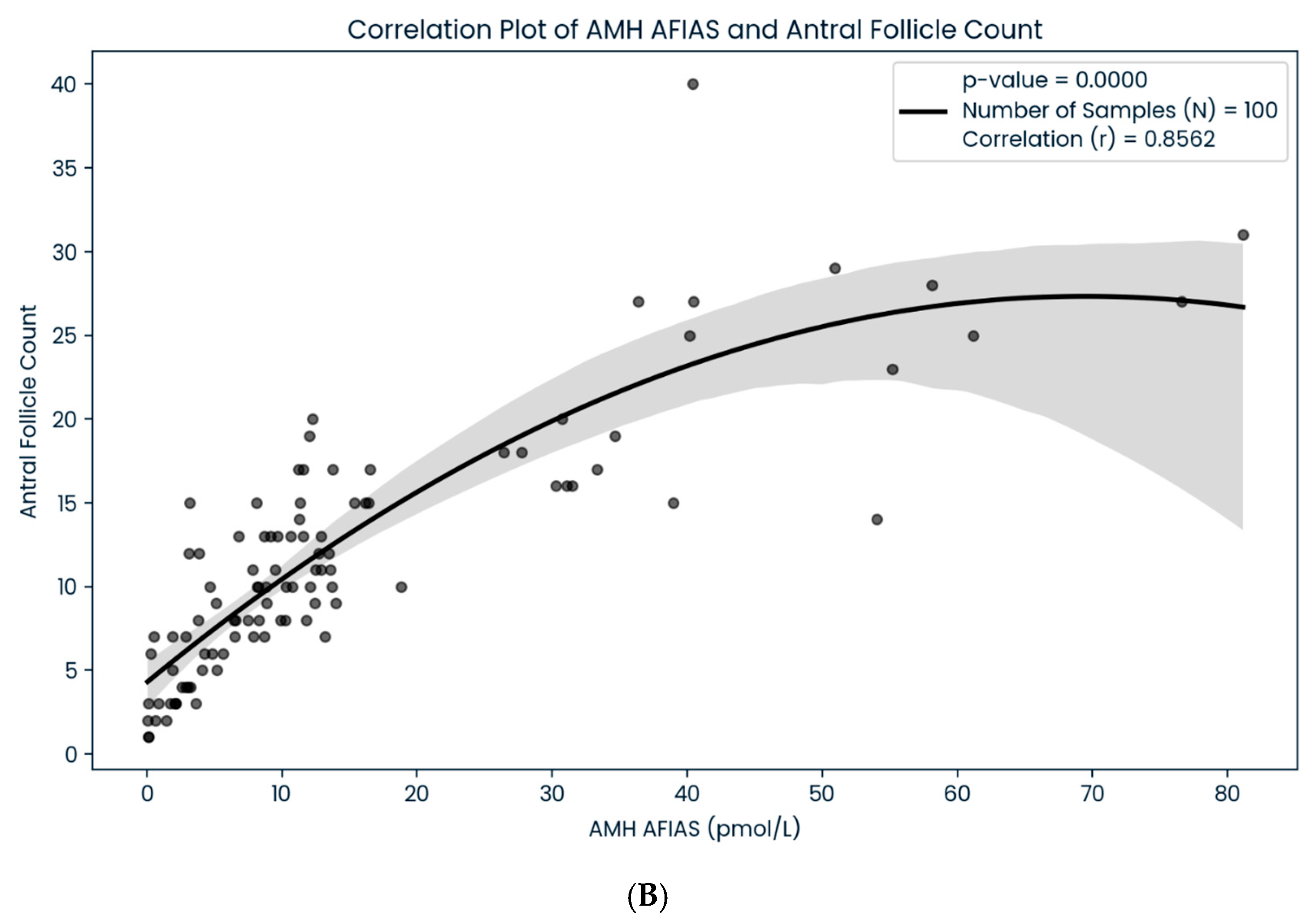
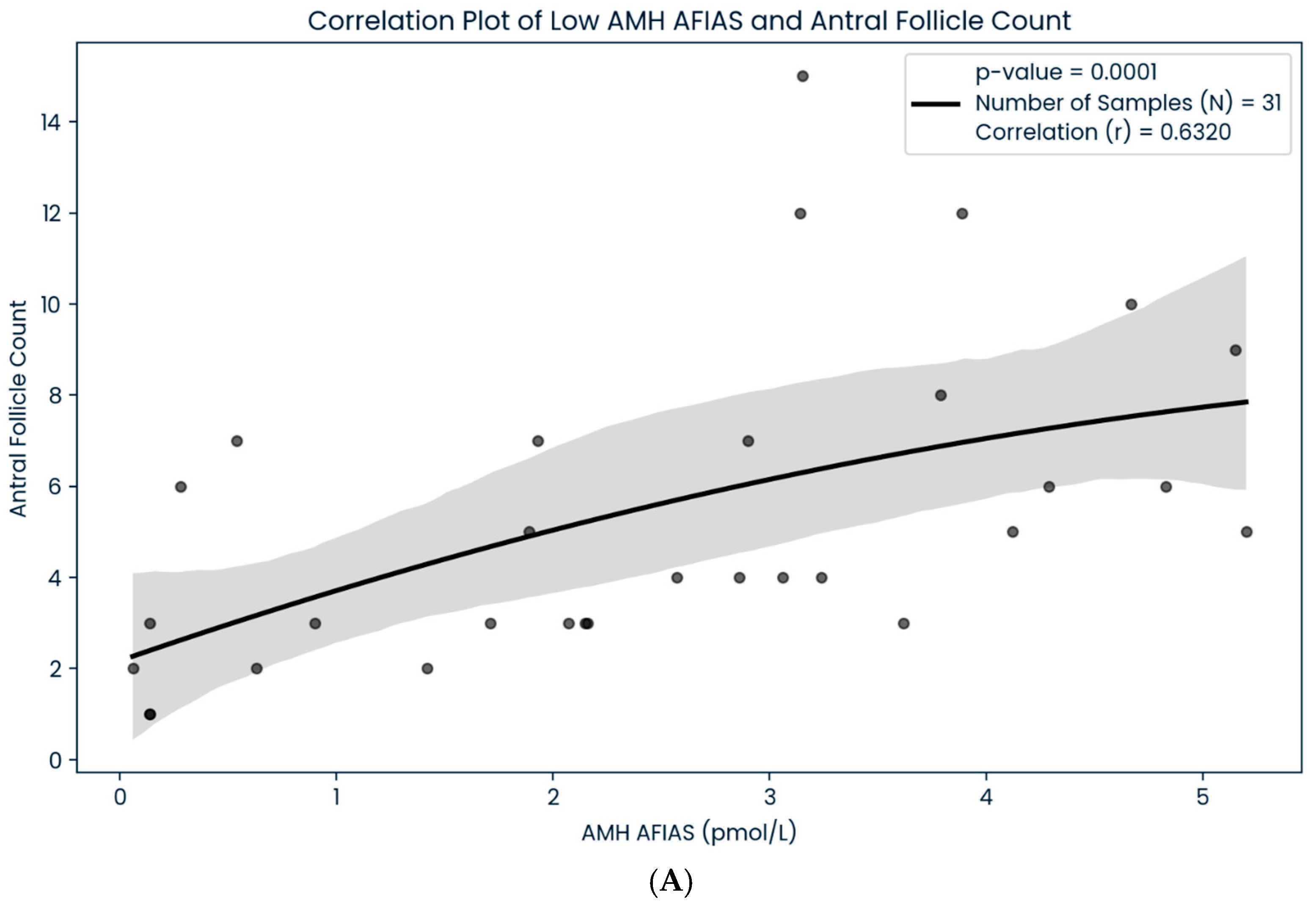
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gleicher, N.; Weghofer, A.; Barad, D.H. Defining ovarian reserve to better understand ovarian aging. Reprod. Biol. Endocrinol. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Keilty, D.; Zhang, Z.F.; Chian, R.C. Mitochondria in oocyte aging: Current understanding. Facts Views Vis. Obgyn 2017, 9, 29–38. [Google Scholar] [PubMed]
- Wang, X.; Jin, L.; Mao, Y.-D.; Shi, J.-Z.; Huang, R.; Jiang, Y.-N.; Zhang, C.-L.; Liang, X.-Y. Evaluation of ovarian reserve tests and age in the prediction of poor ovarian response to controlled ovarian stimulation—A real-world data analysis of 89,002 patients. Front. Endocrinol. 2021, 12, 702061. [Google Scholar] [CrossRef]
- Ferguson, J.; Hockley, J.; Rigsby, P.; Burns, C. Establishment of a WHO Reference Reagent for anti-Mullerian hormone. Reprod. Biol. Endocrinol. 2020, 18, 86. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Haguenauer, O.; Picard, J.; Mattei, M.-G.; Serero, S.; Van Cong, N.; De Tand, M.-F.; Guerrier, D.; Hors-Cayla, M.-C.; Josso, N.; Frézal, J. Mapping of the gene for anti-Müllerian hormone to the short arm of human chromosome 19. Cytogenet. Genome Res. 1987, 44, 2–6. [Google Scholar] [CrossRef]
- van Disseldorp, J.; Lambalk, C.; Kwee, J.; Looman, C.; Eijkemans, M.; Fauser, B.; Broekmans, F. Comparison of inter-and intra-cycle variability of anti-Müllerian hormone and antral follicle counts. Hum. Reprod. 2010, 25, 221–227. [Google Scholar] [CrossRef]
- Sowers, M.; McConnell, D.; Gast, K.; Zheng, H.; Nan, B.; McCarthy, J.D.; Randolph, J.F. Anti-Müllerian hormone and inhibin B variability during normal menstrual cycles. Fertil. Steril. 2010, 94, 1482–1486. [Google Scholar] [CrossRef]
- Gracia, C.R.; Shin, S.S.; Prewitt, M.; Chamberlin, J.S.; Lofaro, L.R.; Jones, K.L.; Clendenin, M.; Manzanera, K.E.; Broyles, D.L. Multi-center clinical evaluation of the Access AMH assay to determine AMH levels in reproductive age women during normal menstrual cycles. J. Assist. Reprod. Genet. 2018, 35, 777–783. [Google Scholar] [CrossRef]
- Li, H.; Wong, B.; Ip, W.; Yeung, W.; Ho, P.; Ng, E. Comparative evaluation of three new commercial immunoassays for anti-Müllerian hormone measurement. Hum. Reprod. 2016, 31, 2796–2802. [Google Scholar] [CrossRef]
- Magnusson, Å.; Oleröd, G.; Thurin-Kjellberg, A.; Bergh, C. The correlation between AMH assays differs depending on actual AMH levels. Hum. Reprod. Open 2017, 2017, hox026. [Google Scholar] [CrossRef]
- Pearson, K.; Long, M.; Prasad, J.; Wu, Y.Y.; Bonifacio, M. Assessment of the Access AMH assay as an automated, high-performance replacement for the AMH Generation II manual ELISA. Reprod. Biol. Endocrinol. 2016, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Smith, K.; Nelson, S.M. A single-centre evaluation of two new anti-Müllerian hormone assays and comparison with the current clinical standard assay. Hum. Reprod. 2014, 29, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Punchoo, R.; Bhoora, S. Variation in the Measurement of Anti-Müllerian Hormone–What Are the Laboratory Issues? Front. Endocrinol. 2021, 12, 719029. [Google Scholar] [CrossRef]
- Szmelskyj, I.; Aquilina, L.; Szmelskyj, A.O. Orthodox medical tests and investigations. In Acupuncture for IVF and Assisted Reproduction; Szmelskyj, I., Aquilina, L., Szmelskyj, A.O., Eds.; Churchill Livingstone: London, UK, 2015; pp. 73–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Xue, Q.; Shang, J.; Yang, X.; Shan, X.; Kuai, Y.; Wang, S.; Zeng, C. Discordance between antral follicle counts and anti-Müllerian hormone levels in women undergoing in vitro fertilization. Reprod. Biol. Endocrinol. 2019, 17, 51. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Electronic address, a.a.o.; Practice Committee of the American Society for Reproductive, M. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef]
- Hyldgaard, J.; Bor, P.; Ingerslev, H.J.; Tørring, N. Comparison of two different methods for measuring anti-mullerian hormone in a clinical series. Reprod. Biol. Endocrinol. 2015, 13, 107. [Google Scholar] [CrossRef]
- Broekmans, F.J.; de Ziegler, D.; Howles, C.M.; Gougeon, A.; Trew, G.; Olivennes, F. The antral follicle count: Practical recommendations for better standardization. Fertil. Steril. 2010, 94, 1044–1051. [Google Scholar] [CrossRef]
- Iliodromiti, S.; Nelson, S.M. Ovarian response biomarkers: Physiology and performance. Curr. Opin. Obstet. Gynecol. 2015, 27, 182–186. [Google Scholar] [CrossRef]
- O’Flynn, N. Assessment and treatment for people with fertility problems: NICE guideline. Br. J. Gen. Pract. 2014, 64, 50–51. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.; Louwers, Y.V.; Laven, J.S.; Visser, J.A. Comparison of 3 different AMH assays with AMH levels and follicle count in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2022, 107, e3714–e3722. [Google Scholar] [CrossRef]
- Han, A.; Suh, B.; Yi, G.; Lee, Y.J.; Kim, S.E. Comparison of the automated fluorescent immunoassay system with Roche Elecsys and Beckman Coulter access 2 assays for anti-Müllerian hormone measurement. Ann. Lab. Med. 2022, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.R.; Robertson, D.M.; Burns, C.; Ledger, W.L. Challenges in measuring AMH in the clinical setting. Front. Endocrinol. 2021, 12, 691432. [Google Scholar] [CrossRef]
- Grynberg, M.; Genro, V.; Arbo, E.; Frydman, N.; Frydman, R.; Fanchin, R. Serum anti-müllerian hormone (AMH) level/antral follicle count ratio: An alternative approach to optimize the predictability of AMH as a marker of IVF-ET outcome. Fertil. Steril. 2009, 92, S177. [Google Scholar] [CrossRef]
- Saleh, H.; Saleh, H. Comparison between antral follicle count and anti-Müllerian hormonal level in the prediction of ovarian response and pregnancy outcome in intracytoplasmic sperm injection patients: Implications in personalizing ovarian stimulation. Clin. Exp. Obstet. Gynecol. 2020, 47, 166–173. [Google Scholar] [CrossRef]
- Dillon, K.E.; Gracia, C.R. What is normal ovarian reserve? In Seminars in Reproductive Medicine; Thieme Medical Publishers: New York, NY, USA, 2013; pp. 427–436. [Google Scholar]
- Ng, E.H.Y.; Yeung, W.S.B.; Fong, D.Y.T.; Ho, P.C. Effects of age on hormonal and ultrasound markers of ovarian reserve in Chinese women with proven fertility. Hum. Reprod. 2003, 18, 2169–2174. [Google Scholar] [CrossRef][Green Version]
- Almog, B.; Shehata, F.; Shalom-Paz, E.; Tan, S.L.; Tulandi, T. Age-related normogram for antral follicle count: McGill reference guide. Fertil. Steril. 2011, 95, 663–666. [Google Scholar] [CrossRef]
- Wiweko, B.; Prawesti, D.M.P.; Hestiantoro, A.; Sumapraja, K.; Natadisastra, M.; Baziad, A. Chronological age vs biological age: An age-related normogram for antral follicle count, FSH and anti-Mullerian hormone. J. Assist. Reprod. Genet. 2013, 30, 1563–1567. [Google Scholar] [CrossRef]
| Parameter | Percentage (%) |
|---|---|
| Age, years old | |
| <35 | 38 (38%) |
| ≥35 | 62 (62%) |
| Race | |
| Malay | 81 (81%) |
| Chinese | 12 (12%) |
| Indian | 6 (6%) |
| Lain—lain | 1(1%) |
| BMI, kg/m2 | |
| Underweight (<18.5) | 1 (1%) |
| Normal (18.5–23.0) | 26 (26%) |
| Overweight (23.0–27.5) | 33 (33%) |
| Obesity (>27.5) | 40 (40%) |
| Type of Infertility | |
| Primary | 77 (77%) |
| Secondary | 23 (23%) |
| Cause of Infertility | |
| PCOS | 17 (17%) |
| Endometriosis | 24 (24%) |
| Uterine fibroid | 1 (1%) |
| Tubal | 13 (13%) |
| Male | 19 (19%) |
| Unexplained | 26 (26%) |
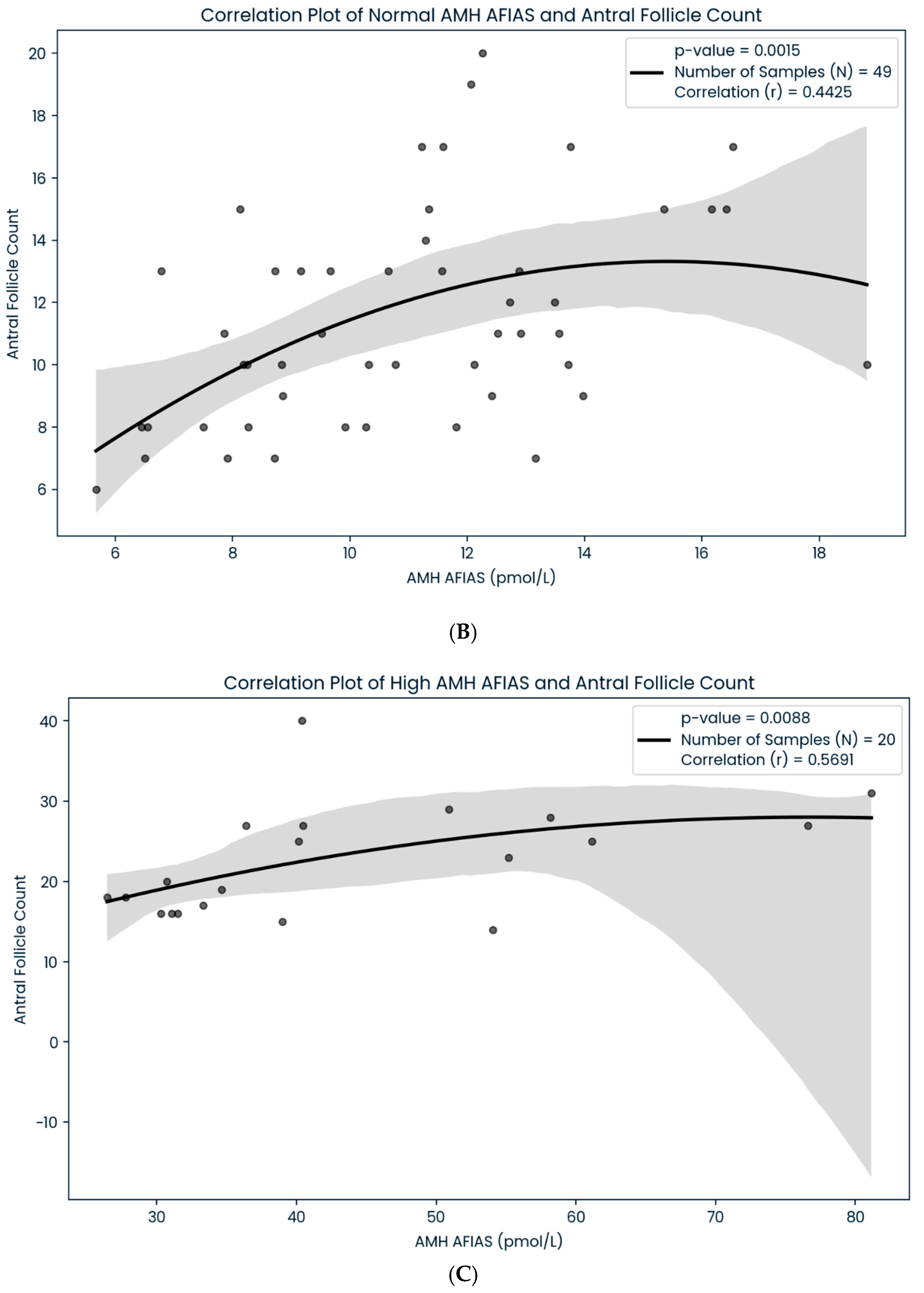
| Scheme 100 | AFIAS-AMH (n:100) Correlation (r) | AMH Plus (n:100) Correlation (r) | p Value |
|---|---|---|---|
| Low (<5.4 pmol/L) | 0.6320 (n: 31) | 0.4516 (n:20) | p < 0.05 |
| Normal (5.5–24.9 pmol/L) | 0.4425 (n: 49) | 0.5135 (n:62) | p < 0.05 |
| High (>25.0 pmol/L) | 0.5691 (n:20) | 0.5965 (n:18) | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.J.; Ahmad, M.F.; Abu, M.A.; Johari, N.S.; Badaruddin, I.A.; Azhar, S.S.; Abdul Karim, A.K. Technical and Performance Characteristics Between Different Anti-Mullerian Hormone (AMH) Assay Methods and Antral Follicle Count (AFC) in Malaysian Women with Infertility: A University-Based Centre Cohort. Life 2025, 15, 383. https://doi.org/10.3390/life15030383
Wen CJ, Ahmad MF, Abu MA, Johari NS, Badaruddin IA, Azhar SS, Abdul Karim AK. Technical and Performance Characteristics Between Different Anti-Mullerian Hormone (AMH) Assay Methods and Antral Follicle Count (AFC) in Malaysian Women with Infertility: A University-Based Centre Cohort. Life. 2025; 15(3):383. https://doi.org/10.3390/life15030383
Chicago/Turabian StyleWen, Chong Jie, Mohd Faizal Ahmad, Muhammad Azrai Abu, Nalisa Shamyra Johari, Izzatul Aliaa Badaruddin, Shah Shamsul Azhar, and Abdul Kadir Abdul Karim. 2025. "Technical and Performance Characteristics Between Different Anti-Mullerian Hormone (AMH) Assay Methods and Antral Follicle Count (AFC) in Malaysian Women with Infertility: A University-Based Centre Cohort" Life 15, no. 3: 383. https://doi.org/10.3390/life15030383
APA StyleWen, C. J., Ahmad, M. F., Abu, M. A., Johari, N. S., Badaruddin, I. A., Azhar, S. S., & Abdul Karim, A. K. (2025). Technical and Performance Characteristics Between Different Anti-Mullerian Hormone (AMH) Assay Methods and Antral Follicle Count (AFC) in Malaysian Women with Infertility: A University-Based Centre Cohort. Life, 15(3), 383. https://doi.org/10.3390/life15030383







