Myocardial Work Analysis in ST-Elevation Myocardial Infarction: Insights into Left Ventricular Ejection Fraction—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Transthoracic Echocardiographic Evaluation
2.3. Myocardial Work Assessment
- Global work index (GWI): Represents the total myocardial work performed from mitral valve closure to its opening, reflecting the area within the pressure-strain loop.
- Global constructive work (GCW): Defined as positive myocardial work during systolic shortening and negative work of diastolic lengthening during isovolumic relaxation.
- Global wasted work (GWW): Refers to negative myocardial work during lengthening of the left ventricle in systole and shortening during isovolumic relaxation.
- Global work efficiency (GWE): Expressed as a percentage, GWE is calculated by dividing GCW by the sum of GCW and GWW.
2.4. Statistical Analysis
3. Results
3.1. Subjects’ Clinical Characteristics
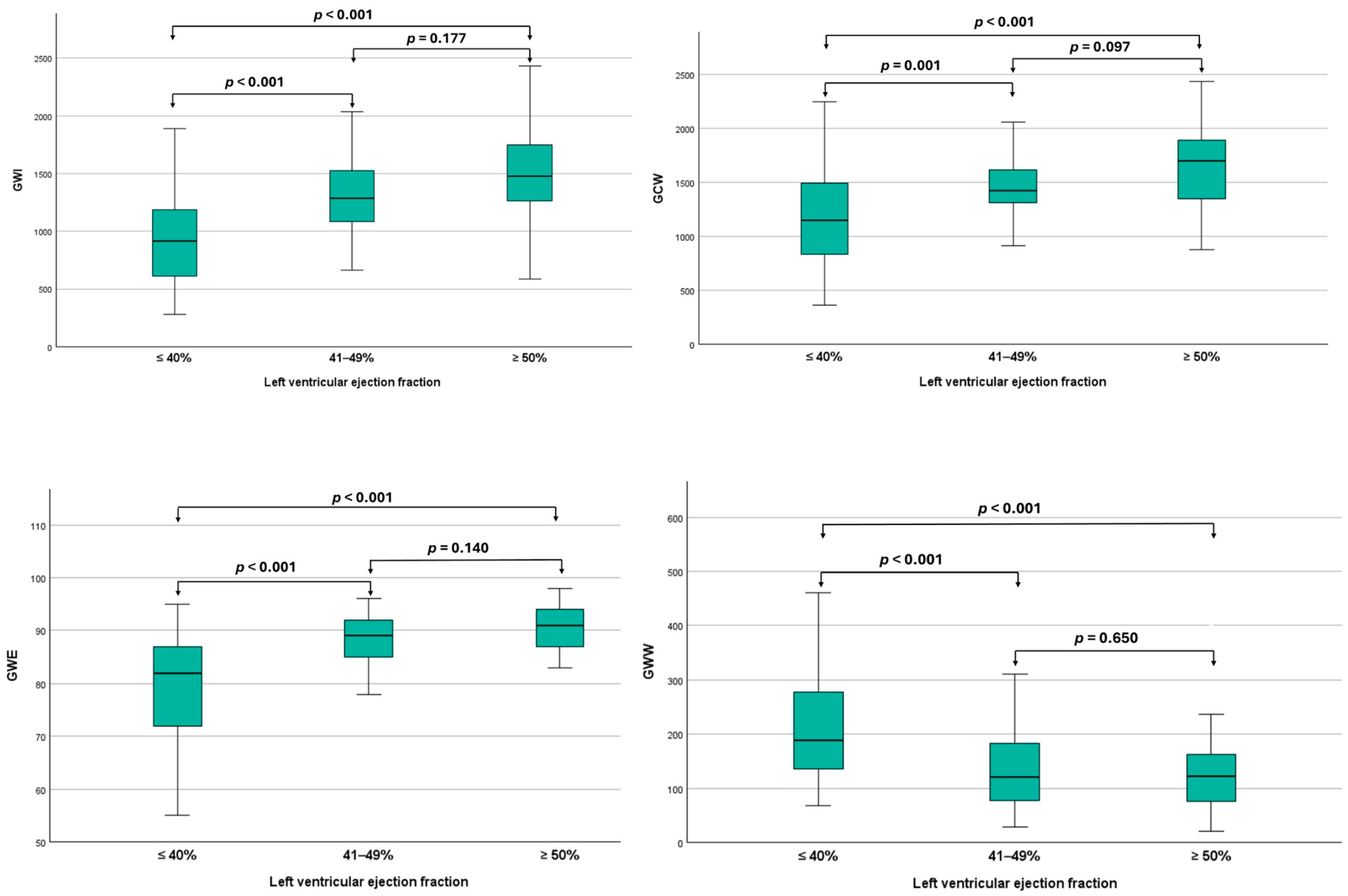
3.2. Myocardial Work Analysis Among Different Ejection Fraction Categories
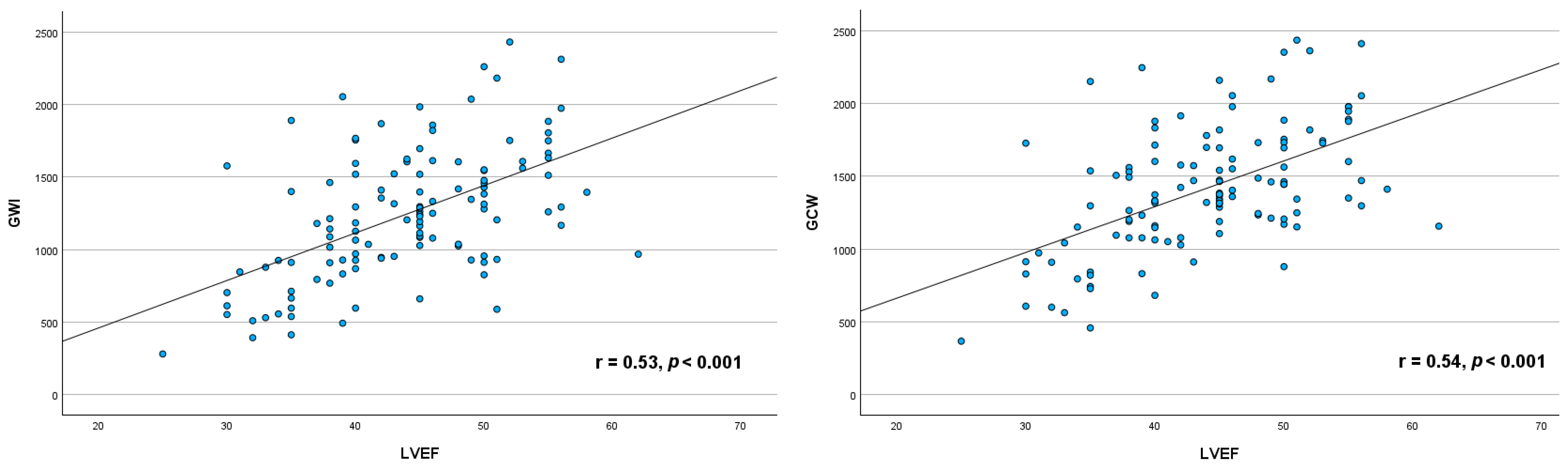
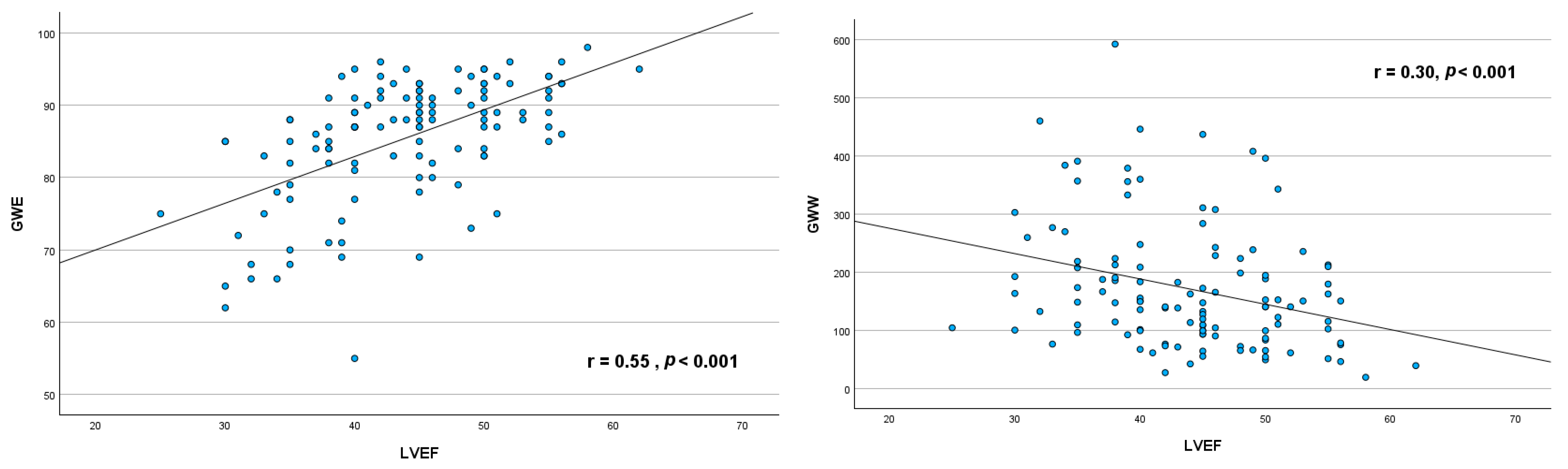
3.3. Relationship Between Myocardial Work Indices and Left Ventricular Ejection Fraction
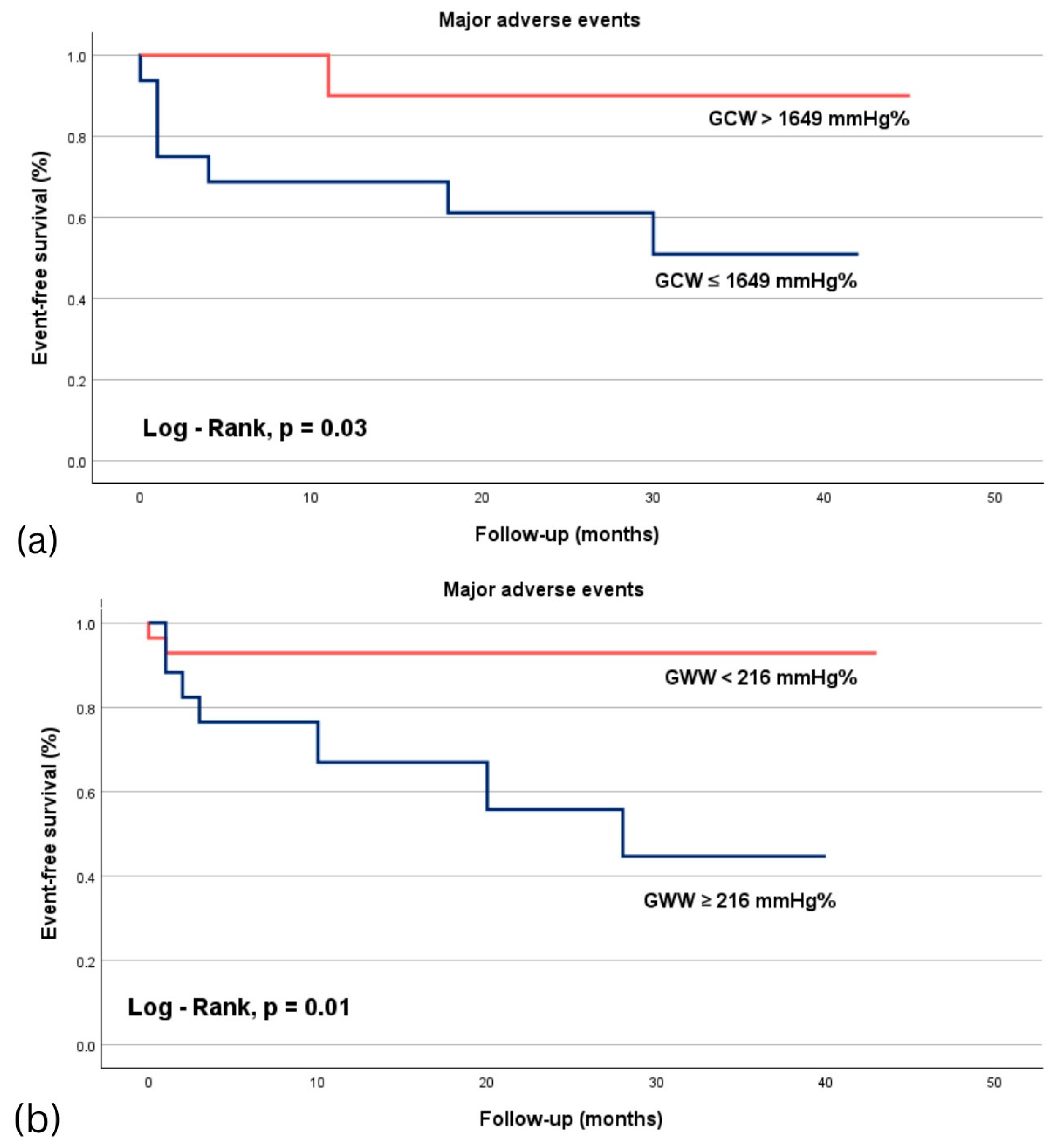
3.4. ROC Analysis in Predicting Major Adverse Events in Acute STEMI Patients
3.5. The Reliability of MW Parameters
4. Discussion
4.1. Differences in MW Indices Across LVEF Categories
4.2. Correlation Between LVEF and MW Indices
4.3. Prognostic Value of MW Indices
4.4. Pathophysiological Implications
4.5. Clinical Implications
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Peak late transmitral flow velocity |
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| e’ | Peak early diastolic mitral annulus velocity |
| E | Peak early diastolic mitral flow velocity |
| GCW | Global Constructive Work |
| GLS | Global Longitudinal Strain |
| GWE | Global Work Efficiency |
| GWI | Global Work Index |
| GWW | Global Wasted work |
| LV | Left ventricle |
| MAE | Major Adverse Event |
| MW | Myocardial Work |
| PCI | Percutaneous Coronary Intervention |
| s’ | Peak systolic mitral annulus velocity |
| STEMI | ST-elevation myocardial infarction |
References
- Otero-García, O.; Cid-Álvarez, A.B.; Juskova, M.; Álvarez-Álvarez, B.; Tasende-Rey, P.; Gude-Sampedro, F.; García-Acuña, J.M.; Agra-Bermejo, R.; López-Otero, D.; Sanmartín-Pena, J.C.; et al. Prognostic impact of left ventricular ejection fraction recovery in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: Analysis of an 11-year all-comers registry. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, M.; Cui, Y.; Wu, M.; Chen, H. Incidence and predictors of left ventricular function change following ST-segment elevation myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1079647. [Google Scholar] [CrossRef] [PubMed]
- Chew, D.S.; Heikki, H.; Schmidt, G.; Kavanagh, K.M.; Dommasch, M.; Bloch Thomsen, P.E.; Sinnecker, D.; Raatikainen, P.; Exner, D.V. Change in Left Ventricular Ejection Fraction Following First Myocardial Infarction and Outcome. JACC Clin. Electrophysiol. 2018, 4, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Solomon, S.D. Classification of Heart Failure According to Ejection Fraction: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 29, 3217–3225. [Google Scholar] [CrossRef]
- Crişan, S.; Petrescu, L.; Lazăr, M.A.; Văcărescu, C.; Nicola, A.R.; Cozma, D.; Mornoş, C.; Luca, C.T. Reduced ejection fraction heart failure—New data from multicenter studies and national registries regarding general and elderly populations: Hopes and disappointments. Clin. Interv. Aging 2018, 13, 651–656. [Google Scholar] [CrossRef]
- Abou, R.; van der Bijl, P.; Bax, J.J.; Delgado, V. Global longitudinal strain: Clinical use and prognostic implications in contemporary practice. Heart 2020, 106, 1438–1444. [Google Scholar] [CrossRef]
- Grapsa, J. Left Ventricular Ejection Fraction and Global Longitudinal Strain: Prognostic When Not Load Dependent? J. Am. Coll. Cardiol. 2018, 28, 1065–1066. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Ilardi, F.; D’Andrea, A.; D’Ascenzi, F.; Bandera, F.; Benfari, G.; Esposito, R.; Malagoli, A.; Mandoli, G.E.; Santoro, C.; Russo, V.; et al. Myocardial Work by Echocardiography: Principles and Applications in Clinical Practice. J. Clin. Med. 2021, 10, 4521. [Google Scholar] [CrossRef]
- Wang, C.L.; Chan, Y.H.; Wu, V.C.; Lee, H.F.; Hsiao, F.C.; Chu, P.H. Incremental prognostic value of global myocardial work over ejection fraction and global longitudinal strain in patients with heart failure and reduced ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 348–356. [Google Scholar] [CrossRef]
- Edwards, N.F.A.; Scalia, G.M.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Khandheria, B.K.; Chan, J. Global myocardial work is superior to global longitudinal strain to predict significant coronary artery disease in patients with normal left ventricular function and wall motion. J. Am. Soc. Echocardiogr. 2019, 32, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 14, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 2023, 44, 3627–3639. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, 996–1003. [Google Scholar] [CrossRef]
- Moya, A.; Buytaert, D.; Penicka, M.; Bartunek, J.; Vanderheyden, M. State-of-the-Art: Noninvasive Assessment of Left Ventricular Function Through Myocardial Work. J. Am. Soc. Echocardiogr. 2023, 36, 1027–1042. [Google Scholar] [CrossRef]
- Papadopoulos, K.; Ozden, T.O.; Mitrousi, K.; Ikonimidis, I. Myocardial work: Methodology and clinical applications. Diagnostics 2021, 11, 573. [Google Scholar] [CrossRef]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef]
- Reindl, M.; Holzknecht, M.; Tiller, C.; Lechner, I.; Schiestl, M.; Simma, F.; Pamminger, M.; Henninger, B.; Mayr, A.; Klug, G.; et al. Impact of infarct location and size on clinical outcome after ST-elevation myocardial infarction treated by primary percutaneous coronary intervention. Int. J. Cardiol. 2020, 15, 14–20. [Google Scholar] [CrossRef]
- Babina, A.; Galimskaya, V.; Golubeva, A.; Korenkova, K.; Burko, N.; Oleinikov, V. Correlation relationships of myocardial work indices with left ventricular ejection fraction and postsystolic strain index in healthy individuals. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 2047–2404. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Y.; Wang, X.; Yang, C.; Li, Y.; Meng, X.; Pei, Z.; Zhang, R.; Zhong, Y.; Wang, F. Myocardial Work by Speckle Tracking Echocardiography Accurately Assesses Left Ventricular Function of Coronary Artery Disease Patients. Front. Cardiovasc. Med. 2021, 8, 727389. [Google Scholar] [CrossRef] [PubMed]
- Chilingaryan, A.; Tunyan, L.G.; Tumasyan, L.R.; Asatryan, A.A.; Sisakyan, J.G.; Kzhdryan, H.K.; Adamyan, K.G.; Zelveyan, P.H. Global constructive myocardial work predicts reduction of ejection fraction in patients with heart failure with preserved ejection fraction. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.786. [Google Scholar] [CrossRef]
- Riolet, C.; Menet, A.; Mailliet, A.; Binda, C.; Altes, A.; Appert, L.; Castel, A.L.; Delelis, F.; Viart, G.; Guyomar, Y.; et al. Clinical Significance of Global Wasted Work in Patients with Heart Failure Receiving Cardiac Resynchronization Therapy. J. Am. Soc. Echocardiogr. 2021, 34, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Barroso, A.C.; Juliano, M.T.H.; Barbosa, M.M.; Gama, G.T.; Melo, R.J.L.; Barbosa, J.B.; Gama, C.A.V.; Filho, J.X.M.; Araujo, A.C. Increased Wasted Myocardial Work as an Indicator of Significant Coronary Lesion. Arq. Bras. Cardiol. Imagem. Cardiovasc. 2022, 35, eabc346. [Google Scholar]
- Samset, E. Evaluation of Segmental Myocardial Work in the Left Ventricle. 2017. Available online: https://www.gehealthcare.com/-/media/8cab29682ace4ed7841505f813001e33.pdf (accessed on 27 December 2024).
- Skulstad, H.; Edvardsen, T.; Urheim, S.; Rabben, S.I.; Stugaard, M.; Lyseggen, E.; Ihlen, H.; Smiseth, O.A. Postsystolic shortening in ischemic myocardium: Active contraction or passive recoil? Circulation 2002, 6, 718–724. [Google Scholar] [CrossRef]
- Brainin, P.; Haahr-Pedersen, S.; Sengeløv, M.; Olsen, F.J.; Fritz-Hansen, T.; Jensen, J.S.; Biering-Sørensen, T. Presence of post-systolic shortening is an independent predictor of heart failure in patients following ST-segment elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2018, 34, 751–760. [Google Scholar] [CrossRef]
- Lustosa, R.P.; Fortuni, F.; van der Bijl, P.; Mahdiui, M.E.; Montero-Cabezas, J.M.; Kostyukevich, M.V.; Knuuti, J.; Marsan, N.A.; Delgado, V.; Bax, J.J. Changes in Global Left Ventricular Myocardial Work Indices and Stunning Detection 3 Months After ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2021, 157, 15–21. [Google Scholar] [CrossRef]
- Colli-Franzone, P.; Gionti, V.; Pavarino, L.F.; Scacchi, S.; Storti, C. Role of infarct scar dimensions, border zone repolarization properties and anisotropy in the origin and maintenance of cardiac reentry. Math. Biosci. 2019, 315, 108228. [Google Scholar] [CrossRef]
- De Sousa Bispo, J.P.; Azevedo, P.; Freitas, P.; Marques, N.; Reis, C.; Horta, E.; Trabulo, M.; Abecasis, J.; Canada, M.; Ribeiras, R.; et al. Mechanical Dispersion as a powerful echocardiographic predictor of outcomes after Myocardial Infarction. Eur. Heart J. 2020, 41 (Suppl. S2), ehaa946.0126. [Google Scholar] [CrossRef]
- Mollema, S.A.; Liem, S.S.; Suffoletto, M.S.; Bleeker, G.B.; van der Hoeven, B.L.; van de Veire, N.R.; Boersma, E.; Holman, E.R.; van der Wall, E.E.; Schalij, M.J.; et al. Left ventricular dyssynchrony acutely after myocardial infarction predicts left ventricular remodeling. J. Am. Coll. Cardiol. 2007, 50, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Mirea, O.; Berceanu, M.; Soldea, S.; Donoiu, I.; Raicea, V. Classification of longitudinal strain curves measured by speckle-tracking echocardiography in normal and pathological myocardial segments. Rom. J. Cardiol. 2023, 33, 4. [Google Scholar] [CrossRef]
- Iwahashi, N.; Kirigaya, J.; Gohbara, M.; Abe, T.; Horii, M.; Hanajima, Y.; Toya, N.; Takahashi, H.; Kirigaya, H.; Minamimoto, Y.; et al. Mechanical dispersion combined with global longitudinal strain estimated by three dimensional speckle tracking in patients with ST elevation myocardial infarction. Int. J. Cardiol. Heart Vasc. 2022, 40, 101028. [Google Scholar] [CrossRef] [PubMed]

| Variable | All Patients (n = 119) | STEMI with EF ≥50% (n = 33) | STEMI with EF 41–49% (n = 41) | STEMI with EF ≤40% (n = 45) | p Value |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Man, n (%) | 93 (78.2) | 28 (84.8) | 33 (80.5) | 32 (71.1) | 0.326 |
| Age, years | 58 ± 11 | 54 ± 9 | 57 ± 12 | 60 ± 11 | 0.339 |
| BMI, kg/m2 | 27.4 (24.9–31.06) | 27.6 (25.9–30.6) | 28.4 (25.9–31.2) | 27.4 (25.1–32.1) | 0.979 |
| BSA, m2 | 2.0 ± 0.2 | 1.9 ± 0.1 | 2 ± 0.2 | 1.9 ± 0.2 | 0.755 |
| SBP, mmHg | 146 ± 24 | 148 ± 28 | 145 ± 22 | 145 ± 22 | 0.734 |
| DBP, mmHg | 88 ± 15 | 89 ± 14 | 90 ± 16 | 86 ± 14 | 0.837 |
| Heart rate, bpm | 79 ± 14 | 77 ± 11 | 78 ± 12 | 82 ± 17 | 0.095 |
| Cardiovascular risk factors | |||||
| Dyslipidemia, n (%) | 113 (95.0) | 32 (97.0) | 39 (95.1) | 42 (93.3) | 0.877 |
| Hypertension, n (%) | 90 (75.6) | 22 (66.7) | 30 (73.2) | 38 (84.4) | 0.176 |
| Smoking, n (%) | 79 (66.4) | 24 (72.7) | 27 (65.9) | 28 (62.2) | 0.622 |
| Diabetes, n (%) | 24 (20.2) | 6 (18.2) | 7 (17.1) | 11 (24.4) | 0.658 |
| Cardiac inheritance, n (%) | 8 (6.7) | 2 (6.1) | 1 (2.4) | 5 (11.1) | 0.340 |
| Previous CAD, n (%) | 4 (3.4) | 0 (0.0) | 3 (7.3) | 1 (2.2) | 0.316 |
| Killip class at admission | |||||
| Class I, n (%) | 99 (83.2) | 31 (93.9) | 38 (92.7) | 30 (66.7) | <0.001 |
| Class II, n (%) | 17 (14.3) | 1 (3.0) | 3 (7.3) | 13 (28.9) | 0.003 |
| Class III, n (%) | 2 (1.7) | 1 (3.0) | 0 (0.0) | 1 (2.2) | 0.735 |
| Class IV, n (%) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 1.000 |
| Culprit vessel | |||||
| Left main coronary artery, n (%) | 1 (0.8) | 0 (0) | 1 (2.4) | 0 (0) | 0.383 |
| Left anterior descending artery, n (%) | 52 (43.7) | 7 (21.2) | 17 (41.5) | 28 (62.2) | 0.001 |
| Circumflex coronary artery, n (%) | 22 (18.5) | 7 (21.2) | 9 (22.0) | 6 (13.3) | 0.527 |
| Right coronary artery, n (%) | 44 (37.0) | 19 (57.6) | 14 (34.1) | 11 (24.4) | 0.010 |
| Number of affected vessels | |||||
| Single-vessel disease, n (%) | 55 (46.2) | 20 (60.6) | 15 (36.6) | 20 (44.4) | 0.114 |
| Two-vessel disease, n (%) | 40 (33.6) | 7 (21.2) | 18 (43.9) | 15 (33.3) | 0.121 |
| Three-vessel disease, n (%) | 24 (20.2) | 6 (18.2) | 8 (19.5) | 10 (22.2) | 0.900 |
| Treatment at discharge | |||||
| Aspirin, n (%) | 120 (99.2) | 33 (100) | 41 (100) | 44 (97.8) | 1.00 |
| P2Y12i, n (%) | 121 (100) | 34 (100) | 42 (100) | 45 (100) | |
| Statins, n (%) | 121 (100) | 30 (100) | 36 (100) | 37 (100) | |
| ACE inhibitor/ARBs n (%) | 79 (66.4) | 24 (72.7) | 32 (78.0) | 23 (51.1) | 0.020 |
| Nitrate, n (%) | 54 (45.4) | 12 (36.4) | 22 (53.7) | 20 (44.4) | 0.328 |
| Betablocker, n (%) | 87 (73.1) | 22 (66.7) | 30 (73.2) | 35 (77.8) | 0.550 |
| Loop diuretics, n (%) | 90 (75.6) | 17 (51.5) | 32 (78.0) | 41 (91.1) | <0.001 |
| MRAs, n (%) | 93 (78.2) | 19 (57.6) | 32 (78.0) | 42 (93.3) | 0.001 |
| SGLT2i, n (%) | 19 (16.0) | 1 (3.0) | 2 (4.9) | 16 (35.6) | <0.001 |
| Variable | All Patients (n = 119) | STEMI with EF ≥50% (n = 33) | STEMI with EF 41–49% (n = 41) | STEMI with EF ≤40% (n = 45) | p Value |
|---|---|---|---|---|---|
| Glycemia, mg/dL | 126 (108–152) | 114 (103–135) | 123 (108–144) | 148 (110–187) | 0.064 |
| Total cholesterol, mg/dL | 189 ± 42 | 190 ± 40 | 191 ± 47 | 185 ± 40 | 0.940 |
| LDLc, mg/dL | 118 ± 29 | 120.9 ± 31.3 | 119.0 ± 27.1 | 116.1 ± 30.1 | 0.875 |
| Triglycerides, mg/dL | 134 (95–169) | 151 (97–200) | 143 (95–172) | 120 (78–151) | 0.156 |
| Creatinine level, mg/dL | 0.95 (0.83–1.13) | 0.90 (0.82–1.04) | 0.92 (0.77–1.10) | 0.95 (0.84–1.17) | 0.699 |
| eGFR, ml/min/1.73 m2 | 85.0 ± 21.6 | 88.8 ± 18.9 | 87.9 ± 21.4 | 79.3 ± 23.2 | 0.468 |
| Hemoglobin, g/dL | 14.6 ± 1.5 | 14.6 ± 1.5 | 14.5 ± 1.5 | 14.5 ± 1.4 | 0.976 |
| Leukocytes, 103/µL | 12.0 ± 3.3 | 11.4 ± 3.5 | 12.2 ± 3.5 | 12.2 ± 3.1 | 0.243 |
| Neutrophiles, 103/µL | 7.44 (6.47–8.17) | 7.44 (6.57–8.15) | 7.52 (6.80–8.18) | 7.97 (7.24–8.52) | 0.377 |
| ESR, mm/h | 12 (6–18) | 10 (6–14) | 10 (6–15) | 10 (5–20) | 0.560 |
| Troponin I, ng/L * | 6849 (333–34,857) | 1405 (188–13,339) | 9337 (1182–40,000) | 6942 (262–39,356) | 0.026 |
| CK-MB, U/L * | 221 (95.5–379) | 181 (61–270) | 249 (118–359) | 259 (115–483) | 0.031 |
| ALT, U/L * | 59 (45–90) | 50 (36–71) | 57 (47–81) | 82 (49–103) | 0.020 |
| AST, U/L * | 229 (113–378) | 182 (65–276) | 217 (95–313) | 312 (173–566) | 0.003 |
| Variable | All Patients (n = 119) | STEMI with EF ≥ 50% (n = 33) | STEMI with EF 41–49% (n = 41) | STEMI with EF ≤ 40% (n = 45) | p Value |
|---|---|---|---|---|---|
| LA volume index (mL/m2) | 31.2 ± 10.9 | 30.4 ± 9.1 | 31.2 ± 10.0 | 31.8 ± 12.9 | 0.827 |
| LVEDV (mL) | 109.4 ± 26.1 | 101 ± 25 | 111 ± 29 | 114 ± 23 | 0.014 |
| LVESV (mL) | 62 (45–72) | 45 (41–61) | 60 (47–70) | 68 (54–77) | <0.001 |
| LVEF (%) | 44 ± 7 | 53 ± 3 | 44 ± 2 | 36 ± 3 | <0.001 |
| IVS (cm) | 1.21 ± 0.2 | 1.14 ± 0.2 | 1.25 ± 0.16 | 1.23 ± 0.17 | 0.254 |
| PWT (cm) | 1.15 ± 0.1 | 1.11 ± 0.1 | 1.16 ± 0.1 | 1.18 ± 0.1 | 0.271 |
| E wave (m/s) | 0.65 ± 0.1 | 0.65 ± 0.2 | 0.68 ± 0.1 | 0.64 ± 0.1 | 0.607 |
| A wave (m/s) | 0.75 ± 0.1 | 0.75 ± 0.1 | 0.76 ± 0.2 | 0.76 ± 0.2 | 0.795 |
| E/A ratio | 0.8 (0.6–1.1) | 0.7 (0.5–1.1) | 0.8 (0.7–1.1) | 0.7 (0.6–1.2) | 0.544 |
| e’ wave (m/s) | 0.06 ± 0.02 | 0.07 ± 0.02 | 0.06 ± 0.01 | 0.05 ± 0.01 | <0.001 |
| s’ wave (m/s) | 0.07 ± 0.01 | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.06 ± 0.01 | <0.001 |
| E/e’ ratio | 10 (7.8–12.2) | 9.2 (6.8–10.9) | 10 (8.1–12.5) | 10 (8.1–15.0) | 0.111 |
| LV global longitudinal strain (%) | −12.8 ± 3.4 | −15.3 ± 2.9 | −13.3 ± 2.3 | −10.4 ± 3.1 | <0.001 |
| LV mechanical dispersion (ms) | 74.0 ± 24.4 | 65.8 ± 22.5 | 74.2 ± 22.0 | 80.4 ± 26.6 | 0.061 |
| GWI, mmHg% | 1276 ± 431 | 1540 ± 414 | 1298 ± 308 | 1048 ± 428 | <0.001 |
| GCW, mmHg% | 1445 ± 412 | 1693 ± 386 | 1454 ± 292 | 1243 ± 4229 | <0.001 |
| GWW, mmHg% | 148 (95–213) | 123 (71–158) | 133 (74–199) | 191 (143–305) | <0.001 |
| GWE, % | 88 (82–92) | 92 (87–94) | 89 (84–92) | 84 (73–87) | <0.001 |
| Parameters | AUC (95% CI) | p Value |
|---|---|---|
| Preserved LVEF (≥50%) | ||
| s’ wave | 0.640 (0.425–0.855) | 0.361 |
| E/e’ ratio | 0.378 (0.170–0.585) | 0.248 |
| Global longitudinal strain | 0.643 (0.384–0.901) | 0.280 |
| Mechanical dispersion | 0.650 (0.419–0.881) | 0.203 |
| GWI | 0.725 (0.491–0.959) | 0.060 |
| GCW | 0.730 (0.516–0.944) | 0.035 |
| GWW | 0.533 (0.292–0.773) | 0.791 |
| GWE | 0.625 (0.385–0.8765) | 0.307 |
| Mildly reduced LVEF (41–49%) | ||
| s’ wave | 0.415 (0.228–0.601) | 0.368 |
| E/e’ ratio | 0.406 (0.212–0.600) | 0.447 |
| Global longitudinal strain | 0.531 (0.321–0.740) | 0.775 |
| Mechanical dispersion | 0.571 (0.355–0.787) | 0.519 |
| GWI | 0.616 (0.398–0.835) | 0.298 |
| GCW | 0.645 (0.429–0.862) | 0.189 |
| GWW | 0.482 (0.270–0.695) | 0.870 |
| GWE | 0.561 (0.353–0.770) | 0.564 |
| Reduced LVEF (≤40%) | ||
| s’ wave | 0.469 (0.278–0.660) | 0.756 |
| E/e’ ratio | 0.520 (0.324–0.716) | 0.841 |
| Global longitudinal strain | 0.571 (0.353–0.789) | 0.524 |
| Mechanical dispersion | 0.725 (0.518–0.933) | 0.033 |
| GWI | 0.519 (0.287–0.751) | 0.876 |
| GCW | 0.451 (0.234–0.667) | 0.667 |
| GWW | 0.787 (0.618–0.956) | 0.001 |
| GWE | 0.657 (0.445–0.870) | 0.147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frișan, A.-C.; Lazăr, M.-A.; Șoșdean, R.; Simonescu, M.; Brie, D.-M.; Mornoș, A.; Luca, S.A.; Ionac, I.; Mornoș, C. Myocardial Work Analysis in ST-Elevation Myocardial Infarction: Insights into Left Ventricular Ejection Fraction—A Pilot Study. Life 2025, 15, 338. https://doi.org/10.3390/life15030338
Frișan A-C, Lazăr M-A, Șoșdean R, Simonescu M, Brie D-M, Mornoș A, Luca SA, Ionac I, Mornoș C. Myocardial Work Analysis in ST-Elevation Myocardial Infarction: Insights into Left Ventricular Ejection Fraction—A Pilot Study. Life. 2025; 15(3):338. https://doi.org/10.3390/life15030338
Chicago/Turabian StyleFrișan, Alexandra-Cătălina, Mihai-Andrei Lazăr, Raluca Șoșdean, Marius Simonescu, Daniel-Miron Brie, Aniko Mornoș, Silvia Ana Luca, Ioana Ionac, and Cristian Mornoș. 2025. "Myocardial Work Analysis in ST-Elevation Myocardial Infarction: Insights into Left Ventricular Ejection Fraction—A Pilot Study" Life 15, no. 3: 338. https://doi.org/10.3390/life15030338
APA StyleFrișan, A.-C., Lazăr, M.-A., Șoșdean, R., Simonescu, M., Brie, D.-M., Mornoș, A., Luca, S. A., Ionac, I., & Mornoș, C. (2025). Myocardial Work Analysis in ST-Elevation Myocardial Infarction: Insights into Left Ventricular Ejection Fraction—A Pilot Study. Life, 15(3), 338. https://doi.org/10.3390/life15030338







