Optimizing Allelopathy Screening Bioassays by Using Nano Silver
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reidsma, P.; Accatino, F.; Appel, F.; Gavrilescu, C.; Krupin, V.; Tasevska, G.M.; Meuwissen, M.P.M.; Peneva, M.; Severini, S.; Soriano, B.; et al. Alternative systems and strategies to improve future sustainability and resilience of farming systems across Europe: From adaptation to transformation. Land Use Policy 2023, 134, 106881. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Ofosu, R.; Agyemang, E.; Márton, A.; Pásztor, G.; Taller, J.; Kazinczi, G. Herbicide resistance: Managing weeds in a changing world. Agronomy 2023, 13, 1595. [Google Scholar] [CrossRef]
- Abu-Nassar, J.; Matzrafi, M. Effect of Herbicides on the Management of the Invasive Weed Solanum rostratum Dunal (Solanaceae). Plants 2021, 10, 284. [Google Scholar] [CrossRef]
- Sharma, A.; Vinod, K.; Babar, S.; Mohsin, T.; Gagan, S.; Neha, H.; Sukhmeen, K.; Poonam, Y.; Aditi, B.; Ripu, P.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Pilet-Nayel, M.-L.; Moury, B.; Caffier, V.; Montarry, J.; Kerlan, M.-C.; Fournet, S.; Durel, C.-E.; Delourme, R. Quantitative resistance to plant pathogens in pyramiding strategies for durable crop protection. Front. Plant Sci. 2017, 8, 1838. [Google Scholar] [CrossRef]
- Raimundo, R.; Silva, T.; Ferreira, A.; Santos, B.; Fonseca, D.C.T.R.; Silva, O.; Castanha, C.; Guerrero, B.; Fonseca, F. Agrochemicals residues in human milk, scientific evidence or overestimated risk? Curr. Nutr. Food Sci. 2020, 17, 189–195. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botlas, C.; Rotheray, E.L. Bee declines driven by combined Stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Patel, S.K.S.; Rasool, K.; Lone, N.; Bhatia, S.K.; Seth, C.S.; Ghodake, G.S. Bioinspired silver nanoparticle-based nanocomposites for effective control of plant pathogens: A review. Sci. Total Environ. 2024, 908, 168318. [Google Scholar] [CrossRef]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and its application as a weed management tool: A review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef]
- MacLaren, C.; Storkey, J.; Menegat, A.; Metcalfe, H.; Dehnen-Schmutz, K. An ecological future for weed science to sustain crop production and the environment. A review. Agron. Sustain. Dev. 2020, 40, 24. [Google Scholar] [CrossRef]
- Mallik, A.; Inderjit, K. Problems and prospects in the study of plant allelochemicals: A brief introduction. In Chemical Ecology of Plants: Allelopathy in Aquatic and Terrestrial Ecosystems; Mallik, A.U., Inderjit, K., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2002; pp. 1–5. [Google Scholar]
- Duke, S.O. Proving allelopathy in crop–weed interactions. Weed Sci. 2015, 63, 121–132. [Google Scholar] [CrossRef]
- Hickman, D.; Comont, D.; Rasmussen, A.; Birkett, M. Novel and holistic approaches are required to realize allelopathic potential for weed management. Ecol. Evol. 2023, 13, e10018. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rajeswari, G.; Nirmal, L.; Jacob, S. Synthesis and extraction routes of allelochemicals from plants and microbes: A review. Rev. Anal. Chem. 2021, 40, 293–311. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, L.; Ye, W. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; Van-Kleunen, M. Effect of allelopathy on plant performance: A meta-analysis. Ecol. Lett. 2021, 24, 348–362. [Google Scholar] [CrossRef]
- Girotto, L.; Franco, A.C.; Nunez, C.V.; Oliveira, S.C.; Scheffer De Souza, M.C.; Fachin-Espinar, M.T.; Ferreira, C.S. Phytotoxicity and allelopathic potential of extracts from rhizomes and leaves of Arundo donax, an invasive grass in neotropical savannas. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12440. [Google Scholar] [CrossRef]
- Borghetti, F.; Coutinho de Lima, E.; Ramos Silva, L. A simple procedure for the purification of active fractions in aqueous extracts of plants with allelopathic properties. Acta Bot. Bras. 2013, 27, 50–53. [Google Scholar] [CrossRef]
- Marinov-Serafimov, P.; Golubinova, I. A study of suitability of some conventional chemical preservatives and natural antimicrobial compounds in allelopathic research. Pestic. I Fitomedicina 2015, 30, 233–241. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Rezaei, Z. Carbon quantum dots-based antifungal coating film for active packaging application of avocado. Food Packag. Shelf Life 2022, 33, 100878. [Google Scholar] [CrossRef]
- Dazon, C.; Fierro, V.; Celzard, A.; Witschger, O. Identification of nanomaterials by the volume specific surface area (VSSA) criterion: Application to powder mixes. Nanoscale Adv. 2020, 2, 4908–4917. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z.; Khan, M.; Khan, M. Engineered Nanomaterials in Soil: Their Impact on Soil Microbiome and Plant Health. Plants 2022, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Bakand, S.; Hayes, A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef] [PubMed]
- Afshar, P.; Sedaghat, S. Bio-synthesis of silver nanoparticles using water extract of Satureja hortensis L. and evaluation of the antibacterial properties. Curr. Nanosci. 2016, 12, 90–93. [Google Scholar] [CrossRef]
- Boverhof, D.; Bramante, C.M.; Butala, J.; Clancy, S.F.; Lafranconi, M.; West, J.; Gordon, S. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Durán, N.; Nakazato, G.; Seabra, A. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: An overview and comments. Appl. Microbiol. Biotechnol. 2016, 100, 6555–6570. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.; Shaheen, M.; El-Nekeety, A.; Abdel-Wahhab, M. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J. Saudi Chem. Soc. 2014, 18, 356–363. [Google Scholar] [CrossRef]
- Budhani, S.; Egboluche, N.; Arslan, Z.; Yu, H.; Deng, H. Phytotoxic effect of silver nanoparticles on seed germination and growth of terrestrial plants. J. Environ. Sci. Health 2019, 37, 330–355. [Google Scholar] [CrossRef]
- Lyu, J.; Park, J.; Pandey, L.; Choi, S.; Lee, H.; Saeger, J.; Depuydt, S.; Han, T. Testing the toxicity of metals, phenol, effluents, and receiving waters by root elongation in Lactuca sativa L. Ecotoxicol. Environ. Saf. 2018, 149, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Mardani, H.; Maninang, J.; Appiah, K.; Oikawa, Y.; Azizi, M.; Fujii, Y. Evaluation of biological response of lettuce (Lactuca sativa L.) and weeds to safranal allelochemical of saffron (Crocus sativus) by using static exposure method. Molecules 2019, 24, 1788. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, K.; Wu, B.; Zhou, J.; Lv, Y. Silver nanoparticles with different particle sizes enhance the allelopathic effects of Canada goldenrod on the seed germination and seedling development of lettuce. Ecotoxicology 2018, 27, 1116–1125. [Google Scholar] [CrossRef]
- Vieira, C.; Droste, A. Biomonitors to evaluate the toxic potential of urban solid waste landfill leachate. Ambiente Água 2019, 14, e2326. [Google Scholar] [CrossRef]
- Vieira, C.; Marcona, C.; Droste, A. Phytotoxic and cytogenotoxic assessment of glyphosate on Lactuca sativa L. Braz. J. Biol. 2024, 84, e257039. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Parvez, S.S.; Parvez, M.; Ohmae, Y.; Iida, O. Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biol. Manag. 2003, 3, 233–241. [Google Scholar] [CrossRef]
- Petrova, S.; Nikolov, B.; Marinov-Serafimov, P.; Golubinova, I.; Valchevа, E. Screening of temperature tolerance and adaptive potential of Sorghum vulgare var. technicum [Körn] genotypes. Вulgarian J. Agric. Sci. 2021, 27 (Suppl. S1), 119–124. [Google Scholar]
- Li, Y.; Feng, Y.; Kang, Z.; Zheng, Y.; Zhang, J.; Chen, Y. Changes in soil microbial communities due to biological invasions can reduce allelopathic effects. J. Appl. Ecol. 2017, 54, 1281–1290. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, R.; Singh, R. Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig. J. Nanomater. Biostructures 2010, 5, 427–432. [Google Scholar]
- Golubinova, I.; Nikolov, B.; Petrova, S.; Velcheva, I.; Valcheva, E.; Marinov-Serafimov, P. Effect of Cycocel 750 SL on Germination and Initial Development of Some Sorghum Species. Еcologia Balk. 2020, 12, 11–19. [Google Scholar]
- Thabet, S.G.; Moursi, Y.S.; Karam, M.A.; Graner, A.; Alqudah, A.M. Genetic basis of drought tolerance during seed germination in barley. PLoS ONE 2018, 13, e0206682. [Google Scholar] [CrossRef]
- Nasr, M.; Mansour, S. The use of allelochemicals to delay germination of Astragalus cycluphyllus seeds. J. Agron. 2005, 4, 147–150. [Google Scholar] [CrossRef]
- Gariglio, N.F.; Buyatti, M.; Pillati, R.; Gonzales, R.D.; Acosta, M. Use a germination biossay to test compost maturity of willow (Salix sp.) sawdust. N. Z. J. Crop Hortic. Sci. 2002, 30, 135–139. [Google Scholar] [CrossRef]
- Ayeb-Zakhama, A.; Harzallah-Skhiri, F. Allelopathic activity of extracts of Citharexylum spinosum L. from Tunisia. J. Plant Breed. Crop Sci. 2016, 8, 189–196. [Google Scholar] [CrossRef]
- Niklas, K. Plant height and the properties of some herbaceous stems. Ann. Bot. 1995, 75, 133–142. [Google Scholar] [CrossRef]
- Niklas, K.; Enquist, B. An allometric model for seed plant reproduction. Evol. Ecol. Res. 2003, 5, 79–88. [Google Scholar]
- Rice, E. Allelopathy; Academic Press: New York, NY, USA; Academic Press: San Francisco, CA, USA; Academic Press: London, UK, 1974; 392p. [Google Scholar]
- Reigosa, M.; Souto, X.; Gonzales, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Dimitrova, T.; Serafimov, P. Ecological approach against invasion of Jonson grass (Sorghum halepense (L.) Pers.) though mixed stands of Lucerne with perennial grasses. Herbologia 2007, 8, 13–20. [Google Scholar]
- Cheng, F.; Cheng, Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Ain, Q.; Mushtaq, W.; Shadab, M.; Siddiqui, M.B. Allelopathy: An alternative tool for sustainable agriculture. Physiol. Mol. Biol. Plants 2023, 29, 495–511. [Google Scholar] [CrossRef]
- Aleksieva, A.; Serafimov, P. A study of allelopathyc effect of Amaranthus retroflexus (L.) and Solanum nigrum (L.) in different soybean genotypes. Herbologia 2008, 9, 47–58. [Google Scholar]
- Treber, I.; Baličević, R.; Ravlić, M. Assessment of allelopathic effect of pale persicaria on two soybean cultivars. Herbologia 2015, 15, 31–38. [Google Scholar] [CrossRef]
- Marinov-Serafimov, P.; Katova, A.; Golubinova, I. Allelopathic activity of rhizosphere soil in some perennial grasses. Proc. Union Sci.—Ruse Sect. Agric. Vet. Sci. 2015, 3, 209–215. [Google Scholar]
- Kadioglu, I.; Yanar, Y.; Asav, U. Alellopathic effects of weeds extracts against seed germination of some plants. J. Environ. Biol. 2005, 26, 169–173. [Google Scholar] [PubMed]
- Steven, J.; Burnside, O.; Specht, J.; Swisher, B. Competition and allelopathy between soybeans and weeds. Agron. J. 1984, 76, 523–528. [Google Scholar]
- Stoimenova, I.; Mikova, A.; Aleksieva, S. Role of allelopathy in weed management of crop production. Agric. Sci. 2008, 41, 3–13. (In Bulgarian) [Google Scholar]
- Colman, B.; Wang, S.; Auffan, M.; Wiesner, M.; Bernhardt, E. Antimicrobial effects of commercial silver nanoparticles are attenuated in natural stream water and sediment. Ecotoxicology 2012, 21, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Rao, S. Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum. J. Clust. Sci. 2015, 26, 693–701. [Google Scholar] [CrossRef]
- Wu, S.; Huang, L.; Head, J.; Chen, D.; Kong, I.C.; Tang, Y. Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. J. Pet. Environ. Biotechnol. 2012, 3, 126. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Gurunathan, S.; Chung, I. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 2015, 252, 1031–1046. [Google Scholar] [CrossRef]
- Elsharkawy, E. Allelopathy effects of silver nanoparticle synthesis by green method from Pulicaria undulate. Annu. Res. Rev. Biol. 2019, 32, 1–9. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Prażak, R.; Święciło, A.; Krzepiłko, A.; Michałek, S.; Arczewska, M. Impact of Ag nanoparticles on seed germination and seedling growth of green beans in normal and chill temperatures. Agriculture 2020, 10, 312. [Google Scholar] [CrossRef]
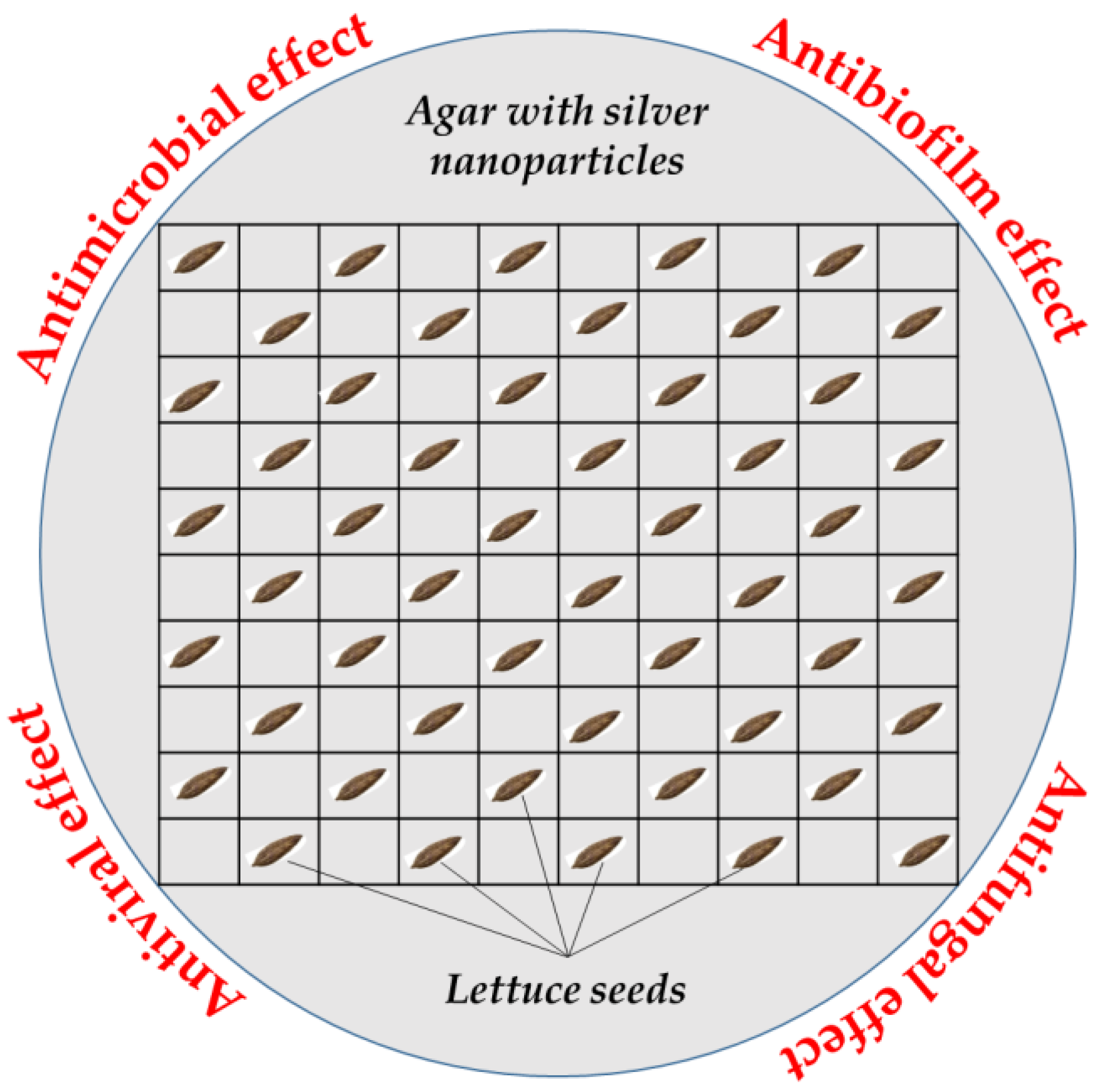
| Parameter/Reference | Formula | Explanation |
|---|---|---|
| Percentage of germinated seeds, GP% Saxena et al. (1996) [39] | NSG—number of germinated seeds TNS—total number of seeds used in all experimental variants and replicates | |
| Length of root, hypocotyl and seedling, cm Golubinova et al. (2020) [40] | I—number of individual measurements of plant organs for all experimental variants and replicates n—number of all measurements | |
| Percentage of inhibition, I% Golubinova et al. (2020) [40] | E1—response of plant seeds into the control E2—response of plant seeds from experimental variants | |
| Reduction of studied parameters, R Thabet et al. (2018) [41] | Average values of biometric indicators of: Gi—experimental variants Gc—control (untreated) variant | |
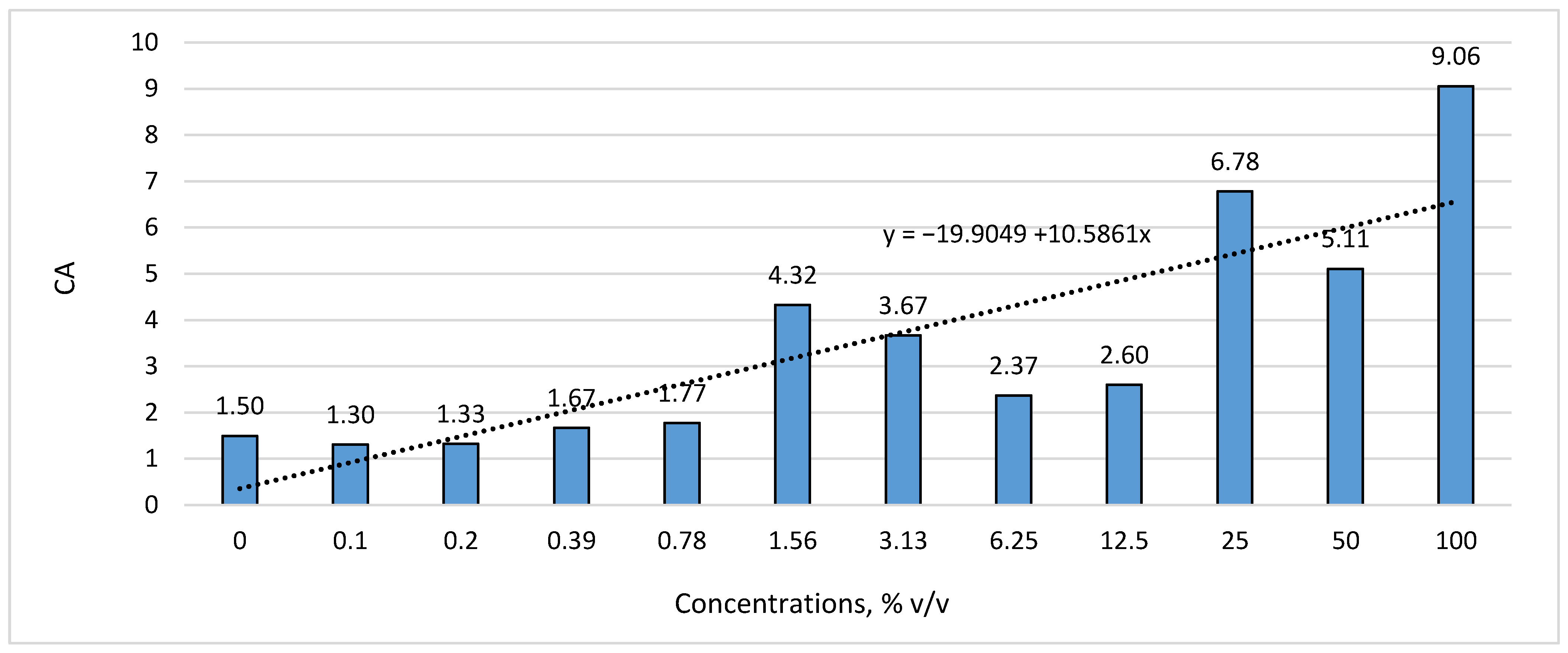
| Coefficient of allometry, CA Nasr and Mansour (2005) [42] | Ls—hypocotyl length, cm Lr—root length, cm | |
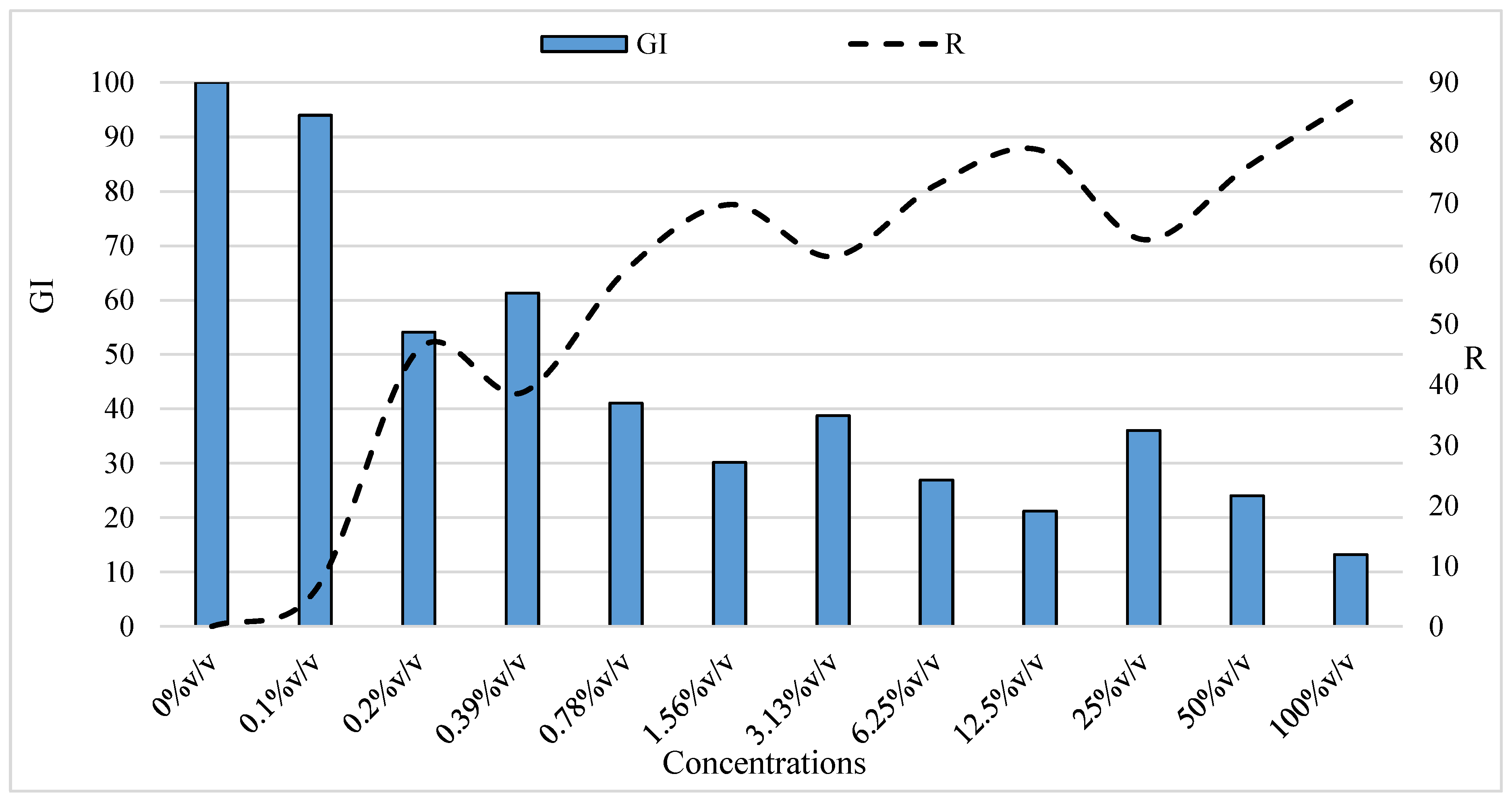
| Global development index, GI Gariglio et al. (2002) [43] | G and G0—germinated seeds in the experimental variants and the control (%); L—seedling length in the experimental variants; L0—seedling length in the control variant, taken as 100% |
| Concentration, % (v/v) | Percentage of Germinated Seeds, GP% | ±Standard Error, SE | Reduction of Percentage Germinated Seeds, R | Percentage of Inhibition, I% |
|---|---|---|---|---|
| 0.0 * | 77.1 cd | 2.4 | 0.0 | 0.0 |
| 0.10 | 83.2 е | 3.75 | −6.1 | −7.91 |
| 0.20 | 71.6 bc | 2.89 | 5.5 | 7.13 |
| 0.39 | 71.6 bc | 2.04 | 5.5 | 7.13 |
| 0.78 | 69.1 c | 2.30 | 8 | 10.38 |
| 1.56 | 60.0 a | 3.48 | 17.1 | 22.18 |
| 3.13 | 63.4 ab | 3.75 | 13.7 | 17.77 |
| 6.25 | 60.0 a | 2.89 | 17.1 | 22.18 |
| 12.5 | 63.4 ab | 4.04 | 13.7 | 17.77 |
| 25.0 | 63.4 ab | 2.31 | 13.7 | 17.77 |
| 50.0 | 56.8 a | 3.21 | 20.3 | 26.33 |
| 100.0 | 56.8 a | 2.15 | 20.3 | 26.33 |
| Parameter | 0.0 * % v/v | 0.10% v/v | 0.20% v/v | 0.39% v/v | 0.78% v/v | 1.56% v/v | 3.13% v/v | 6.25% v/v | 12.5% v/v | 25.0% v/v | 50.0% v/v | 100.0% v/v |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root, cm | 1.60 e | 1.51 de | 1.00 cd | 0.99 cd | 0.66 bc | 0.31 ab | 0.38 ab | 0.38 abc | 0.29 ab | 0.22 ab | 0.21 ab | 0.07 a |
| ±SE | 0.13 | 0.18 | 0.20 | 0.20 | 0.18 | 0.22 | 0.20 | 0.25 | 0.21 | 0.20 | 0.20 | 0.22 |
| R | 0.00 | 0.09 | 0.6 | 0.61 | 0.94 | 1.29 | 1.23 | 1.22 | 1.31 | 1.38 | 1.39 | 1.53 |
| I% | 0.00 | 5.6 | 37.5 | 38.3 | 58.8 | 80.4 | 76.6 | 76.3 | 82.1 | 85.9 | 86.7 | 95.6 |
| Hypocotyl, cm | 2.39 d | 1.97 cd | 1.32 abc | 1.65 bcd | 1.17 abc | 1.36 abc | 1.38 abc | 0.90 ab | 0.74 ab | 1.53 abc | 1.09 ab | 0.64 a |
| ±SE | 0.21 | 0.29 | 0.32 | 0.32 | 0.28 | 0.34 | 0.32 | 0.4 | 0.34 | 0.32 | 0.32 | 0.34 |
| R | 0.00 | 0.42 | 1.07 | 0.74 | 1.22 | 1.04 | 1.02 | 1.49 | 1.65 | 0.87 | 1.31 | 1.75 |
| I% | 0.00 | 17.7 | 44.7 | 31.1 | 51.1 | 43.3 | 42.6 | 62.4 | 69.0 | 36.3 | 54.6 | 73.1 |
| Seedling, cm | 3.99 e | 3.48 de | 2.33 bcd | 2.64 cd | 1.83 abc | 1.67 abc | 1.75 abc | 1.28 abc | 1.03 ab | 1.75 abc | 1.30 abc | 0.71 a |
| ±SE | 0.33 | 0.44 | 0.49 | 0.49 | 0.44 | 0.52 | 0.50 | 0.63 | 0.53 | 0.50 | 0.49 | 0.52 |
| R | 0.51 | 1.67 | 1.36 | 2.16 | 2.32 | 2.24 | 2.71 | 2.97 | 2.24 | 2.69 | 3.28 | |
| I% | I% | 12.9 | 41.8 | 34.0 | 54.2 | 58.2 | 56.2 | 68.0 | 74.2 | 56.2 | 67.5 | 82.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinov-Serafimov, P.; Golubinova, I.; Zapryanova, N.; Valcheva, E.; Nikolov, B.; Petrova, S. Optimizing Allelopathy Screening Bioassays by Using Nano Silver. Life 2024, 14, 687. https://doi.org/10.3390/life14060687
Marinov-Serafimov P, Golubinova I, Zapryanova N, Valcheva E, Nikolov B, Petrova S. Optimizing Allelopathy Screening Bioassays by Using Nano Silver. Life. 2024; 14(6):687. https://doi.org/10.3390/life14060687
Chicago/Turabian StyleMarinov-Serafimov, Plamen, Irena Golubinova, Nadezhda Zapryanova, Ekaterina Valcheva, Bogdan Nikolov, and Slaveya Petrova. 2024. "Optimizing Allelopathy Screening Bioassays by Using Nano Silver" Life 14, no. 6: 687. https://doi.org/10.3390/life14060687
APA StyleMarinov-Serafimov, P., Golubinova, I., Zapryanova, N., Valcheva, E., Nikolov, B., & Petrova, S. (2024). Optimizing Allelopathy Screening Bioassays by Using Nano Silver. Life, 14(6), 687. https://doi.org/10.3390/life14060687









