Monoclonal Antibodies as a Therapeutic Strategy against Multidrug-Resistant Bacterial Infections in a Post-COVID-19 Era
Abstract
1. Escalating Challenge of Antimicrobial Resistance in the Post-COVID Era
2. Developmental Challenges in Bacterial Vaccines
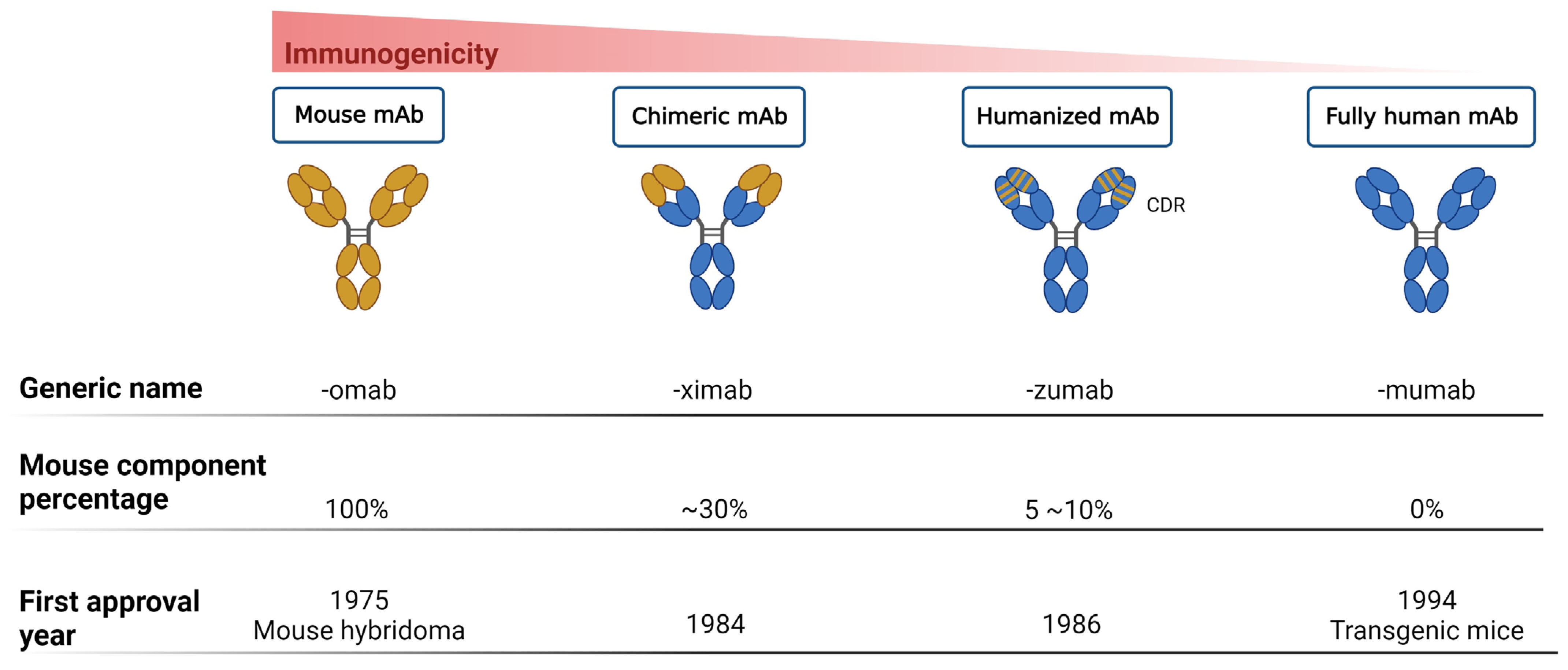
3. Developmental Process of Antibacterial Monoclonal Antibodies
4. Current Research and Clinical Trials
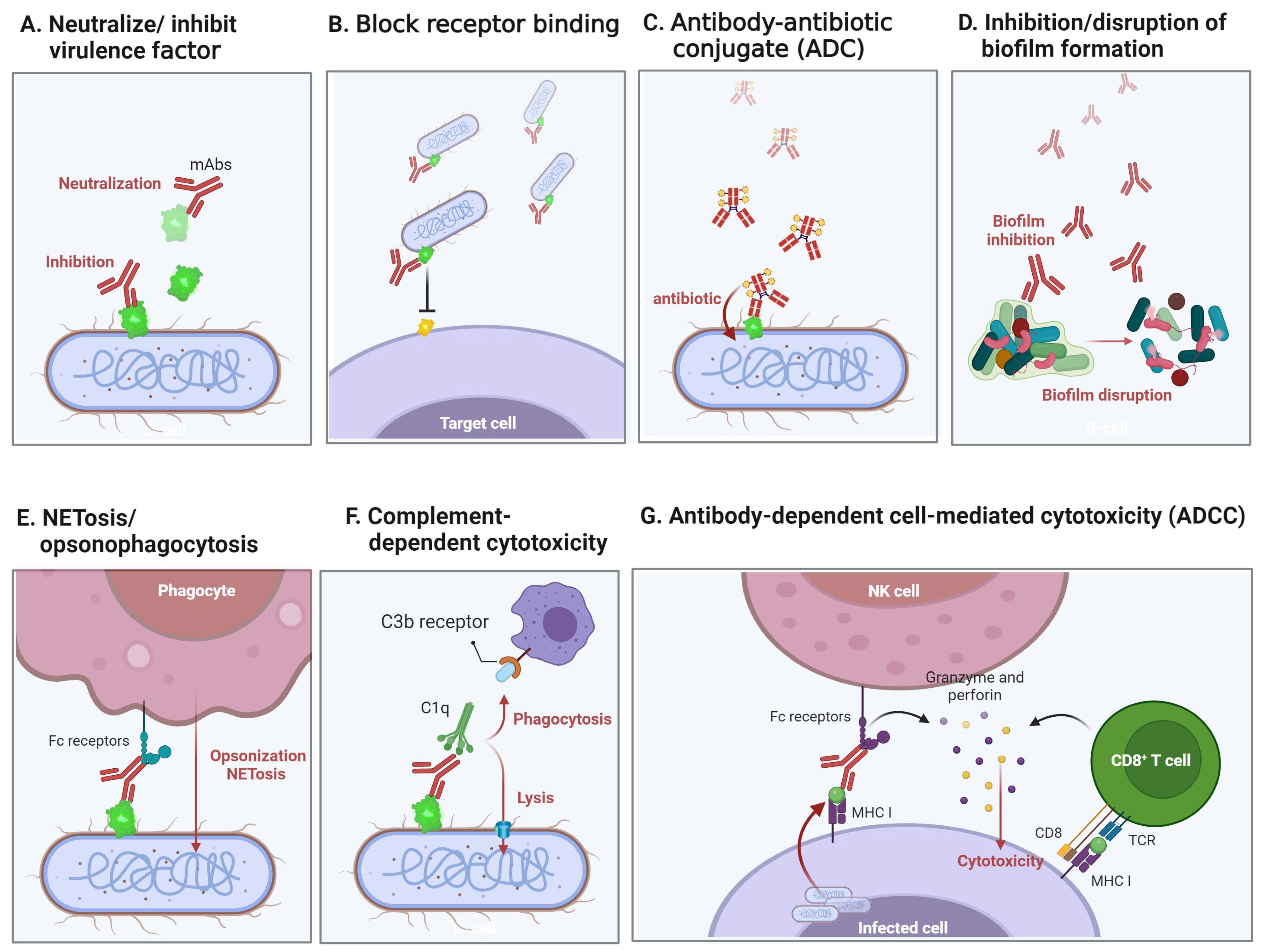
5. Novel Monoclonal Antibody Formats in Combatting Bacterial Infection
6. Shortcomings and Future Prospects of Antibacterial Monoclonal Antibodies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassini, A.; Hogberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States 2019. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 20 February 2023).
- O’Neill, J. Review on Antimicrobial Resistance Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; The UK Prime Minister: London, UK, 2014. [Google Scholar]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022; CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. iScience 2021, 24, 102304. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Raghavan, S.; Thomas, B.; Shastry, B.A. Elizabethkingia meningoseptica: Emerging multidrug resistance in a nosocomial pathogen. BMJ Case Rep. 2017, 2017, bcr2017221076. [Google Scholar] [CrossRef]
- Butler, M.S.; Henderson, I.R.; Capon, R.J.; Blaskovich, M.A.T. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 2023, 76, 431–473. [Google Scholar] [CrossRef]
- Bekeredjian-Ding, I. Challenges for Clinical Development of Vaccines for Prevention of Hospital-Acquired Bacterial Infections. Front. Immunol. 2020, 11, 1755. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Holm, M.; Frost, I.; Hasso-Agopsowicz, M.; Abbas, K. Global and regional burden of attributable and associated bacterial antimicrobial resistance avertable by vaccination: Modelling study. BMJ Glob. Health 2023, 8, e011341. [Google Scholar] [CrossRef] [PubMed]
- Frost, I.; Sati, H.; Garcia-Vello, P.; Hasso-Agopsowicz, M.; Lienhardt, C.; Gigante, V.; Beyer, P. The role of bacterial vaccines in the fight against antimicrobial resistance: An analysis of the preclinical and clinical development pipeline. Lancet Microbe 2023, 4, e113–e125. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Bausch-Jurken, M. The Burden of COVID-19 in the Immunocompromised Patient: Implications for Vaccination and Needs for the Future. J. Infect. Dis. 2023, 228, S4–S12. [Google Scholar] [CrossRef]
- Lee, J.-S.; Mogasale, V.; Kim, S.; Cannon, J.; Giannini, F.; Abbas, K.; Excler, J.-L.; Kim, J.H. The potential global cost-effectiveness of prospective Strep A vaccines and associated implementation efforts. npj Vaccines 2023, 8, 128. [Google Scholar] [CrossRef]
- Anderson, J.D.I.V.; Bagamian, K.H.; Pecenka, C.J.; Muhib, F.; Puett, C.A.; Hausdorff, W.P.; Scheele, S. Potential impact and cost-effectiveness of Shigella vaccination in 102 low-income and middle-income countries in children aged 5 years or younger: A modelling study. Lancet Glob. Health 2023, 11, e880–e891. [Google Scholar] [CrossRef]
- Bajema, K.L.; Gierke, R.; Farley, M.M.; Schaffner, W.; Thomas, A.; Reingold, A.L.; Harrison, L.H.; Lynfield, R.; Burzlaff, K.E.; Petit, S.; et al. Impact of Pneumococcal Conjugate Vaccines on Antibiotic-Nonsusceptible Invasive Pneumococcal Disease in the United States. J. Infect. Dis. 2022, 226, 342–351. [Google Scholar] [CrossRef]
- Saha, S.K.; Tabassum, N.; Saha, S. Typhoid Conjugate Vaccine: An Urgent Tool to Combat Typhoid and Tackle Antimicrobial Resistance. J. Infect. Dis. 2021, 224, S788–S791. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial Metabolism and Antibiotic Efficacy. Cell Metab 2019, 30, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Buchy, P.; Ascioglu, S.; Buisson, Y.; Datta, S.; Nissen, M.; Tambyah, P.A.; Vong, S. Impact of vaccines on antimicrobial resistance. Int. J. Infect. Dis. 2020, 90, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Flasche, S.; Jit, M.; Atkins, K.E. Modeling the effect of vaccination on selection for antibiotic resistance in Streptococcus pneumoniae. Sci. Transl. Med. 2021, 13, eaaz8690. [Google Scholar] [CrossRef] [PubMed]
- López-Siles, M.; Corral-Lugo, A.; McConnell, M.J. Vaccines for multidrug resistant Gram negative bacteria: Lessons from the past for guiding future success. FEMS Microbiol. Rev. 2020, 45, fuaa054. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Chiang, M.H.; Sung, W.C.; Lien, S.P.; Chen, Y.Z.; Lo, A.F.; Huang, J.H.; Kuo, S.C.; Chong, P. Identification of novel vaccine candidates against Acinetobacter baumannii using reverse vaccinology. Hum. Vaccins Immunother. 2015, 11, 1065–1073. [Google Scholar] [CrossRef]
- Michalik, M.; Djahanshiri, B.; Leo, J.C.; Linke, D. Reverse Vaccinology: The Pathway from Genomes and Epitope Predictions to Tailored Recombinant Vaccines. Methods Mol. Biol. 2016, 1403, 87–106. [Google Scholar] [CrossRef]
- Pasero, D.; Cossu, A.P.; Terragni, P. Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19. Microorganisms 2021, 9, 1773. [Google Scholar] [CrossRef]
- McConnell, M.J. Where are we with monoclonal antibodies for multidrug-resistant infections? Drug Discov. Today 2019, 24, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Martinez, A.; Gambin, Y.; Sierecki, E. Thinking Outside the Bug: Molecular Targets and Strategies to Overcome Antibiotic Resistance. Int. J. Mol. Sci. 2019, 20, 1255. [Google Scholar] [CrossRef] [PubMed]
- Wang-Lin, S.X.; Balthasar, J.P. Pharmacokinetic and Pharmacodynamic Considerations for the Use of Monoclonal Antibodies in the Treatment of Bacterial Infections. Antibodies 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Tan, M.W. Antibody-Antibiotic Conjugates: A Novel Therapeutic Platform against Bacterial Infections. Trends Mol. Med. 2017, 23, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Seong, B.L. Solubility, Stability, and Avidity of Recombinant Antibody Fragments Expressed in Microorganisms. Front. Microbiol. 2020, 11, 1927. [Google Scholar] [CrossRef]
- Hwang, W.Y.; Foote, J. Immunogenicity of engineered antibodies. Methods 2005, 36, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Motley, M.P.; Banerjee, K.; Fries, B.C. Monoclonal antibody-based therapies for bacterial infections. Curr. Opin. Infect. Dis. 2019, 32, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Pietrzykowski, M.; Flanagan, W.; Pizzi, V.; Brown, A.; Sinclair, A.; Monge, M. An environmental life cycle assessment comparison of single-use and conventional process technology for the production of monoclonal antibodies. J. Clean. Prod. 2013, 41, 150–162. [Google Scholar] [CrossRef]
- Nowak, C.; Cheung, J.K.; Dellatore, S.M.; Katiyar, A.; Bhat, R.; Sun, J.; Ponniah, G.; Neill, A.; Mason, B.; Beck, A.; et al. Forced degradation of recombinant monoclonal antibodies: A practical guide. mAbs 2017, 9, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Szijártó, V.; Nagy, E.; Nagy, G. Directly Bactericidal Anti-Escherichia coli Antibody. Trends Microbiol. 2018, 26, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, M.; Castanho, M.; Neves, V. The Use of Antibody-Antibiotic Conjugates to Fight Bacterial Infections. Front. Microbiol. 2022, 13, 835677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lehar, S.; Gutierrez, J.; Rosenberger, C.M.; Ljumanovic, N.; Dinoso, J.; Koppada, N.; Hong, K.; Baruch, A.; Carrasco-Triguero, M.; et al. Pharmacokinetics and pharmacodynamics of DSTA4637A: A novel THIOMAB™ antibody antibiotic conjugate against Staphylococcus aureus in mice. mAbs 2016, 8, 1612–1619. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nielsen, J.; Stephanopoulos, G. Protein Engineering: Tools and Applications; WILEY-VCH GmbH: Weinheim, Germany, 2021; pp. 317–351. [Google Scholar]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. mAbs 2010, 2, 256–265. [Google Scholar] [CrossRef]
- Klemm, J.; Pekar, L.; Krah, S.; Zielonka, S. Antibody Display Systems. In Introduction to Antibody Engineering; Rüker, F., Wozniak-Knopp, G., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 65–96. [Google Scholar]
- National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov (accessed on 7 August 2023).
- Chiang, M.H.; Yang, Y.S.; Sun, J.R.; Wang, Y.C.; Kuo, S.C.; Lee, Y.T.; Chuang, Y.P.; Chen, T.L. Confronting tigecycline-resistant Acinetobacter baumannii via immunization against conserved resistance determinants. Front. Microbiol. 2020, 11, 536. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; Lu, H. Anti-bacterial monoclonal antibodies: Next generation therapy against superbugs. Appl. Microbiol. Biotechnol. 2022, 106, 3957–3972. [Google Scholar] [CrossRef]
- Zurawski, D.V.; McLendon, M.K. Monoclonal Antibodies as an Antibacterial Approach against Bacterial Pathogens. Antibiotics 2020, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Hiroshi Morisaki, J.; et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Moir, D.T.; Opperman, T.J.; Aron, Z.D.; Bowlin, T.L. Adjunctive therapy for multidrug-resistant bacterial infections: Type III secretion system and efflux inhibitors. Drug Discov. Today 2021, 26, 2173–2181. [Google Scholar] [CrossRef]
- Akshay, S.D.; Deekshit, V.K.; Mohan Raj, J.; Maiti, B. Outer Membrane Proteins and Efflux Pumps Mediated Multi-Drug Resistance in Salmonella: Rising Threat to Antimicrobial Therapy. ACS Infect. Dis. 2023, 9, 2072–2092. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Q.; Li, W.; Chen, Y.; Shu, C.; Li, Q.; Zhou, J.; Ye, C.; Bai, H.; Sun, W.; et al. Anti-outer Membrane Vesicle Antibodies Increase Antibiotic Sensitivity of Pan-Drug-Resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1379. [Google Scholar] [CrossRef]
- Yadav, P.D.; Mendiratta, S.K.; Mohandas, S.; Singh, A.K.; Abraham, P.; Shete, A.; Bandyopadhyay, S.; Kumar, S.; Parikh, A.; Kalita, P.; et al. ZRC3308 Monoclonal Antibody Cocktail Shows Protective Efficacy in Syrian Hamsters against SARS-CoV-2 Infection. Viruses 2021, 13, 2424. [Google Scholar] [CrossRef] [PubMed]
- Gilchuk, P.; Murin, C.D.; Milligan, J.C.; Cross, R.W.; Mire, C.E.; Ilinykh, P.A.; Huang, K.; Kuzmina, N.; Altman, P.X.; Hui, S.; et al. Analysis of a Therapeutic Antibody Cocktail Reveals Determinants for Cooperative and Broad Ebolavirus Neutralization. Immunity 2020, 52, 388–403.e312. [Google Scholar] [CrossRef]
- Buckley, P.T.; Chan, R.; Fernandez, J.; Luo, J.; Lacey, K.A.; DuMont, A.L.; O’Malley, A.; Brezski, R.J.; Zheng, S.; Malia, T.; et al. Multivalent human antibody-centyrin fusion protein to prevent and treat Staphylococcus aureus infections. Cell Host Microbe 2023, 31, 751–765.e711. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Read, A.F. Why does drug resistance readily evolve but vaccine resistance does not? Proc. Biol. Sci. 2017, 284, 12878–12886. [Google Scholar] [CrossRef]
- Batchelder, J.I.; Hare, P.J.; Mok, W.W.K. Resistance-resistant antibacterial treatment strategies. Front. Antibiot. 2023, 2, 1093156. [Google Scholar] [CrossRef]
- Tvilum, A.; Johansen, M.I.; Glud, L.N.; Ivarsen, D.M.; Khamas, A.B.; Carmali, S.; Mhatre, S.S.; Sogaard, A.B.; Faddy, E.; de Vor, L.; et al. Antibody-Drug Conjugates to Treat Bacterial Biofilms via Targeting and Extracellular Drug Release. Adv. Sci. 2023, 10, e2301340. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Keller, A.E.; Gao, C.; Rainey, G.J.; Warrener, P.; Camara, M.M.; Bonnell, J.; Fleming, R.; Bezabeh, B.; Dimasi, N.; et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 2014, 6, 262ra155. [Google Scholar] [CrossRef]
- Amro, W.A.; Al-Qaisi, W.; Al-Razem, F. Production and purification of IgY antibodies from chicken egg yolk. J. Genet. Eng. Biotechnol. 2018, 16, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Satheeshkumar, P.K. Expression of Single Chain Variable Fragment (scFv) Molecules in Plants: A Comprehensive Update. Mol. Biotechnol. 2020, 62, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, K.; Christophersen, L.; Bjarnsholt, T.; Jensen, P.O.; Moser, C.; Hoiby, N. Anti-Pseudomonas aeruginosa IgY antibodies augment bacterial clearance in a murine pneumonia model. J. Cyst. Fibros. 2016, 15, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.H.; Jin, L.J.; Guo, J.; Li, X.Y.; Li, Z.; Fang, R.; Xu, Y.P. Characterization of specific egg yolk immunoglobulin (IgY) against mastitis-causing Staphylococcus aureus. J. Appl. Microbiol. 2008, 105, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Tadayoni, R.; Sararols, L.; Weissgerber, G.; Verma, R.; Clemens, A.; Holz, F.G. Brolucizumab: A Newly Developed Anti-VEGF Molecule for the Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmologica 2021, 244, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Nyakatura, E.K.; Soare, A.Y.; Lai, J.R. Bispecific antibodies for viral immunotherapy. Hum. Vaccins Immunother. 2017, 13, 836–842. [Google Scholar] [CrossRef]
- Sedykh, S.E.; Prinz, V.V.; Buneva, V.N.; Nevinsky, G.A. Bispecific antibodies: Design, therapy, perspectives. Drug Des. Dev. Ther. 2018, 12, 195–208. [Google Scholar] [CrossRef]
- Lee, L.; Samardzic, K.; Wallach, M.; Frumkin, L.R.; Mochly-Rosen, D. Immunoglobulin Y for Potential Diagnostic and Therapeutic Applications in Infectious Diseases. Front. Immunol. 2021, 12, 696003. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, Y.; Sivaccumar, J.P.; An, Z.; Xia, N.; Luo, W. Research progress and applications of nanobody in human infectious diseases. Front. Pharmacol. 2022, 13, 963978. [Google Scholar] [CrossRef]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.; Hamid, M. scFv antibody: Principles and clinical application. Clin. Dev. Immunol. 2012, 2012, 980250. [Google Scholar] [CrossRef]
- Muñoz-López, P.; Ribas-Aparicio, R.M.; Becerra-Báez, E.I.; Fraga-Pérez, K.; Flores-Martínez, L.F.; Mateos-Chávez, A.A.; Luria-Pérez, R. Single-Chain Fragment Variable: Recent Progress in Cancer Diagnosis and Therapy. Cancers 2022, 14, 4206. [Google Scholar] [CrossRef]
- Kim, E.G.; Kim, K.M. Strategies and Advancement in Antibody-Drug Conjugate Optimization for Targeted Cancer Therapeutics. Biomol. Ther. 2015, 23, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Beckett, V.V.; Konstan, M.W.; Accurso, F.J.; Burns, J.L.; Mayer-Hamblett, N.; Milla, C.; VanDevanter, D.R.; Chmiel, J.F.; Group, K.A.S. KB001-A, a novel anti-inflammatory, found to be safe and well-tolerated in cystic fibrosis patients infected with Pseudomonas aeruginosa. J. Cyst. Fibros. 2018, 17, 484–491. [Google Scholar] [CrossRef]
- Raj, G.M.; Priyadarshini, R.; Murugesan, S.; Adhimoolam, M. Monoclonal Antibodies Against Infectious Microbes: So Long and Too Little! Infect Disord Drug Targets 2021, 21, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Ozdilek, A.; Huang, J.; Babb, R.; Paschall, A.V.; Middleton, D.R.; Duke, J.A.; Pirofski, L.A.; Mousa, J.J.; Avci, F.Y. A Structural Model for the Ligand Binding of Pneumococcal Serotype 3 Capsular Polysaccharide-Specific Protective Antibodies. mBio 2021, 12, e0080021. [Google Scholar] [CrossRef] [PubMed]
- Cywes-Bentley, C.; Skurnik, D.; Zaidi, T.; Roux, D.; Deoliveira, R.B.; Garrett, W.S.; Lu, X.; O’Malley, J.; Kinzel, K.; Zaidi, T.; et al. Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, E2209–E2218. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.C.; Cywes-Bentley, C.; Moeller, T.D.; Weyant, K.B.; Putnam, D.; Chang, Y.F.; Jones, B.D.; Pier, G.B.; DeLisa, M.P. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, E3106–E3115. [Google Scholar] [CrossRef] [PubMed]
| Agents | Bacterial Species | mAb Target | Sponsor (s) | Phase of Trial | NCT Number | Origin |
|---|---|---|---|---|---|---|
| Tefibazumab | Staphylococcus aureus | clumping factor A | Bristol Myers Squibb | II | NCT00198289 | Humanized |
| 514G3 | cell wall moiety Protein A (SpA) | XBiotech | II | NCT02357966 | Human (isolated and cloned from a healthy human donor) | |
| MEDI4893 (Suvratoxumab) | alpha-hemolysin | Medimmune | II | NCT02296320 | Human (VelocImmune mice) | |
| ASN-100 (ASN-1 and ASN-2) | alpha-hemolysin, gamma-hemolysin, bicomponent leucocidin (HlgAB, HlgCB, LukED, LukSF, and LukGH) | Arsansis | II | NCT02940626 | Human | |
| AR-301 (Tosatoxumab) | alpha toxin | Aridis Pharmaceuticals | III | NCT03816956 | Human (convalescent patient B-cell) | |
| DSTA-4637S | Teichoic acid (Antibody–Antibiotic Conjugate) | Genentech and Roche | I | NCT03162250 | Human | |
| Aurograb® | ABC transporter GrfA | NeuTec Pharma/Novartis | III | NCT00217841 | scFv | |
| KB001 | Pseudomonas aeruginosa | type III secretion system, PcrV | KaloBios | II | NCT00638365 | Humanized PEGylated Fab |
| PsAer-IgY | surface protein (Flagellin) | Mukoviszidose Institut gGmbH | III | NCT01455675 | Chicken egg yolk | |
| AR-105 (Aerucin) | alginate | Aridis Pharmaceuticals | II | NCT03027609 | Human | |
| KBPA-101 (Aermab) | LPS O-antigen (serotype O11) | Aridis (Kenta Biotech) | II | NCT00851435 | Human | |
| MEDI3902 | type III secretion system, PcrV, exopolysaccharide, Psl | Medimmune | II | NCT02696902 | Human (bispecific phage display and VelocImmune mouse) | |
| MK-3415A (actoxumab-bezlotoxumab) | Clostridium difficile | toxin A/B | Merck Sharp & Dohme | III | NCT01513239 | Human |
| Bezlotoxumab (Zinplava®) | toxin B | Merck Sharp & Dohme | IV | NCT03880539 | Human | |
| GS-CDA1/MDX-1388 | toxin A/C-terminal toxin B fragment | MassBiologics/Merck | II | NCT00350298 | human | |
| Raxibacumab (ABthrax®/Anthrin®) | Bacillus anthracis | protective antigen (PA) component of anthrax toxin | Human Genome Sciences | IV | NCT02177721 | Human (phage display) |
| Obiltoxaximab (Anthim®, ETI-204) | PA component of anthrax toxin | Elusys Therapeutics | IV | NCT03088111 | Human–mouse (hybridoma) | |
| MDX-1303 (Valortim®) | uncleaved and cleaved PA | PharmAthene | I | NCT00964561 | Human | |
| AVP-21D9 (ThravixaTM) | PA component of anthrax toxin | Emergent BioSolutions | I | NCT01202695 | Human | |
| NTM-1632/3 | Clostridium botulinum | botulinum neurotoxin type B | NIAID | I | NCT02779140 | Humanized |
| XOMA 3ab | botulinum neurotoxin type B | XOMA/NIAID | I | NCT01357213 | Humanized | |
| TRL1068 | Biofilm—multiple species | biofilm scaffolding proteins DNABII | Trellis Bioscience | I | NCT04763759 | Human |
| F598 | Multiple species | poly-N-acetylglucosamine (PNAG) | Alopexx Pharmaceuticals | II | NCT03222401 | Human |
| Pagibaximab (BSYX-A110) | Staphylococcal Sepsis | lipoteichoic acid | Biosynexus | III | NCT00646399 | Humanized |
| cαStx1/cαStx2 | Shiga Toxin-Producing E. coli | shiga toxins | Thallion Pharmaceuticals | II | NCT01252199 | Humanized |
| Novel mAb Types | Characteristics | Examples | Advantages | Disadvantages | Refs. | |
|---|---|---|---|---|---|---|
| Antibody–Drug conjugation | 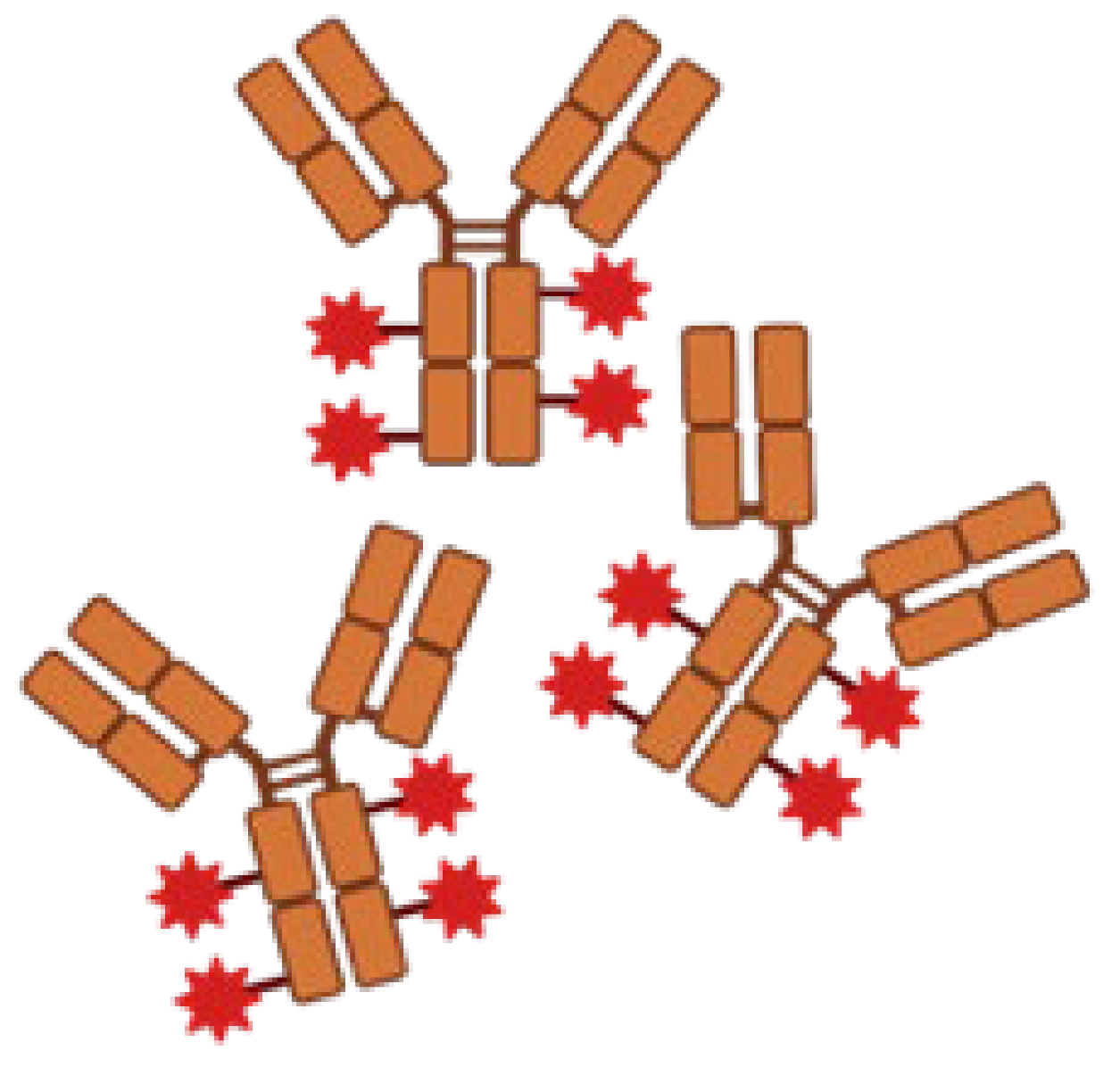 | Delivering drugs directly to specific antigens. | DSTA4637A/MRSA/Human IgG1-dmDNA31 |
|
| [32,39] |
| Bispecific mAbs |  | Simultaneously binds to two different epitopes using an mAb. | MEDI3902/P. aeruginosa PcrV and Psl |
|
| [60,67,68] |
| IgY | 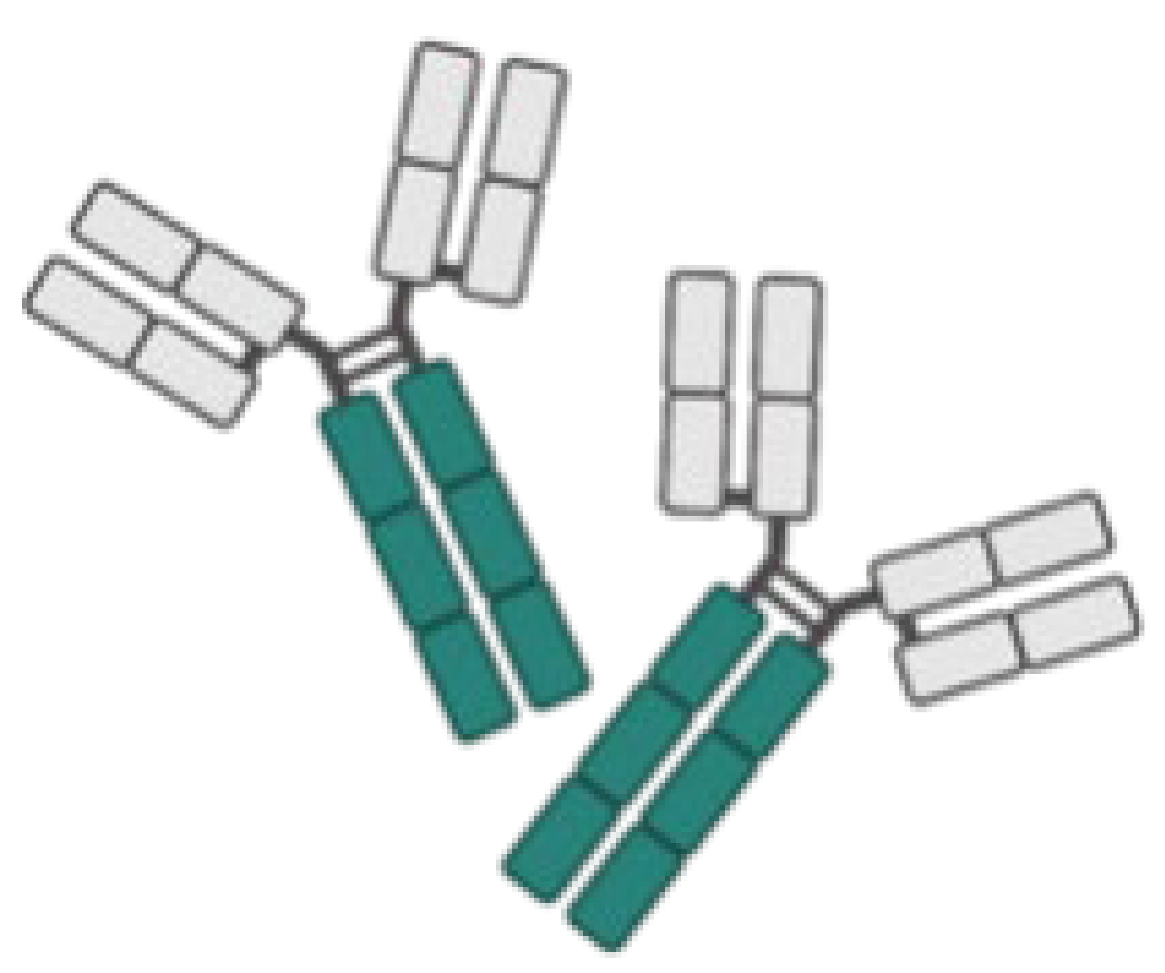 | Highly conserved to human IgG. | PsAer-IgY/P. aeruginosa surface protein (Flagellin) |
|
| [64,69] |
| Nanobody | 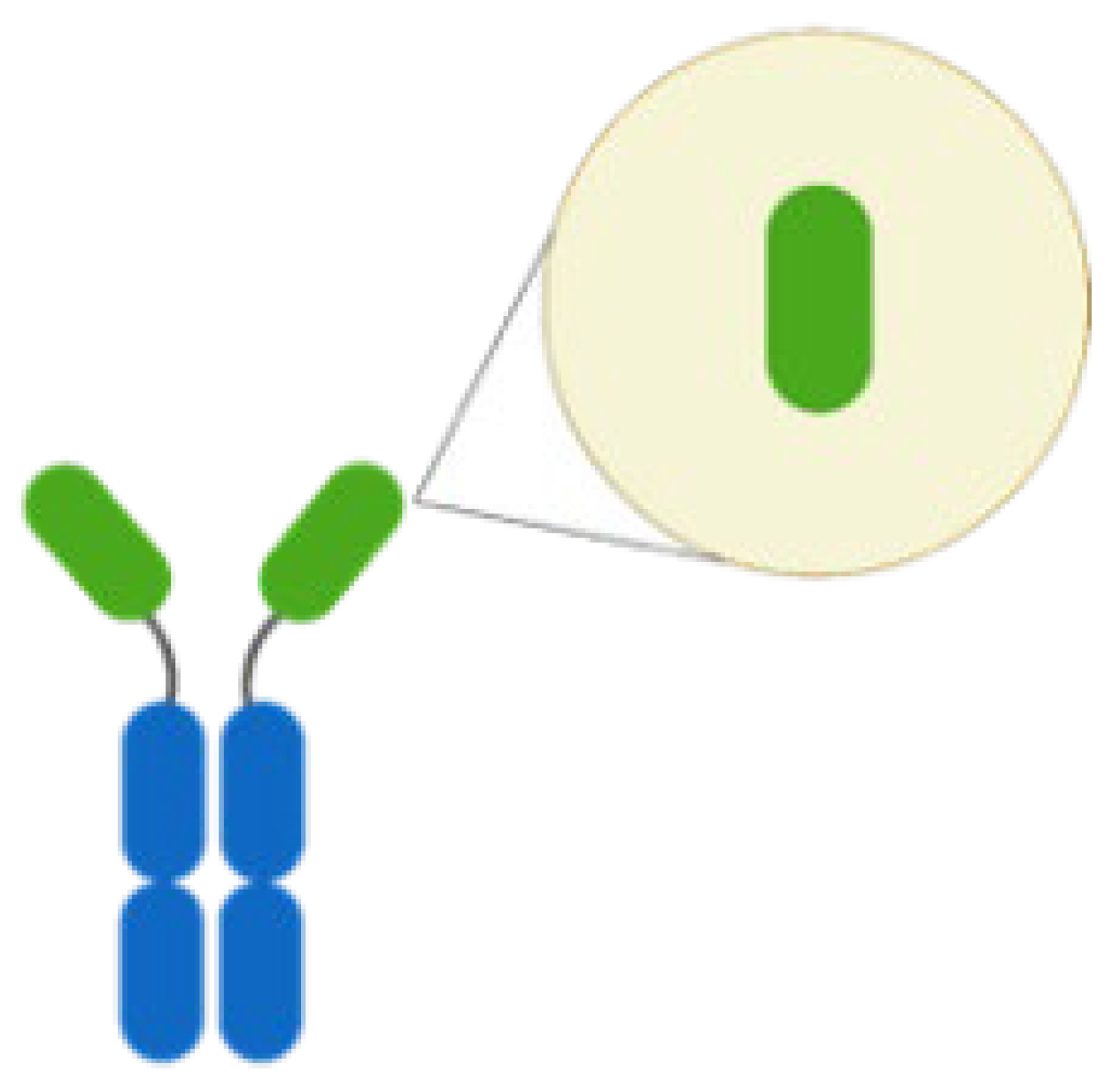 | Antibody lack of light chain and constant domain. | NbD7/Ehrlichia chaffeensis translocated factor-1 |
|
| [62,70] |
| scFv | 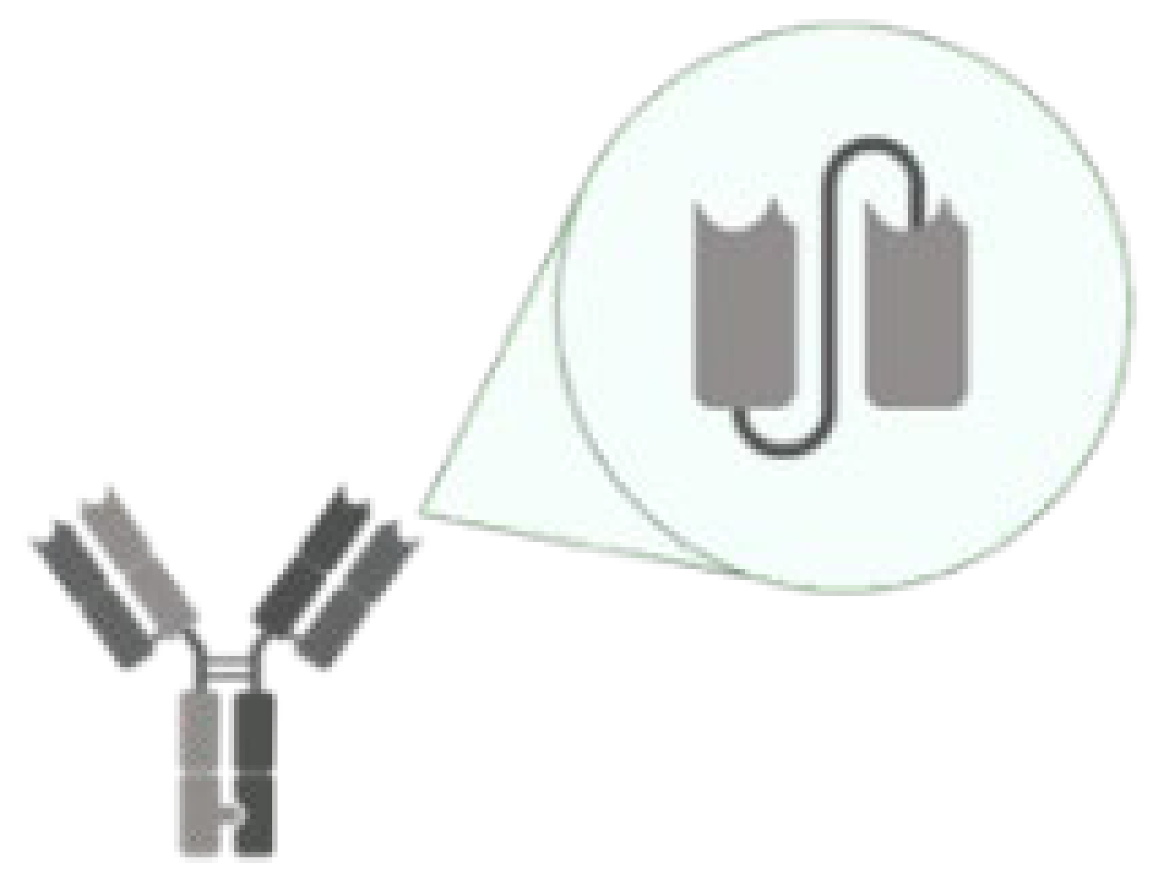 | An antibody comprises only the variable regions of the heavy and light chains. | Brolucizumab/Neovascular Age-related Macular Degeneration VEGF-A |
|
| [66,71,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-C.; Pan, Y.-L.; Chen, Y.; Yang, T.-H.; Hsu, E.-T.; Huang, Y.-T.; Chiang, M.-H. Monoclonal Antibodies as a Therapeutic Strategy against Multidrug-Resistant Bacterial Infections in a Post-COVID-19 Era. Life 2024, 14, 246. https://doi.org/10.3390/life14020246
Chen H-C, Pan Y-L, Chen Y, Yang T-H, Hsu E-T, Huang Y-T, Chiang M-H. Monoclonal Antibodies as a Therapeutic Strategy against Multidrug-Resistant Bacterial Infections in a Post-COVID-19 Era. Life. 2024; 14(2):246. https://doi.org/10.3390/life14020246
Chicago/Turabian StyleChen, Hsiao-Chun, Yu-Ling Pan, Ying Chen, Tsung-Hsuan Yang, Erh-Tung Hsu, Yu-Ting Huang, and Ming-Hsien Chiang. 2024. "Monoclonal Antibodies as a Therapeutic Strategy against Multidrug-Resistant Bacterial Infections in a Post-COVID-19 Era" Life 14, no. 2: 246. https://doi.org/10.3390/life14020246
APA StyleChen, H.-C., Pan, Y.-L., Chen, Y., Yang, T.-H., Hsu, E.-T., Huang, Y.-T., & Chiang, M.-H. (2024). Monoclonal Antibodies as a Therapeutic Strategy against Multidrug-Resistant Bacterial Infections in a Post-COVID-19 Era. Life, 14(2), 246. https://doi.org/10.3390/life14020246








