Chemical Analysis and Biological Activities of Extracts Isolated from Symbiotic L. japonicus Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material, Microbial Inoculation, and Growth Conditions
2.2. Testing Root Colonization by Microsymbionts
2.3. Treatments, Tissue Harvesting, and Extraction
- Non-inoculated plants grown at 21 °C (control sample “C1”)
- Non-inoculated plants grown at 24–25 °C (control sample “C2”)
- Plants inoculated with the Rhizobium bacterium M. loti (“R” sample)
- Plants inoculated with the Greece-isolated R. irregularis strain (“AMF” sample)
- Plants inoculated with the R. irregularis DAOM strain (“AMF-D” sample)
- Plants double-inoculated with Rhizobium and AMF (DAOM) (“R + D” sample)
2.4. Chemical Analysis of the Extracts
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Anti-Inflammatory Activity Assessment
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Lotus Japonicus Metabolite Profiling under Different Microbial Inoculation Treatments
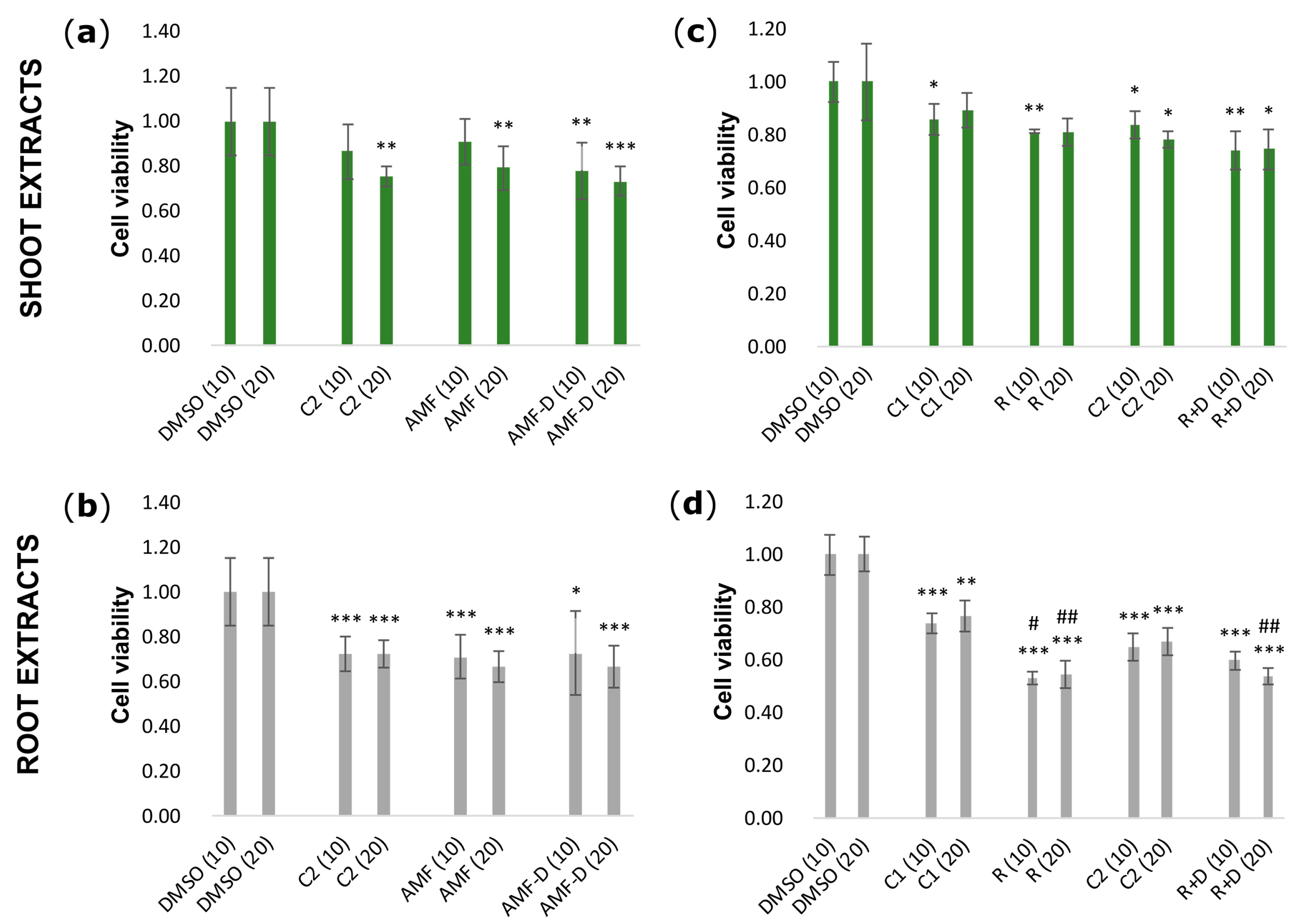
3.2. Effects of L. japonicus Extracts on HEK-293 Cell Viability
3.3. Apoptotic Activities of L. japonicus Extracts
3.4. Anti-Inflammatory Properties of L. japonicus Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Hartmann, T. From Waste Products to Ecochemicals: Fifty Years Research of Plant Secondary Metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Méteignier, L.-V.; Nützmann, H.-W.; Papon, N.; Osbourn, A.; Courdavault, V. Emerging Mechanistic Insights into the Regulation of Specialized Metabolism in Plants. Nat. Plants 2023, 9, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Smith, S.E., Read, D., Eds.; Academic Press: London, UK, 2008; ISBN 978-0-12-370526-6. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cartabia, A.; Lalaymia, I.; Declerck, S. Arbuscular Mycorrhizal Fungi and Production of Secondary Metabolites in Medicinal Plants. Mycorrhiza 2022, 32, 221–256. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Habibi Machiani, R.; Sadeghpour, A. Arbuscular Mycorrhizal Fungi and Changes in Primary and Secondary Metabolites. Plants 2022, 11, 2183. [Google Scholar] [CrossRef]
- Schweiger, R.; Müller, C. Leaf Metabolome in Arbuscular Mycorrhizal Symbiosis. Curr. Opin. Plant Biol. 2015, 26, 120–126. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kapoor, R.; Bhatnagar, A.K. Effectiveness of Two Arbuscular Mycorrhizal Fungi on Concentrations of Essential Oil and Artemisinin in Three Accessions of Artemisia annua L. Appl. Soil. Ecol. 2008, 40, 174–181. [Google Scholar] [CrossRef]
- Domokos, E.; Jakab-Farkas, L.; Darkó, B.; Bíró-Janka, B.; Mara, G.; Albert, C.; Balog, A. Increase in Artemisia annua Plant Biomass Artemisinin Content and Guaiacol Peroxidase Activity Using the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis. Front. Plant Sci. 2018, 9, 478. [Google Scholar] [CrossRef]
- Domokos, E.; Bíró-Janka, B.; Bálint, J.; Molnár, K.; Fazakas, C.; Jakab-Farkas, L.; Domokos, J.; Albert, C.; Mara, G.; Balog, A. Arbuscular Mycorrhizal Fungus Rhizophagus irregularis Influences Artemisia annua Plant Parameters and Artemisinin Content under Different Soil Types and Cultivation Methods. Microorganisms 2020, 8, 899. [Google Scholar] [CrossRef]
- Sailo, G.L.; Bagyaraj, D.J. Influence of Different AM-Fungi on the Growth, Nutrition and Forskolin Content of Coleus forskohlii. Mycol. Res. 2005, 109, 795–798. [Google Scholar] [CrossRef]
- Singh, R.; Soni, S.K.; Kalra, A. Synergy between Glomus fasciculatum and a Beneficial Pseudomonas in Reducing Root Diseases and Improving Yield and Forskolin Content in Coleus forskohlii Briq. under Organic Field Conditions. Mycorrhiza 2013, 23, 35–44. [Google Scholar] [CrossRef]
- Bulut, M.; Wendenburg, R.; Bitocchi, E.; Bellucci, E.; Kroc, M.; Gioia, T.; Susek, K.; Papa, R.; Fernie, A.R.; Alseekh, S. A Comprehensive Metabolomics and Lipidomics Atlas for the Legumes Common Bean, Chickpea, Lentil and Lupin. Plant J. 2023, 116, 1152–1171. [Google Scholar] [CrossRef] [PubMed]
- Serventi, L.; Cai, X.; Chen, R.; Dilrukshi, N.; Su, J.; Tuange, R.P.N.; Ham, E.E. Anticancer Properties of Aqueous Extracts from Leguminosae. Nutraceuticals 2022, 2, 323–334. [Google Scholar] [CrossRef]
- Sánchez-Chino, X.; Jiménez-Martínez, C.; Dávila-Ortiz, G.; Álvarez-González, I.; Madrigal-Bujaidar, E. Nutrient and Nonnutrient Components of Legumes, and Its Chemopreventive Activity: A Review. Nutr. Cancer 2015, 67, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Guang, C.; Chen, J.; Sang, S.; Cheng, S. Biological Functionality of Soyasaponins and Soyasapogenols. J. Agric. Food Chem. 2014, 62, 8247–8255. [Google Scholar] [CrossRef]
- Ranner, J.L.; Schalk, S.; Martyniak, C.; Parniske, M.; Gutjahr, C.; Stark, T.D.; Dawid, C. Primary and Secondary Metabolites in Lotus japonicus. J. Agric. Food Chem. 2023, 71, 11277–11303. [Google Scholar] [CrossRef]
- Forslund, K.; Morant, M.; Jørgensen, B.; Olsen, C.E.; Asamizu, E.; Sato, S.; Tabata, S.; Bak, S. Biosynthesis of the Nitrile Glucosides Rhodiocyanoside A and D and the Cyanogenic Glucosides Lotaustralin and Linamarin in Lotus japonicus. Plant Physiol. 2004, 135, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Motawia, M.S.; Olsen, C.E.; Møller, B.L.; Bak, S. Biosynthesis of Rhodiocyanosides in Lotus japonicus: Rhodiocyanoside A Is Synthesized from (Z)-2-Methylbutanaloxime via 2-Methyl-2-Butenenitrile. Phytochemistry 2012, 77, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Tsikou, D.; Tsiknia, M.; Nikolaou, C.N.; Ehaliotis, C.; Papadopoulou, K.K. The Effect of Rhizophagus irregularis and Mesorhizobium Loti Co-Inoculation on Lotus japonicus. J. Exp. Mol. Biol. 2019, 20, 1–6. [Google Scholar]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition. In Commonwealth Bureau of Horticulture and Plantation Crops. East Malling, England. Technical Communications, 2nd ed.; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1966. [Google Scholar]
- Hoagland, D.R. The Water-Culture Method for Growing Plants without Soil; Arnon, D.I., Ed.; College of Agriculture, University of California: Berkeley, CA, USA, 1950. [Google Scholar]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and Vinegar, a Simple Staining Technique for Arbuscular-Mycorrhizal Fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kalousi, F.D.; Pollastro, F.; Christodoulou, E.C.; Karra, A.G.; Tsialtas, I.; Georgantopoulos, A.; Salamone, S.; Psarra, A.-M.G. Apoptotic, Anti-Inflammatory Activities and Interference with the Glucocorticoid Receptor Signaling of Fractions from Pistacia lentiscus L. Var. Chia Leaves. Plants 2022, 11, 934. [Google Scholar] [CrossRef]
- Bunim, J.J. A Decade of Anti-Inflammatory Steroids, from Cortisone to Dexamethasone. Summary. Ann. N. Y Acad. Sci. 1959, 82, 1012–1013. [Google Scholar] [CrossRef]
- Liu, C.-W.; Murray, J.D. The Role of Flavonoids in Nodulation Host-Range Specificity: An Update. Plants 2016, 5, 33. [Google Scholar] [CrossRef]
- Tian, B.; Pei, Y.; Huang, W.; Ding, J.; Siemann, E. Increasing Flavonoid Concentrations in Root Exudates Enhance Associations between Arbuscular Mycorrhizal Fungi and an Invasive Plant. ISME J. 2021, 15, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Siemann, E.; Tian, B.; Ding, J. Root Flavonoids Are Related to Enhanced AMF Colonization of an Invasive Tree. AoB Plants 2020, 12, plaa002. [Google Scholar] [CrossRef]
- Veitch, N.C. Isoflavonoids of the Leguminosae. Nat. Prod. Rep. 2007, 24, 417–464. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic Activity, Biological Effect and Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; Zin El-Abedin, T.K.A.; Mattar, M.A.; Ekiert, H. Phenolic Compounds of Catalpa speciosa, Taxus cuspidate, and Magnolia acuminata Have Antioxidant and Anticancer Activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef]
- Kim, K.M.; Heo, D.R.; Kim, Y.-A.; Lee, J.; Kim, N.S.; Bang, O.-S. Coniferaldehyde Inhibits LPS-Induced Apoptosis through the PKC α/β II/Nrf-2/HO-1 Dependent Pathway in RAW264.7 Macrophage Cells. Environ. Toxicol. Pharmacol. 2016, 48, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-J.; Lu, L.; Yang, J.-J.; Wang, X.-X.; Su, G.; Wang, Z.-L.; Chen, G.-H.; Sun, H.-M.; Wang, M.-Y.; Yang, Y. Lariciresinol Induces Apoptosis in HepG2 Cells via Mitochondrial-Mediated Apoptosis Pathway. Eur. J. Pharmacol. 2018, 821, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed]
- Tay, K.-C.; Tan, L.T.-H.; Chan, C.K.; Hong, S.L.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Formononetin: A Review of Its Anticancer Potentials and Mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic Application of Betalains: A Review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic Acid Induces Apoptosis and Autophagy in the Treatment of Tongue Squamous Cell Carcinomas. Sci. Rep. 2017, 7, 11277. [Google Scholar] [CrossRef]
- Palomino, O.M.; Giordani, V.; Chowen, J.; Alfonso, S.F.; Goya, L. Physiological Doses of Oleic and Palmitic Acids Protect Human Endothelial Cells from Oxidative Stress. Molecules 2022, 27, 5217. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Kamal, A.; Khan, F.; Jamali, K.S.; Saify, Z.S. Gallic and Vanillic Acid Suppress Inflammation and Promote Myelination in an In Vitro Mouse Model of Neurodegeneration. Mol. Biol. Rep. 2019, 46, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory Role in the Cardiovascular System. IUBMB Life 2019, 71, 507–515. [Google Scholar] [CrossRef] [PubMed]


| Metabolites | Shoot Extracts | Root Extracts | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| * Control | AMF | AMF-D | R | R + D | Control | AMF | AMF-D | R | R + D | |
| Phenolics | ||||||||||
| vanillic acid | 1 | 0.4 | 1.1 | 1.2 | 0.9 | 1 | 0.5 | 0.2 | 3.0 | 1.5 |
| apigenin 7-glucoside | 1 | 0.4 | 0.3 | 5.4 | 3.4 | 1 | 1.9 | 2.1 | 0.4 | 2.0 |
| cyanidin3-rytinoside | 1 | 0.4 | 0.1 | 2.4 | 1.7 | 1 | 1.3 | 0.7 | 0.6 | 6.8 |
| hydroxycaffeic acid | 1 | 0.6 | 1.1 | 3.4 | 1.7 | 1 | 0.0 | 0.4 | 10.7 | 7.4 |
| isoorientin | 1 | 0.6 | 0.5 | 3.2 | 0.8 | 1 | 0.9 | 0.8 | 1.2 | 0.9 |
| isorhoifolin | 1 | 0.3 | 0.3 | 5.1 | 2.9 | 1 | 0.5 | 0.2 | 4.6 | 3.1 |
| lariciresinol | 1 | 0.6 | 0.6 | 0.6 | 1.0 | 1 | 1.3 | 2.5 | 1.7 | 5.3 |
| luteolin-5-glucoside | 1 | 0.6 | 0.5 | 3.2 | 0.8 | 1 | 0.9 | 0.8 | 1.2 | 0.9 |
| quercetin-3-rutinoside | 1 | 0.2 | 0.3 | 4.7 | 1.3 | N/A | N/A | N/A | N/A | N/A |
| gamma tocopherol | 1 | 0.0 | 1.0 | 7.2 | 1.1 | 1 | 0.1 | 0.2 | 2.3 | 2.3 |
| m-coumaric acid | 1 | 0.6 | 0.3 | 1.8 | 1.0 | 1 | 0.9 | 0.9 | 19.5 | 8.2 |
| maachiain | 1 | 0.1 | 0.3 | 0.4 | 0.4 | 1 | 0.2 | 0.2 | 0.5 | 11.3 |
| glycinol | 1 | 1.3 | 1.2 | 1.2 | 1.2 | 1 | 1.0 | 0.9 | 1.1 | 1.0 |
| phaseollin | 1 | 0.6 | 3.5 | 1.8 | 1 | 0.1 | 0.0 | 0.4 | 2.8 | |
| vestitol | 1 | 0.0 | 0.4 | 0.1 | 0.2 | 1 | 0.1 | 0.1 | 16.8 | 4.5 |
| sophorol | 1 | 0.0 | 0.6 | 0.4 | 1.5 | 1 | 0.1 | 0.1 | 0.3 | 8.5 |
| (6aR,11aR)-3,9-Dihydroxypterocarpan | 1 | 0.2 | 0.3 | 0.7 | 0.2 | 1 | 1.0 | 0.2 | 6.6 | 2.4 |
| 2′-Hydroxyformononetin | 1 | 0.1 | 0.3 | 0.4 | 0.4 | 1 | 0.2 | 0.2 | 0.5 | 11.3 |
| astragalin | 1 | 0.6 | 0.5 | 3.2 | 0.8 | 1 | 0.9 | 0.8 | 1.2 | 0.9 |
| chalconaringenin | 1 | 1.3 | 1.2 | 1.2 | 1.2 | 1 | 1.0 | 0.9 | 1.1 | 1.0 |
| delphinidin | 1 | 0.1 | 0.1 | 0.1 | 0.1 | 1 | 0.3 | 0.1 | 0.1 | 0.0 |
| eugenol | 1 | 60.9 | 72.2 | 80.8 | 79.1 | 1 | 1.0 | 1.2 | 2.2 | 1.6 |
| formononetin | 1 | 0.0 | 0.4 | 0.2 | 0.3 | 1 | 0.3 | 0.0 | 2.8 | 1.6 |
| genistein | 1 | 0.2 | 0.1 | 0.1 | 0.1 | 1 | 0.4 | 0.2 | 0.6 | 0.4 |
| genistin | 1 | 0.7 | 0.5 | 0.0 | 2.1 | 1 | 1.9 | 2.1 | 1.7 | 2.0 |
| homogentistic acid | 1 | 0.4 | 1.1 | 1.2 | 0.9 | 1 | 0.5 | 0.2 | 3.0 | 1.5 |
| leucopelargonidin | 1 | 5.2 | 4.6 | 4.4 | 4.3 | 1 | 1.0 | 1.1 | 1.0 | 1.0 |
| lotaustralin | 1 | 0.3 | 0.7 | 1.0 | 1.8 | 1 | 0.4 | 0.1 | 8.0 | 9.5 |
| pelargonidin | 1 | 0.2 | 0.1 | 0.1 | 0.1 | 1 | 0.4 | 0.2 | 0.6 | 0.0 |
| phenylalcetaldehyde | 1 | 0.6 | 0.6 | 6.2 | 1.3 | 1 | 0.1 | 0.0 | 12.6 | 5.8 |
| sinapaldehyde | 1 | 0.8 | 0.9 | 0.4 | 0.8 | 1 | 1.0 | 1.0 | 1.0 | 0.6 |
| vestitone | 1 | 0.8 | 0.7 | 0.6 | 0.7 | 1 | 0.9 | 0.8 | 1.0 | 0.9 |
| coniferyl acetate | 1 | 1.2 | 1.1 | 1.2 | 1.1 | 1 | 1.0 | 1.0 | 1.1 | 1.1 |
| astragalin | 1 | 0.6 | 0.5 | 3.2 | 0.8 | 1 | 0.9 | 0.8 | 1.2 | 0.9 |
| caffeic acid hexoside | 1 | 0.6 | 1.1 | 3.4 | 1.7 | 1 | 0.0 | 0.4 | 10.7 | 7.4 |
| coniferaldehyde | 1 | 0.5 | 0.4 | 3.1 | 1.6 | 1 | 0.9 | 0.1 | 3.2 | 4.5 |
| diosmetin | 1 | 0.0 | 0.6 | 0.4 | 1.5 | 1 | 0.1 | 0.1 | 0.3 | 8.5 |
| eriodictyol | N/A | N/A | N/A | 1 | N/A | N/A | N/A | N/A | 5.3 | N/A |
| N-containing compounts | ||||||||||
| caffeine | 1 | 0.5 | 0.4 | 1.4 | 2.2 | 1 | 0.3 | 0.3 | 2.1 | 3.5 |
| cinchonidine | 1 | 1.1 | 1.0 | 1.2 | 1.0 | 1 | 1.1 | 0.4 | 0.1 | 1.1 |
| theobromine | 1 | 0.8 | 0.1 | 0.2 | 0.8 | 1 | 0.2 | 0.3 | 0.8 | 2.2 |
| portulacaxanthin | 1 | 4.0 | 9.0 | 26.2 | 0.5 | 1 | 0.6 | 2.4 | 8.4 | 1.2 |
| Terpenes | ||||||||||
| oleanolic acid | 1 | 0.3 | 0.6 | 2.4 | 0.9 | 1 | 0.1 | 0.0 | 1.8 | 0.7 |
| ursolic acid | 1 | 0.3 | 0.6 | 2.4 | 0.9 | 1 | 0.1 | 0.0 | 1.8 | 0.7 |
| menthone | 1 | 0.4 | 0.7 | 2.0 | 1.1 | 1 | 0.7 | 0.8 | 0.9 | 0.8 |
| Sterols | ||||||||||
| desmosterol | 1 | 1.2 | 1.3 | 0.7 | 0.6 | 1 | 1.2 | 1.0 | 0.6 | 0.5 |
| Fatty acids | ||||||||||
| myristoleic | 1 | 0.4 | 0.5 | 0.6 | 1.8 | 1 | 0.7 | 0.7 | 1.3 | 8.0 |
| octadecanoic acid | 1 | 0.6 | 0.8 | 0.7 | 0.7 | 1 | 1.1 | 0.8 | 0.8 | 1.8 |
| oleic acid | 1 | 0.6 | 0.5 | 0.8 | 0.6 | 1 | 0.7 | 1.1 | 2.9 | 2.0 |
| palmitoleic acid | 1 | 0.7 | 0.7 | 0.6 | 1.4 | 1 | 1.1 | 1.0 | 1.4 | 6.9 |
| pentadecanoic acid | 1 | 0.8 | 0.8 | 0.6 | 1.2 | 1 | 0.9 | 0.7 | 1.1 | 2.8 |
| glycerol tributanoate | 1 | 0.5 | 0.5 | 0.7 | 1.0 | 1 | 1.0 | 2.6 | 2.3 | 5.9 |
| Metabolites | Root/Shoot Ratios | ||||
|---|---|---|---|---|---|
| * Control | AMF | AMF-D | R | R + D | |
| Phenolics | |||||
| vanillic acid | 10.4 | 14.4 | 2.1 | 27.2 | 17.0 |
| apigenin 7-glucoside | 0.3 | 1.8 | 2.5 | 0.0 | 0.2 |
| cyanidin3-rytinoside | 0.1 | 0.5 | 0.9 | 0.0 | 0.6 |
| hydroxycaffeic acid | 0.3 | 0.0 | 0.1 | 0.9 | 1.3 |
| isoorientin | 0.5 | 0.8 | 0.9 | 0.2 | 0.6 |
| isorhoifolin | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| lariciresinol | 0.7 | 1.6 | 2.7 | 1.9 | 3.4 |
| luteolin-5-glucoside | 0.5 | 0.8 | 0.9 | 0.2 | 0.6 |
| quercetin-3-rutinoside | Shoot sp. | Shoot sp. | Shoot sp. | Shoot sp. | Shoot sp. |
| gamma tocopherol | 20.0 | 123.1 | 5.0 | 6.4 | 42.0 |
| m-coumaric acid | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| maachiain | 1.0 | 1.8 | 0.5 | 1.3 | 26.1 |
| glycinol | 1.1 | 0.9 | 0.9 | 1.0 | 1.0 |
| phaseollin | 94.4 | 14.3 | 10.1 | 149.3 | |
| vestitol | 0.0 | 0.0 | 0.0 | 0.5 | 0.1 |
| sophorol | 0.7 | 1.5 | 0.1 | 0.5 | 4.0 |
| (6aR,11aR)-3,9-Dihydroxypterocarpan | 1.1 | 4.5 | 0.7 | 10.9 | 10.2 |
| 2′-Hydroxyformononetin | 1.0 | 1.8 | 0.5 | 1.3 | 26.1 |
| astragalin | 0.5 | 0.8 | 0.9 | 0.2 | 0.6 |
| chalconaringenin | 1.1 | 0.9 | 0.9 | 1.0 | 1.0 |
| delphinidin | 0.3 | 1.2 | 0.4 | 0.2 | 0.1 |
| eugenol | 49.0 | 0.8 | 0.8 | 1.3 | 1.0 |
| formononetin | 0.2 | 1.6 | 0.0 | 2.5 | 0.9 |
| genistein | 0.3 | 0.6 | 0.9 | 2.4 | 1.5 |
| genistin | 0.2 | 0.6 | 0.8 | 56.9 | 0.2 |
| homogentistic acid | 10.4 | 14.4 | 2.1 | 27.2 | 17.0 |
| leucopelargonidin | 4.7 | 0.9 | 1.1 | 1.1 | 1.1 |
| lotaustralin | 0.1 | 0.1 | 0.0 | 0.6 | 0.4 |
| pelargonidin | 0.3 | 0.6 | 0.9 | 2.4 | 0.1 |
| phenylalcetaldehyde | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| sinapaldehyde | 0.8 | 1.0 | 1.0 | 2.0 | 0.6 |
| vestitone | 0.8 | 0.9 | 1.0 | 1.3 | 1.1 |
| coniferyl acetate | 1.1 | 0.9 | 1.0 | 1.1 | 1.1 |
| astragalin | 0.5 | 0.8 | 0.9 | 0.2 | 0.6 |
| caffeic acid hexoside | 0.3 | 0.0 | 0.1 | 0.9 | 1.3 |
| coniferaldehyde | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| diosmetin | 0.7 | 1.5 | 0.1 | 0.5 | 4.0 |
| eriodictyol | - | - | - | 5.4 | - |
| N-containing compounts | |||||
| caffeine | 0.5 | 0.3 | 0.3 | 0.8 | 0.8 |
| cinchonidine | 0.9 | 1.0 | 0.3 | 0.1 | 1.0 |
| theobromine | 0.6 | 0.1 | 1.2 | 2.2 | 1.6 |
| portulacaxanthin | 0.5 | 0.1 | 0.1 | 0.1 | 1.2 |
| Terpenes | |||||
| oleanolic acid | 1.9 | 0.6 | 0.0 | 1.4 | 1.6 |
| ursolic acid | 1.9 | 0.6 | 0.0 | 1.4 | 1.6 |
| menthone | 0.6 | 1.1 | 0.6 | 0.3 | 0.4 |
| Sterols | |||||
| desmosterol | 1.3 | 1.4 | 1.1 | 1.2 | 1.0 |
| Fatty acids | |||||
| myristoleic | 0.9 | 1.5 | 1.2 | 2.0 | 3.9 |
| octadecanoic acid | 0.9 | 1.6 | 1.0 | 1.0 | 2.3 |
| oleic acid | 1.0 | 1.1 | 2.0 | 3.5 | 3.2 |
| palmitoleic acid | 0.9 | 1.4 | 1.3 | 1.9 | 4.4 |
| pentadecanoic acid | 1.3 | 1.4 | 1.1 | 2.3 | 3.2 |
| glycerol tributanoate | 0.5 | 1.2 | 2.8 | 1.7 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalousi, F.D.; Tsakos, M.; Nikolaou, C.N.; Georgantopoulos, A.; Psarra, A.-M.G.; Tsikou, D. Chemical Analysis and Biological Activities of Extracts Isolated from Symbiotic L. japonicus Plants. Life 2024, 14, 189. https://doi.org/10.3390/life14020189
Kalousi FD, Tsakos M, Nikolaou CN, Georgantopoulos A, Psarra A-MG, Tsikou D. Chemical Analysis and Biological Activities of Extracts Isolated from Symbiotic L. japonicus Plants. Life. 2024; 14(2):189. https://doi.org/10.3390/life14020189
Chicago/Turabian StyleKalousi, Foteini D., Michail Tsakos, Christina N. Nikolaou, Achilleas Georgantopoulos, Anna-Maria G. Psarra, and Daniela Tsikou. 2024. "Chemical Analysis and Biological Activities of Extracts Isolated from Symbiotic L. japonicus Plants" Life 14, no. 2: 189. https://doi.org/10.3390/life14020189
APA StyleKalousi, F. D., Tsakos, M., Nikolaou, C. N., Georgantopoulos, A., Psarra, A.-M. G., & Tsikou, D. (2024). Chemical Analysis and Biological Activities of Extracts Isolated from Symbiotic L. japonicus Plants. Life, 14(2), 189. https://doi.org/10.3390/life14020189






