Phylogeographic Analysis for Understanding Origin, Speciation, and Biogeographic Expansion of Invasive Asian Hornet, Vespa velutina Lepeletier, 1836 (Hymenoptera, Vespidae)
Abstract
1. Introduction
2. Material and Methods
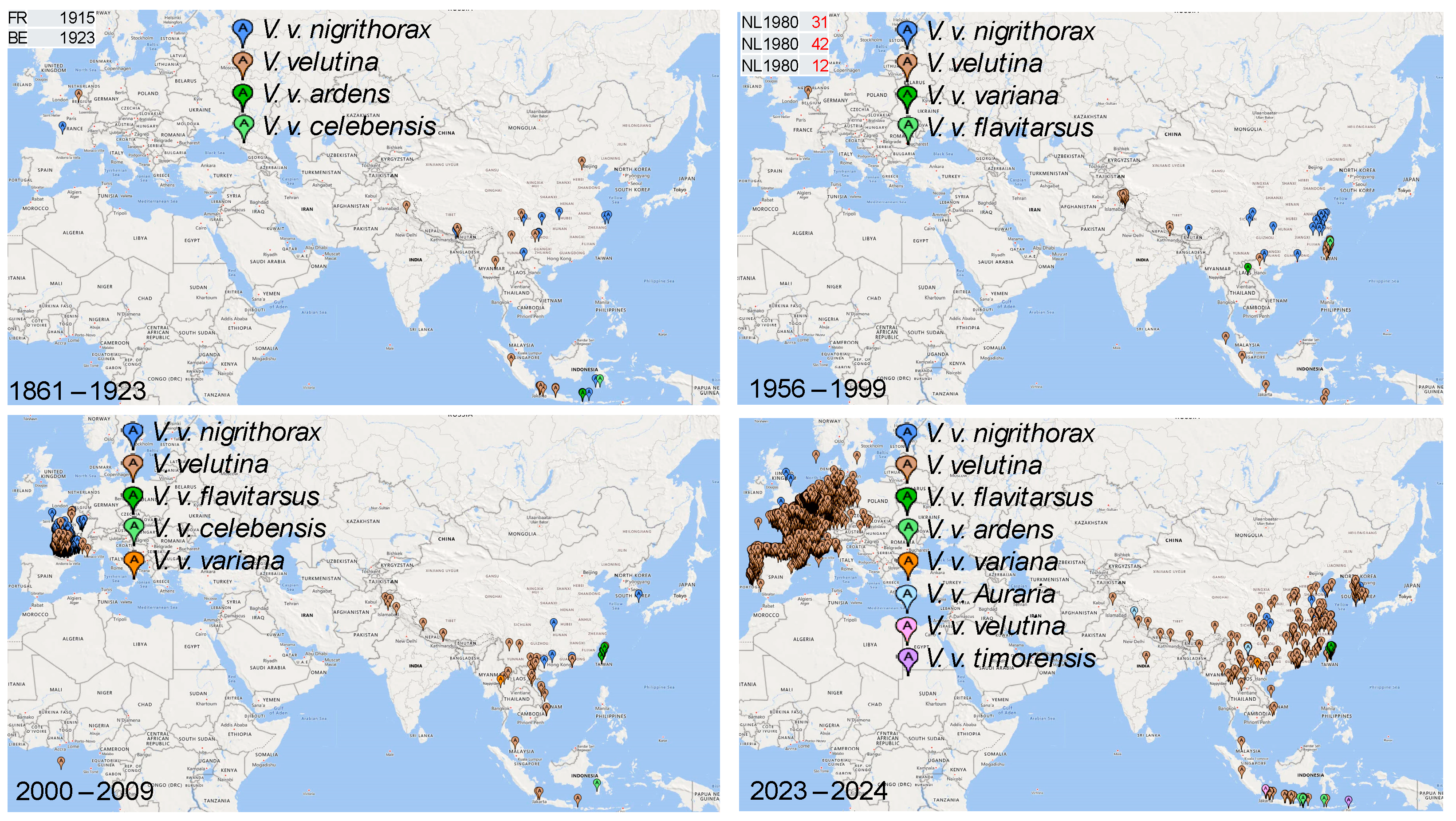
3. Results
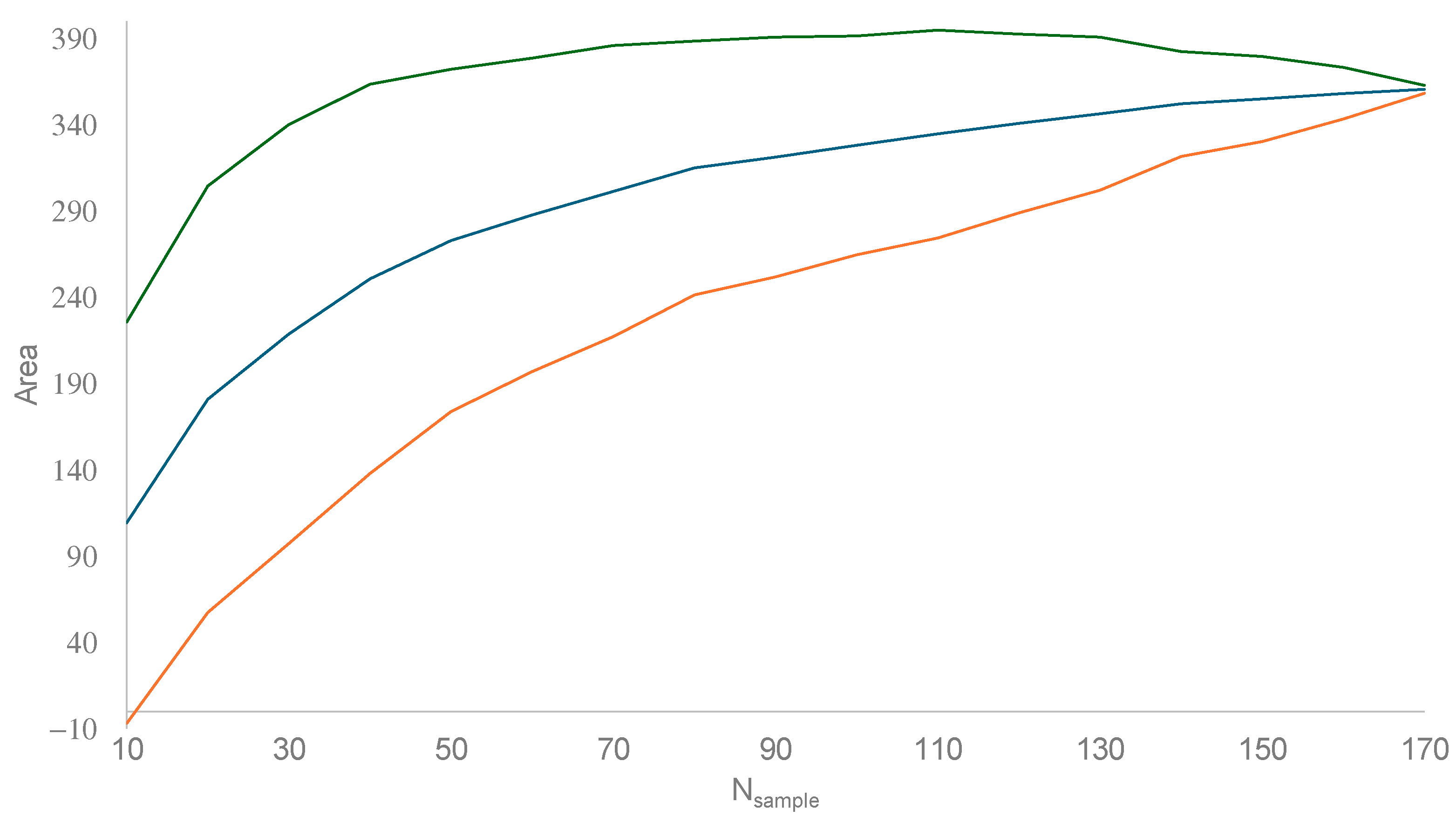
3.1. Estimating Invasion Time () and Instantaneous Rate of Growth (r)
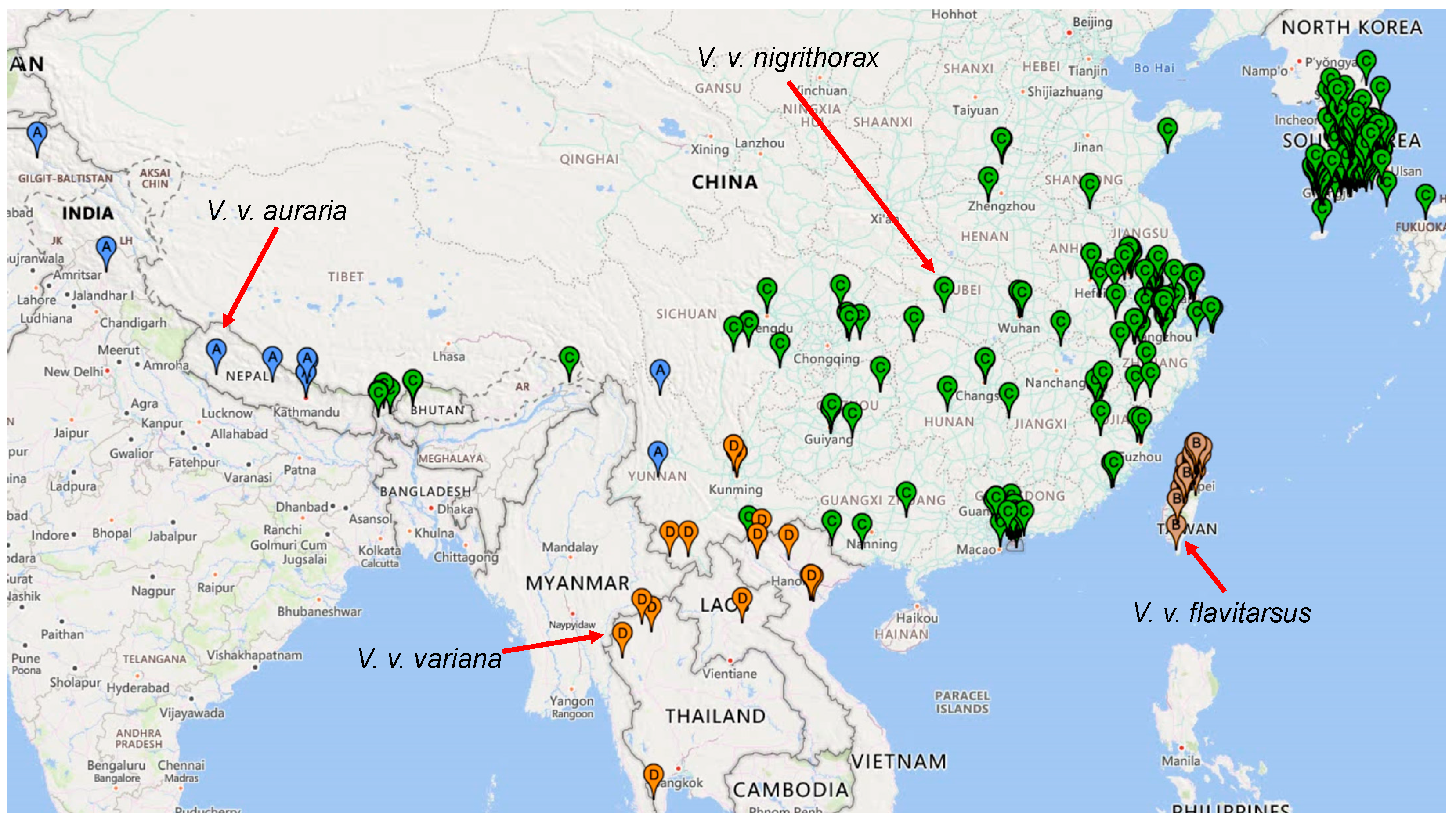
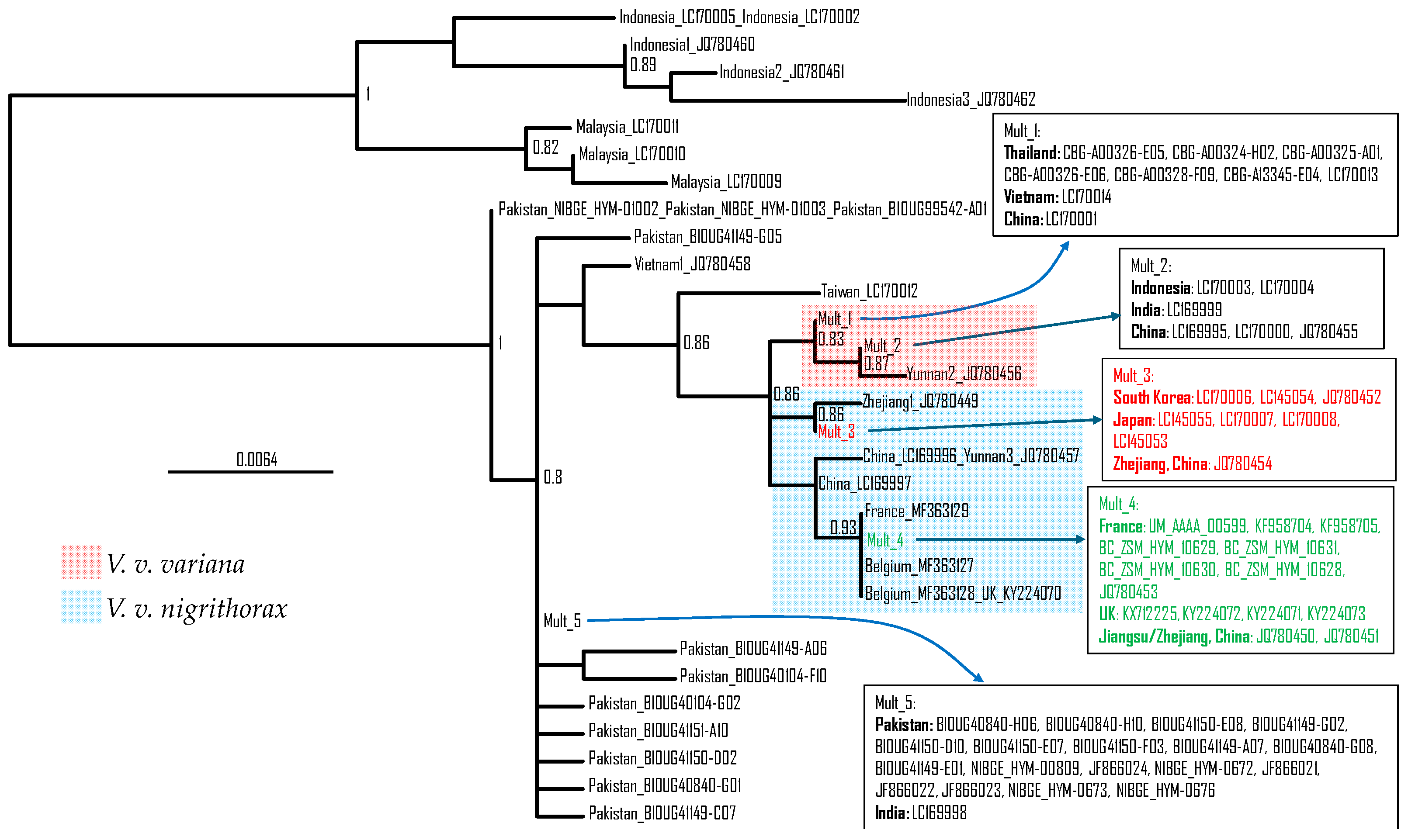
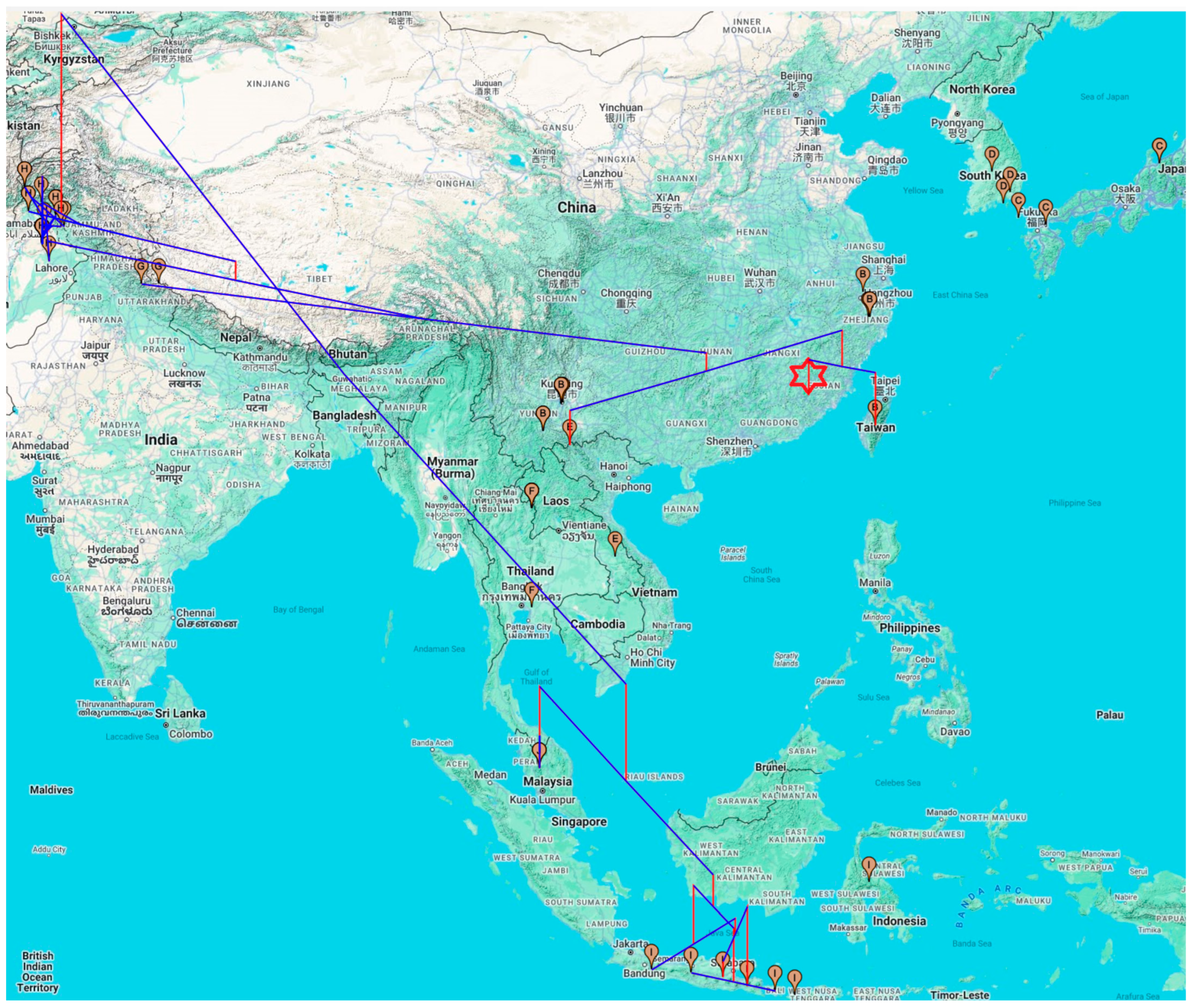
3.2. Phylogeographic Analysis
4. Discussion
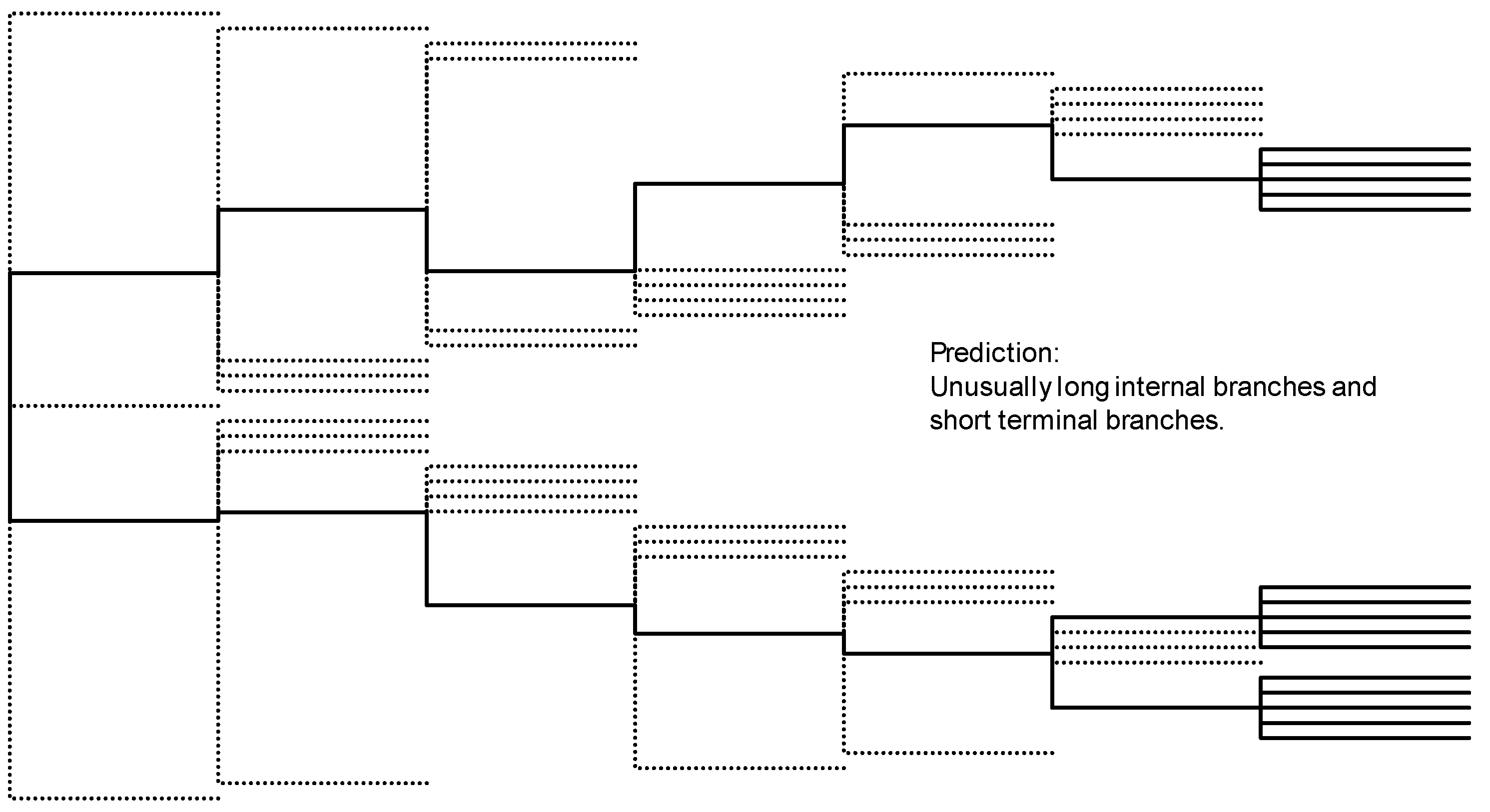
4.1. Species Invasion as a Natural Experiment Opens a Window into a Hidden Part of Nature
4.2. A Proposal for an Index of Invasiveness
4.3. Pros and Cons of a Multi-Loci Phylogeny Versus a Single-Locus Phylogeny
4.4. The Taxonomy of Vespa Velutina Lineages
4.5. Invasive Species and Ecological Niche
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bass, A.; Needham, K.; Bennett, A.M.R. First record of Vespa crabro Linnaeus (Hymenoptera: Vespidae) in western North America with a review of recorded species of Vespa Linnaeus in Canada. Zootaxa 2022, 5154, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Otis, G.W.; Taylor, B.A.; Mattila, H.R. Invasion potential of hornets (Hymenoptera: Vespidae: Vespa spp.). Front. Insect Sci. 2023, 3, 1145158. [Google Scholar] [CrossRef] [PubMed]
- Beggs, J.R.; Brockerhoff, E.G.; Corley, J.C.; Kenis, M.; Masciocchi, M.; Muller, F.; Rome, Q.; Villemant, C. Ecological effects and management of invasive alien Vespidae. BioControl 2011, 56, 505–526. [Google Scholar] [CrossRef]
- Alaniz, A.J.; Carvajal, M.A.; Vergara, P.M. Giants are coming? Predicting the potential spread and impacts of the giant Asian hornet (Vespa mandarinia, Hymenoptera:Vespidae) in the USA. Pest Manag. Sci. 2021, 77, 104–112. [Google Scholar] [CrossRef]
- Nuñez-Penichet, C.; Osorio-Olvera, L.; Gonzalez, V.H.; Cobos, M.E.; Jiménez, L.; DeRaad, D.A.; Alkishe, A.; Contreras-Díaz, R.G.; Nava-Bolaños, A.; Utsumi, K.; et al. Geographic potential of the world’s largest hornet, Vespa mandarinia Smith (Hymenoptera: Vespidae), worldwide and particularly in North America. PeerJ 2021, 9, e10690. [Google Scholar] [CrossRef]
- Bérubé, C. Giant alien insect invasion averted. Am. Bee J. 2020, 160, 209–214. [Google Scholar]
- Zhu, G.; Gutierrez Illan, J.; Looney, C.; Crowder, D.W. Assessing the ecological niche and invasion potential of the Asian giant hornet. Proc. Natl. Acad. Sci. USA 2020, 117, 24646–24648. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.; Sakagami, S.F. A Bionomic Sketch of the Giant Hornet, Vespa mandarinia, a Serious Pest for Japanese Apiculture. J. Faa. Sci. Hokkaido Univ. Ser. VI Zool. 1973, 19, 125–162. [Google Scholar]
- Matsuura, M.; Yamane, S. Biology of the Vespine Wasps; Springer: Heidelberg, Germany, 1990; p. XIX, 323. [Google Scholar]
- Brodmann, J.; Twele, R.; Francke, W.; Yi-bo, L.; Xi-qiang, S.; Ayasse, M. Orchid mimics honey bee alarm pheromone in order to attract hornets for pollination. Curr. Biol. 2009, 19, 1368–1372. [Google Scholar] [CrossRef]
- Monceau, K.; Bonnard, O.; Thiéry, D. Vespa velutina: A new invasive predator of honeybees in Europe. J. Pest Sci. 2014, 87, 1–16. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Salles, J.-M.; Courchamp, F. The economic cost of control of the invasive yellow-legged Asian hornet. NeoBiota 2020, 55, 11–25. [Google Scholar] [CrossRef]
- Ono, M.; Igarashi, T.; Ohno, E.; Sasaki, M. Unusual thermal defence by a honeybee against mass attack by hornets. Nature 1995, 377, 334–336. [Google Scholar] [CrossRef]
- Ono, M.; Okada, I.; Sasaki, M. Heat production by balling in the Japanese honeybee, Apis cerana japonica as a defensive behavior against the hornet, Vespa simillima xanthoptera (Hymenoptera: Vespidae). Experientia 1987, 43, 1031–1034. [Google Scholar] [CrossRef]
- McClenaghan, B.; Schlaf, M.; Geddes, M.; Mazza, J.; Pitman, G.; McCallum, K.; Rawluk, S.; Hand, K.; Otis, G.W. Behavioral responses of honey bees, Apis cerana and Apis mellifera, to Vespa mandarinia marking and alarm pheromones. J. Apic. Res. 2019, 58, 141–148. [Google Scholar] [CrossRef]
- Wilson, T.M.; Takahashi, J.; Spichiger, S.-E.; Kim, I.; van Westendorp, P. First Reports of Vespa mandarinia (Hymenoptera: Vespidae) in North America Represent Two Separate Maternal Lineages in Washington State, United States, and British Columbia, Canada. Ann. Entomol. Soc. Am. 2020, 113, 468–472. [Google Scholar] [CrossRef]
- Papachristoforou, A.; Rortais, A.; Zafeiridou, G.; Theophilidis, G.; Garnery, L.; Thrasyvoulou, A.; Arnold, G. Smothered to death: Hornets asphyxiated by honeybees. Curr. Biol. 2007, 17, R795–R796. [Google Scholar] [CrossRef] [PubMed]
- Baracchi, D.; Cusseau, G.; Pradella, D.; Turillazzi, S. Defence reactions of Apis mellifera ligustica against attacks from the European hornet Vespa crabro. Ethol. Ecol. Evol. 2010, 22, 281–294. [Google Scholar] [CrossRef]
- Tan, K.; Radloff, S.E.; Li, J.J.; Hepburn, H.R.; Yang, M.X.; Zhang, L.J.; Neumann, P. Bee-hawking by the wasp, Vespa velutina, on the honeybees Apis cerana and A. mellifera. Naturwissenschaften 2007, 94, 469–472. [Google Scholar] [CrossRef]
- Archer, M.E.; Penney, D. Vespine Wasps of the World: Behaviour, Ecology and Taxonomy of the Vespinae; Siri Scientif Press: Manchester, UK, 2012; p. 352. [Google Scholar]
- Smith-Pardo, A.H.; Carpenter, J.M.; Kimsey, L. The Diversity of Hornets in the Genus Vespa (Hymenoptera: Vespidae; Vespinae), Their Importance and Interceptions in the United States. Insect Syst. Divers. 2020, 4, 1–27. [Google Scholar] [CrossRef]
- Archer, M. Taxonomy, distribution and nesting biology of the Vespa bicolor group (Hym., Vespinae). Entomol. Mon. Mag. 1994, 130, 149–158. [Google Scholar]
- Perrard, A.; Arca, M.; Rome, Q.; Muller, F.; Tan, J.; Bista, S.; Nugroho, H.; Baudoin, R.; Baylac, M.; Silvain, J.-F.; et al. Geographic Variation of Melanisation Patterns in a Hornet Species: Genetic Differences, Climatic Pressures or Aposematic Constraints? PLoS ONE 2014, 9, e94162. [Google Scholar] [CrossRef] [PubMed]
- van der Vecht, J. The Vespinae of the Indo-Malayan and Papuan Areas (Hymenoptera, Vespidae). Zool. Verh. 1957, 34, 1–82. [Google Scholar]
- Villemant, C.; Muller, F.; Haubois, S.; Perrard, A.; Darrouzet, E.; Rome, Q. Bilan dês travaux (MNHN et IRBI) sur l’invasion en France de Vespa velutina, le frelon asiatique prédateur d’abeilles. In Journée Scientifique Apicole; Barbançon, J.-M., L’hostis, M., Eds.; JSA: Nantes, France, 2011; pp. 3–12. [Google Scholar]
- Ueno, T. Establishment of the Invasive Hornet Vespa velutina (Hymenoptera: Vespidae) in Japan. Int. J. Chem. Environ. Biol. Sci. 2014, 2, 220–222. [Google Scholar]
- Kim, J.K.; Moon, T.Y.; Yoon, I.B. Systematics of Vespine wasps from Korea, 1. Genus Vespa Linnaeus (Vespidae, Hymenoptera). Korean J. Entomol. 1994, 24, 107–115. [Google Scholar]
- Choi, M.B.; Martin, S.J.; Lee, J.W. Distribution, spread, and impact of the invasive hornet Vespa velutina in South Korea. J. Asia-Pac. Entomol. 2012, 15, 473–477. [Google Scholar] [CrossRef]
- Gabín-García, L.B.; Bartolomé, C.; Guerra-Tort, C.; Rojas-Nossa, S.V.; Llovo, J.; Maside, X. Identification of pathogens in the invasive hornet Vespa velutina and in native Hymenoptera (Apidae, Vespidae) from SW-Europe. Sci. Rep. 2021, 11, 11233. [Google Scholar] [CrossRef] [PubMed]
- Macià, F.X.; Menchetti, M.; Corbella, C.; Grajera, J.; Vila, R. Exploitation of the invasive Asian Hornet Vespa velutina by the European Honey Buzzard Pernis apivorus. Bird Study 2019, 66, 425–429. [Google Scholar] [CrossRef]
- Onofre, N.; Portugal, E.C.M.I.; Nave, A.; Cadima, I.S.P.; Ferreira, M.; Godinho, J. On the Evidence of the European Bee-Eater (Merops apiaster) as a Predator of the Yellow-Legged Hornet (Vespa velutina) and Its Possible Contribution as a Biocontrol Agent. Animals 2023, 13, 1906. [Google Scholar] [CrossRef]
- Rakesh, M.; Aris-Brosou, S.; Xia, X. Testing alternative hypotheses on the origin and speciation of Hawaiian katydids. BMC Ecol. Evol. 2022, 22, 83. [Google Scholar] [CrossRef]
- Freeman, A.; Xia, X. Phylogeographic Reconstruction to Trace the Source Population of Asian Giant Hornet Caught in Nanaimo in Canada and Blaine in the USA. Life 2024, 14, 283. [Google Scholar] [CrossRef]
- Franklin, D.N.; Brown, M.A.; Datta, S.; Cuthbertson, A.G.S.; Budge, G.E.; Keeling, M.J. Invasion dynamics of Asian hornet, Vespa velutina (Hymenoptera: Vespidae): A case study of a commune in south-west France. Appl. Entomol. Zool. 2017, 52, 221–229. [Google Scholar] [CrossRef] [PubMed]
- GBIF.org 2024. GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0071789-240506114902167 (accessed on 24 September 2024).
- Haxaire, J.; Bouguet, J.-P.; Tamisier, J.-P. Vespa velutina Lepeletier, 1836, une redoutable nouveauté pour la faune de France (Hymenoptera, Vespidae). Bull. Société Entomol. Fr. 2006, 111, 194. [Google Scholar] [CrossRef]
- Villemant, C.; Streito, J.-C.; Haxaire, J. Premier bilan de l’invasion de Vespa velutina Lepeletier en France (Hymenoptera, Vespidae). Bsef 2006, 111, 535–538. [Google Scholar] [CrossRef]
- Rortais, A.; Villemant, C.; Gargominy, O.; Rome, Q.; Haxaire, J.; Papachristoforou, A.; Arnold, G. A new enemy of honeybees in Europe: The Asian hornet Vespa velutina. Atlas Biodivers. Risks–Eur. Globe Stories Maps. Sofia Mosc. Pensoft 2010, 11, 181. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Wares, J.P.; Hughes, A.R.; Grosberg, R.K. Mechanisms that drive evolutionary change: Insights from species introductions and invasions. In Species Invasions: Insights into Ecology, Evolution and Biogeography; Sax, D.F., Stachowicz, J.J., Gaines, S.D., Eds.; Sinauer: Sunderland, MA, USA, 2005; pp. 229–257. [Google Scholar]
- Stockland, P.-E. Statecraft and Insect Oeconomies in the Global French Enlightenment (1670–1815); Columbia University: New York, NY, USA, 2018. [Google Scholar]
- Arca, M.; Mougel, F.; Guillemaud, T.; Dupas, S.; Rome, Q.; Perrard, A.; Muller, F.; Fossoud, A.; Capdevielle-Dulac, C.; Torres-Leguizamon, M.; et al. Reconstructing the invasion and the demographic history of the yellow-legged hornet, Vespa velutina, in Europe. Biol. Invasions 2015, 17, 2357–2371. [Google Scholar] [CrossRef]
- Takeuchi, T.; Takahashi, R.; Kiyoshi, T.; Nakamura, M.; Minoshima, Y.N.; Takahashi, J. The origin and genetic diversity of the yellow-legged hornet, Vespa velutina introduced in Japan. Insectes Sociaux 2017, 64, 313–320. [Google Scholar] [CrossRef]
- Xia, X. How Trustworthy Are Genomic Sequences of SARS-CoV-2 in GenBank? Preprints 2024, 2024081963. [Google Scholar]
- Xia, X. DAMBE6: New Tools for Microbial Genomics, Phylogenetics, and Molecular Evolution. J. Hered. 2017, 108, 431–437. [Google Scholar] [CrossRef]
- Perrard, A.; Pickett, K.; Villemant, C.; Kojima, J.-i.; Carpenter, J.M. Phylogeny of hornets: A total evidence approach (Hymenoptera, Vespidae, Vespinae, Vespa). J. Hymenopt. Res. 2013, 32, 1–15. [Google Scholar] [CrossRef]
- Urtgam, S.; Jongjitvimol, T. Genetic Evolution of Asian Predatory Wasp, Vespa velutina, in Northernof Thailand Based on Cytochrome Oxidase Subunit I DNA Barcoding. NU. Int. J. Sci. 2020, 17, 101–113. [Google Scholar]
- Mohamadzade Namin, S.; Jung, C. Genetic diversity of genus Vespa including an invaded species of V. velutina (Hymenoptera: Vespidae) in Korea inferred from DNA barcoding data. J. Asia-Pac. Entomol. 2020, 23, 540–545. [Google Scholar] [CrossRef]
- Mattila, H.R.; Nguyen, L.T.P.; Perrard, A.; Bain, M.; Otis, G.W. Biology of the southern giant hornet, Vespa soror: Nest architecture, morphological differences among castes, and the genetic structure of colonies. Front. Insect Sci. 2023, 3, 1136297. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Asimenos, G.; Toh, H. Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 2009, 537, 39–64. [Google Scholar] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Yang, Z. Statistical properties of the maximum likelihood method of phylogenetic estimation and comparison with distance matrix method. Syst. Biol. 1994, 43, 329–342. [Google Scholar] [CrossRef]
- Xia, X. Information-theoretic indices and an approximate significance test for testing the molecular clock hypothesis with genetic distances. Mol. Phylogenet. Evol. 2009, 52, 665–676. [Google Scholar] [CrossRef]
- Xia, X. A Mathematical Primer of Molecular Phylogenetics; CRC Press: New York, NY, USA, 2020; p. 380. [Google Scholar]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Tavaré, S. Some Probabilistic and Statistical Problems in the Analysis of DNA Sequences; American Mathematical Society: Providence, RI, USA, 1986; Volume 17, pp. 57–86. [Google Scholar]
- Lanave, C.; Preparata, G.; Saccone, C.; Serio, G. A new method for calculating evolutionary substitution rates. J. Mol. Evol. 1984, 20, 86–93. [Google Scholar] [CrossRef]
- Graham, R.L. An efficient algorith for determining the convex hull of a finite planar set. Inf. Process. Lett. 1972, 1, 132–133. [Google Scholar] [CrossRef]
- Xia, X. PGT: Visualizing temporal and spatial biogeographic patterns. Glob. Ecol. Biogeogr. 2019, 28, 1195–1199. [Google Scholar] [CrossRef]
- le Ministre de l’Agriculture; de l’Agroalimentaire et de la Forêt; la Ministre de l’Ecologie (Eds.) French Government, Stéphane Le Foll et Delphine Batho Annoncent le Classement du Frelon Asiatique en Espèce Envahissante et Nuisible. Ministere de ‘Agriculture et de la Souverainete Alimentaire, 2012. Available online: https://agriculture.gouv.fr/stephane-le-foll-et-delphine-batho-annoncent-le-classement-du-frelon-asiatique-en-espece (accessed on 24 September 2024).
- Kim, J.-K.; Choi, M.; Moon, T.-Y. Occurrence of Vespa velutina Lepeletier from Korea, and a revised key for Korean Vespa species (Hymenoptera: Vespidae). Entomol. Res. 2006, 36, 112–115. [Google Scholar] [CrossRef]
- Carisio, L.; Cerri, J.; Lioy, S.; Bianchi, E.; Bertolino, S.; Porporato, M. Impacts of the invasive hornet Vespa velutina on native wasp species: A first effort to understand population-level effects in an invaded area of Europe. J. Insect Conserv. 2022, 26, 663–671. [Google Scholar] [CrossRef]
- Rome, Q.; Dambrine, L.; Onate, C.; Muller, F.; Villemant, C.; García-Pérez, A.; Maia, M.; Carvalho-Esteves, P.; Bruneau, E. Spread of the invasive hornet Vespa velutina Lepeletier, 1836, in Europe in 2012 (Hym., Vespidae). Bull. Société Entomol. Fr. 2013, 118, 21–22. [Google Scholar] [CrossRef]
- Sakai, Y.; Takahashi, J. Discovery of a worker of Vespa velutina (Hymenoptera: Vespidae) from Tsushima Island, Japan. Jpn J. Entomol. NS. 2014, 17, 32–36. [Google Scholar]
- Minoshima, Y.N.; Yamane, S.; Ueno, T. An invasive alien hornet, Vespa velutina nigrithorax du Buysson (Hymenoptera, Vespidae), found in Kitakyushu, Kyushu Island: A first record of the species from mainland Japan. Jpn J. Syst. Entomol. 2015, 21, 259–261. [Google Scholar]
- Herrera, C.; Ferragut, J.F.; Leza, M.; Jurado-Rivera, J.A. Invasion genetics of the yellow-legged hornet Vespa velutina in the Westernmost Mediterranean archipelago. J. Pest Sci. 2024, 97, 645–656. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Bessa, A.S.; Carvalho, J.; Gomes, A.; Santarém, F. Climate and land-use drivers of invasion: Predicting the expansion of Vespa velutina nigrithorax into the Iberian Peninsula. Insect Conserv. Divers. 2016, 9, 27–37. [Google Scholar] [CrossRef]
- Takahashi, R.; Okuyama, H.; Minoshima, Y.N.; Takahashi, J.I. Complete mitochondrial DNA sequence of the alien hornet Vespa velutina (Insecta: Hymenoptera) invading Kyushu Island, Japan. Mitochondrial DNA. Part B Resour. 2018, 3, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, Y.; Morita, K.; Sugiura, T.; Okada, Y. Cutaneous hemorrhage or necrosis findings after Vespa mandarinia (wasp) stings may predict the occurrence of multiple organ injury: A case report and review of literature. Clin. Toxicol. 2007, 45, 803–807. [Google Scholar] [CrossRef] [PubMed]
- de Haro, L.; Labadie, M.; Chanseau, P.; Cabot, C.; Blanc-Brisset, I.; Penouil, F. Medical consequences of the Asian black hornet (Vespa velutina) invasion in Southwestern France. Toxicon 2010, 55, 650–652. [Google Scholar] [CrossRef]
- Mayr, E. Systematics and the Origin of Species; Columbia University Press: New York, NY, USA, 1942. [Google Scholar]
- Templeton, A.R. The theory of speciation via the founder principle. Genetics 1980, 94, 1011–1038. [Google Scholar] [CrossRef]
- Wright, S. Statistical genetics and evolution. Bull. Am. Math. Soc. 1942, 48, 223–246. [Google Scholar] [CrossRef][Green Version]
- García-Dorado, A. Understanding and predicting the fitness decline of shrunk populations: Inbreeding, purging, mutation, and standard selection. Genetics 2012, 190, 1461–1476. [Google Scholar] [CrossRef]
- Grossen, C.; Guillaume, F.; Keller, L.F.; Croll, D. Purging of highly deleterious mutations through severe bottlenecks in Alpine ibex. Nat. Commun. 2020, 11, 1001. [Google Scholar] [CrossRef] [PubMed]
- Kleinman-Ruiz, D.; Lucena-Perez, M.; Villanueva, B.; Fernández, J.; Saveljev, A.P.; Ratkiewicz, M.; Schmidt, K.; Galtier, N.; García-Dorado, A.; Godoy, J.A. Purging of deleterious burden in the endangered Iberian lynx. Proc. Natl. Acad. Sci. USA 2022, 119, e2110614119. [Google Scholar] [CrossRef]
- Mortimer, R.K.; Romano, P.; Suzzi, G.; Polsinelli, M. Genome renewal: A new phenomenon revealed from a genetic study of 43 strains of Saccharomyces cerevisiae derived from natural fermentation of grape musts. Yeast 1994, 10, 1543–1552. [Google Scholar] [CrossRef]
- Masel, J.; Lyttle, D.N. The consequences of rare sexual reproduction by means of selfing in an otherwise clonally reproducing species. Theor. Popul. Biol. 2011, 80, 317–322. [Google Scholar] [CrossRef][Green Version]
- Dlugosch, K.M.; Parker, I.M. Founding events in species invasions: Genetic variation, adaptive evolution, and the role of multiple introductions. Mol. Ecol. 2008, 17, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Girish Kumar, P.; Srinivasan, G. Taxonomic Studies of Hornet Wasps (Hymenoptera: Vespidae) Vespa Linnaeus of India. Rec. Zool. Surv. India 2010, 110, 57–80. [Google Scholar] [CrossRef]
- Carpenter, J.M.; Kojima, J. Checklist of the Species in the Subfamily Vespinae (Insecta: Hymenoptera: Vespidae). Nat. Hist. Bull Ibaraki Univ. 1997, 1, 51–92. [Google Scholar]
- Xia, X. Horizontal Gene Transfer and Drug Resistance Involving Mycobacterium tuberculosis. Antibiotics 2023, 12, 1367. [Google Scholar] [CrossRef]
- Askari Rad, M.; Kruglikov, A.; Xia, X. Three-Way Alignment Improves Multiple Sequence Alignment of Highly Diverged Sequences. Algorithms 2024, 17, 205. [Google Scholar] [CrossRef]
- Noah, K.; Hao, J.; Li, Y.; Sun, X.; Foley, B.T.; Yang, Q.; Xia, X. Major revisions in arthropod phylogeny through improved supermatrix, with support for two possible waves of land invasion by chelicerates. Evol. Bioinform. 2020, 16, 1–12. [Google Scholar] [CrossRef]
- Hughes, W.O.H.; Ratnieks, F.L.W.; Oldroyd, B.P. Multiple paternity or multiple queens: Two routes to greater intracolonial genetic diversity in the eusocial Hymenoptera. J. Evol. Biol. 2008, 21, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Bjenning, C.; Wang, F.; Wang, N.; Kream, R.M. Mitochondrial Heteroplasmy. Adv. Exp. Med. Biol. 2017, 982, 577–594. [Google Scholar]
- Wilton, P.R.; Zaidi, A.; Makova, K.; Nielsen, R. A Population Phylogenetic View of Mitochondrial Heteroplasmy. Genetics 2018, 208, 1261–1274. [Google Scholar] [CrossRef]
- Holland, M.M.; Makova, K.D.; McElhoe, J.A. Deep-Coverage MPS Analysis of Heteroplasmic Variants within the mtGenome Allows for Frequent Differentiation of Maternal Relatives. Genes 2018, 9, 124. [Google Scholar] [CrossRef]
- Bedoya, G.; Montoya, P.; García, J.; Soto, I.; Bourgeois, S.; Carvajal, L.; Labuda, D.; Alvarez, V.; Ospina, J.; Hedrick, P.W.; et al. Admixture dynamics in Hispanics: A shift in the nuclear genetic ancestry of a South American population isolate. Proc. Natl. Acad. Sci. USA 2006, 103, 7234–7239. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.K.; Dorey, J.B.; Stevens, M.I.; Gardner, M.G.; Bradford, T.M.; Schwarz, M.P. Unparalleled mitochondrial heteroplasmy and Wolbachia co-infection in the non-model bee, Amphylaeus morosus. Curr. Res. Insect Sci. 2022, 2, 100036. [Google Scholar] [CrossRef] [PubMed]
- Mastrantonio, V.; Porretta, D.; Urbanelli, S.; Crasta, G.; Nascetti, G. Dynamics of mtDNA introgression during species range expansion: Insights from an experimental longitudinal study. Sci. Rep. 2016, 6, 30355. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.E. A Key to the World Species of the Vespinae (Hymenoptera); College of Ripon & York St John: York, UK, 1989. [Google Scholar]
- Nguyen, L.T.P.; Carpenter, J.M. Vespidae Of Vietnam (Insecta: Hymenoptera) 1. Vespinae. J. N. Y. Entomol. Soc. 2002, 110, 113, 199–211. [Google Scholar]
- Nguyen, L.T.P.; Saito, F.; Kojima, J.-I.; Carpenter, J.M. Vespidae of Viet Nam (Insecta: Hymenoptera) 2. Taxonomic Notes on Vespinae. Zool. Sci. 2006, 23, 95–104, 110. [Google Scholar] [CrossRef]
- Tan, C.-C.; Li, J.-C. Inheritance of the Elytral Color Patterns of the Lady-Bird Beetle, Harmonia axyridis Pallas. Am. Nat. 1934, 68, 252–265. [Google Scholar] [CrossRef]
- Tan, C.C. Mosaic Dominance in the Inheritance of Color Patterns in the Lady-Bird Beetle, Harmonia Axyridis. Genetics 1946, 31, 195–210. [Google Scholar] [CrossRef]
- Tan, C.C. Identification of the Salivary Gland Chromosomes in Drosophila Pseudoobscura. Proc. Natl. Acad. Sci. USA 1935, 21, 200–202. [Google Scholar] [CrossRef]
- Pocheville, A. The Ecological Niche: History and Recent Controversies. In Handbook of Evolutionary Thinking in the Sciences; Heams, T., Lecointre, G., Huneman, P., Silberstein, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 547–586. [Google Scholar]
- Schoener, T.W. Ecological niche. In The Princeton Guide to Ecology; Levin, S.A., Carpenter, S.R., Godfray, H.C.J., Kinzig, A.P., Loreau, M., Losos, J.B., Walker, B., Wilcove, D.S., Eds.; Princeton University Press: Princeton, NJ, USA, 2009. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Paine, R.T. Food Web Complexity and Species Diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2023, 21, 195–210. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X. Phylogeographic Analysis for Understanding Origin, Speciation, and Biogeographic Expansion of Invasive Asian Hornet, Vespa velutina Lepeletier, 1836 (Hymenoptera, Vespidae). Life 2024, 14, 1293. https://doi.org/10.3390/life14101293
Xia X. Phylogeographic Analysis for Understanding Origin, Speciation, and Biogeographic Expansion of Invasive Asian Hornet, Vespa velutina Lepeletier, 1836 (Hymenoptera, Vespidae). Life. 2024; 14(10):1293. https://doi.org/10.3390/life14101293
Chicago/Turabian StyleXia, Xuhua. 2024. "Phylogeographic Analysis for Understanding Origin, Speciation, and Biogeographic Expansion of Invasive Asian Hornet, Vespa velutina Lepeletier, 1836 (Hymenoptera, Vespidae)" Life 14, no. 10: 1293. https://doi.org/10.3390/life14101293
APA StyleXia, X. (2024). Phylogeographic Analysis for Understanding Origin, Speciation, and Biogeographic Expansion of Invasive Asian Hornet, Vespa velutina Lepeletier, 1836 (Hymenoptera, Vespidae). Life, 14(10), 1293. https://doi.org/10.3390/life14101293






