Temperature-Induced Seasonal Dynamics of Brain Gangliosides in Rainbow Trout (Oncorhynchus mykiss Walbaum) and Common Carp (Cyprinus carpio L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals, Ethics, and Tissue Collection
2.2. Methods
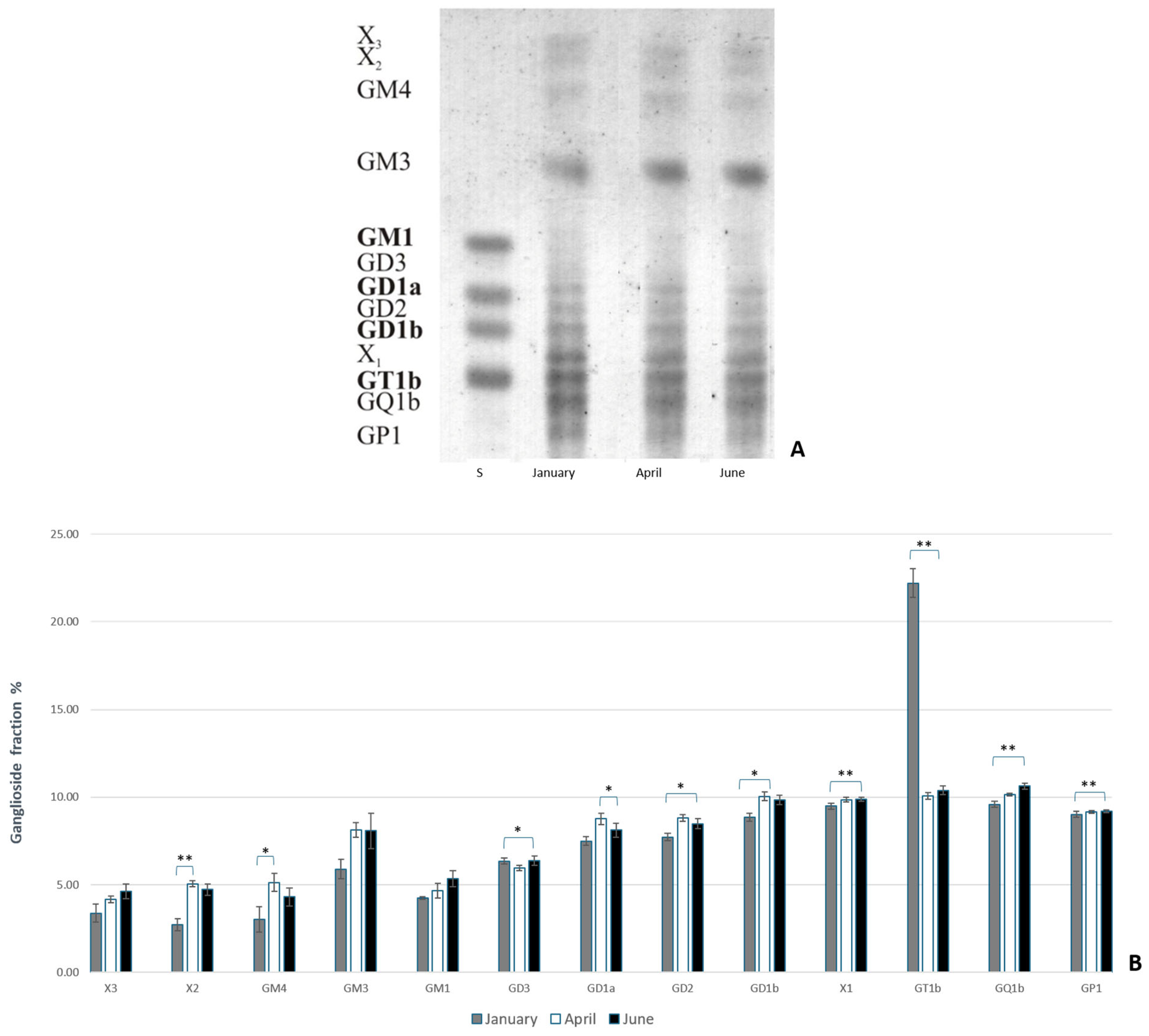
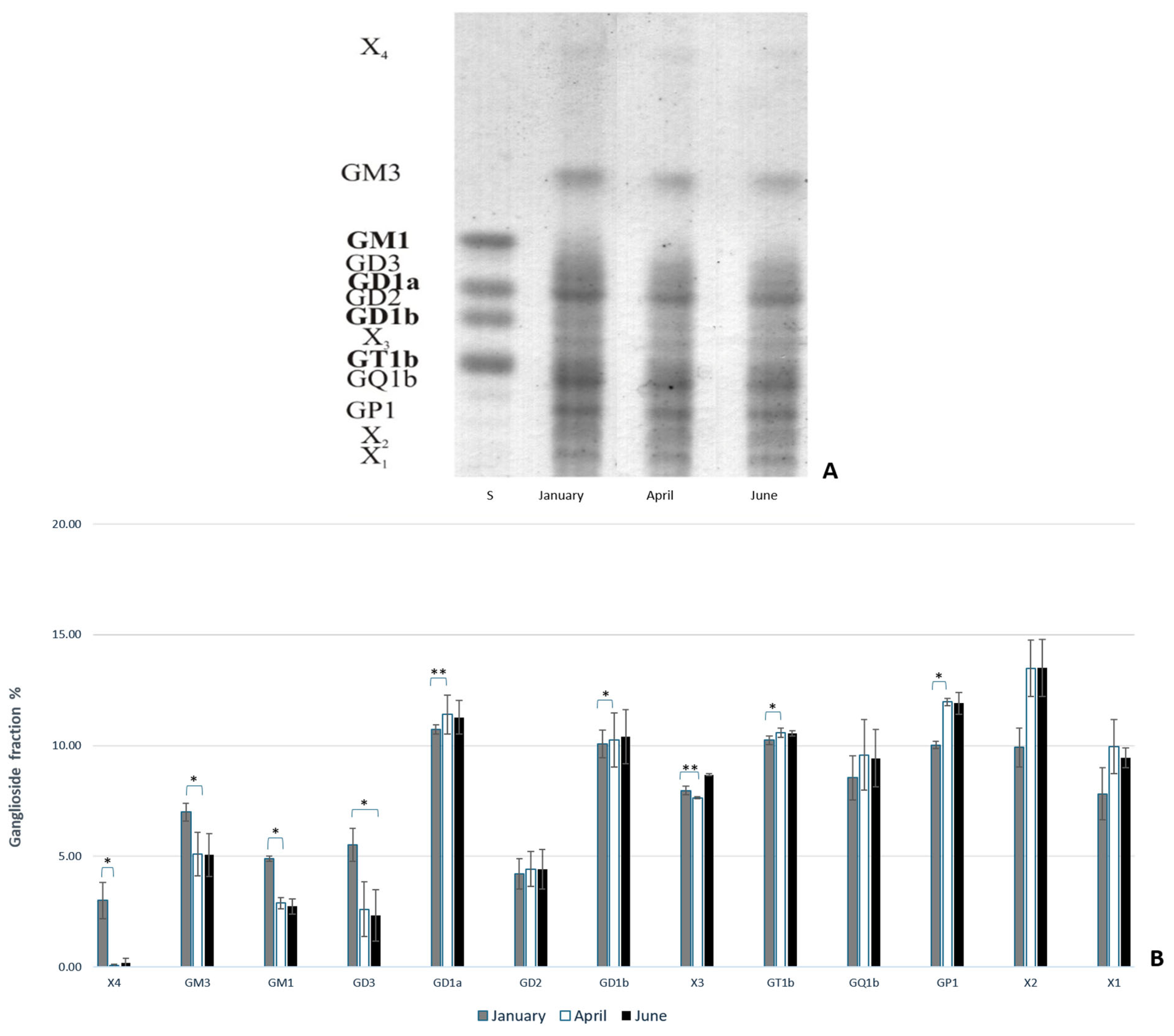
2.2.1. Ganglioside Extraction
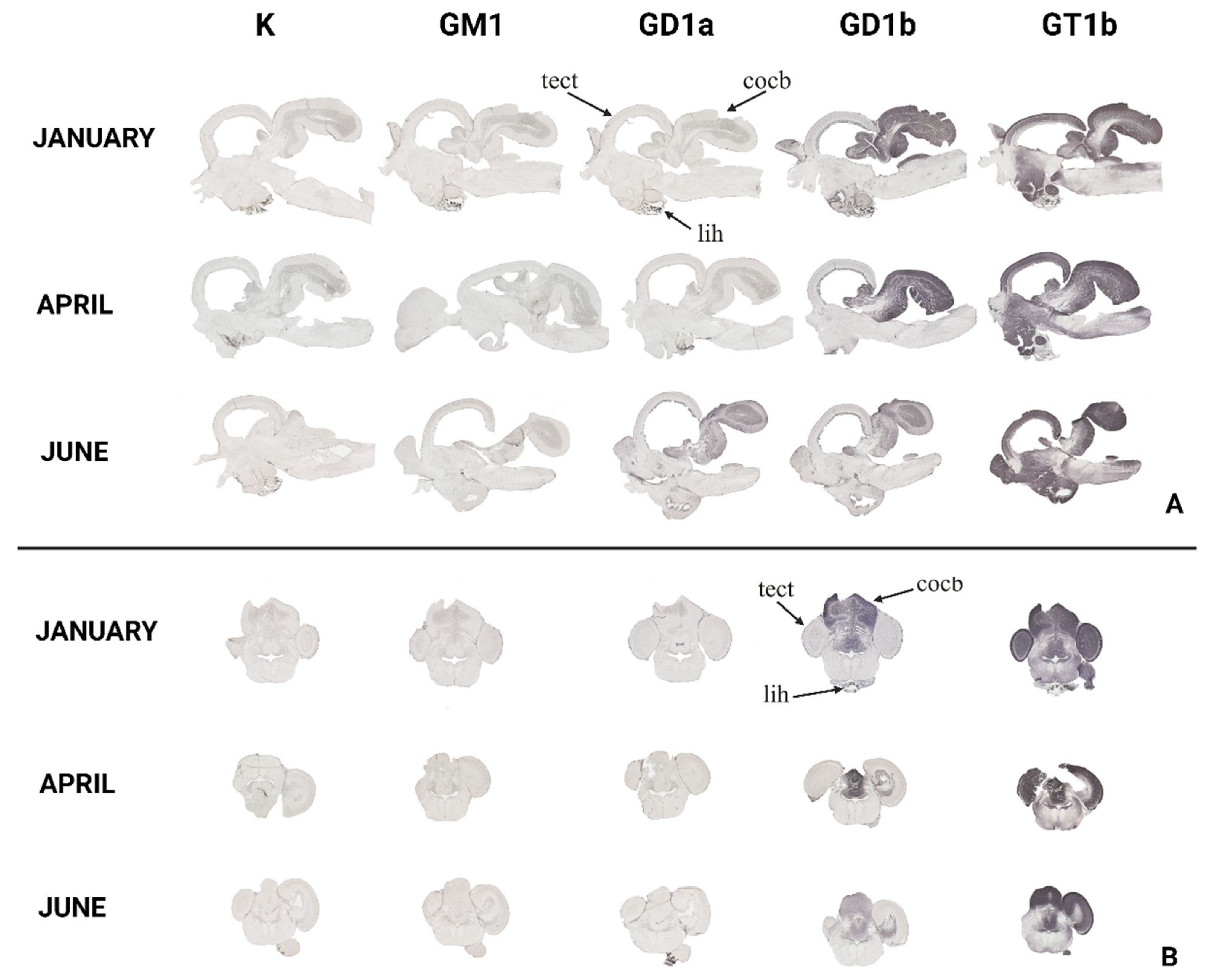
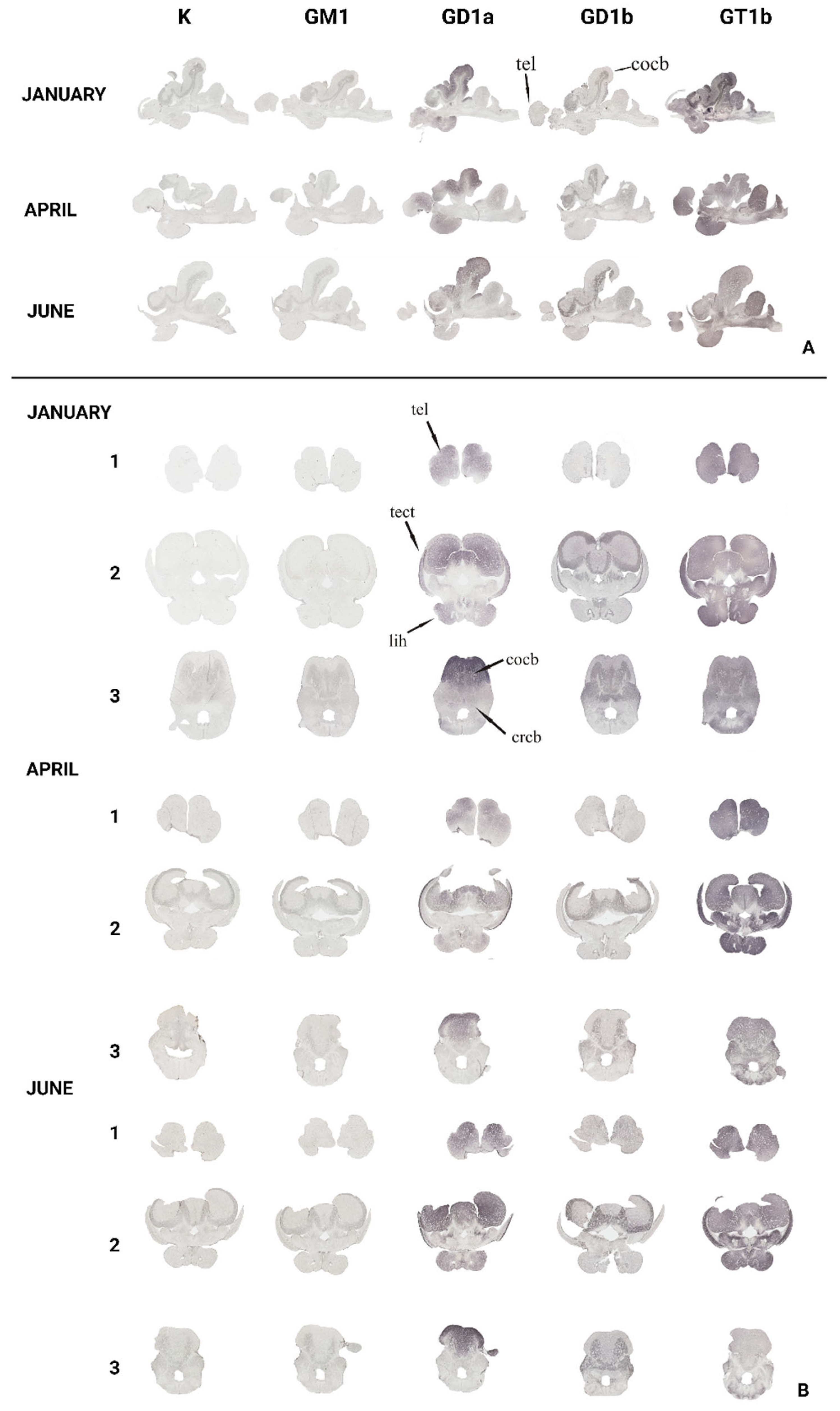
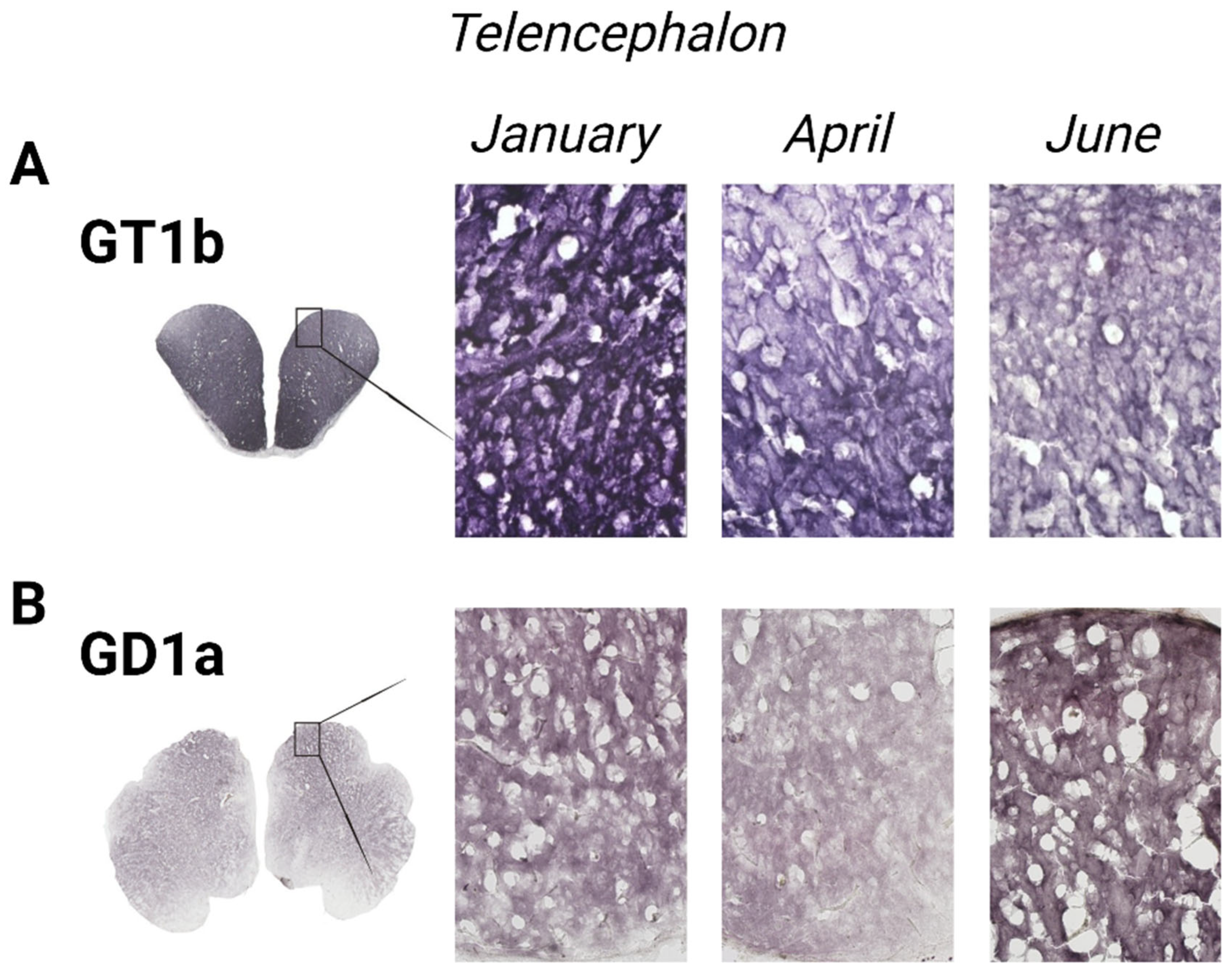
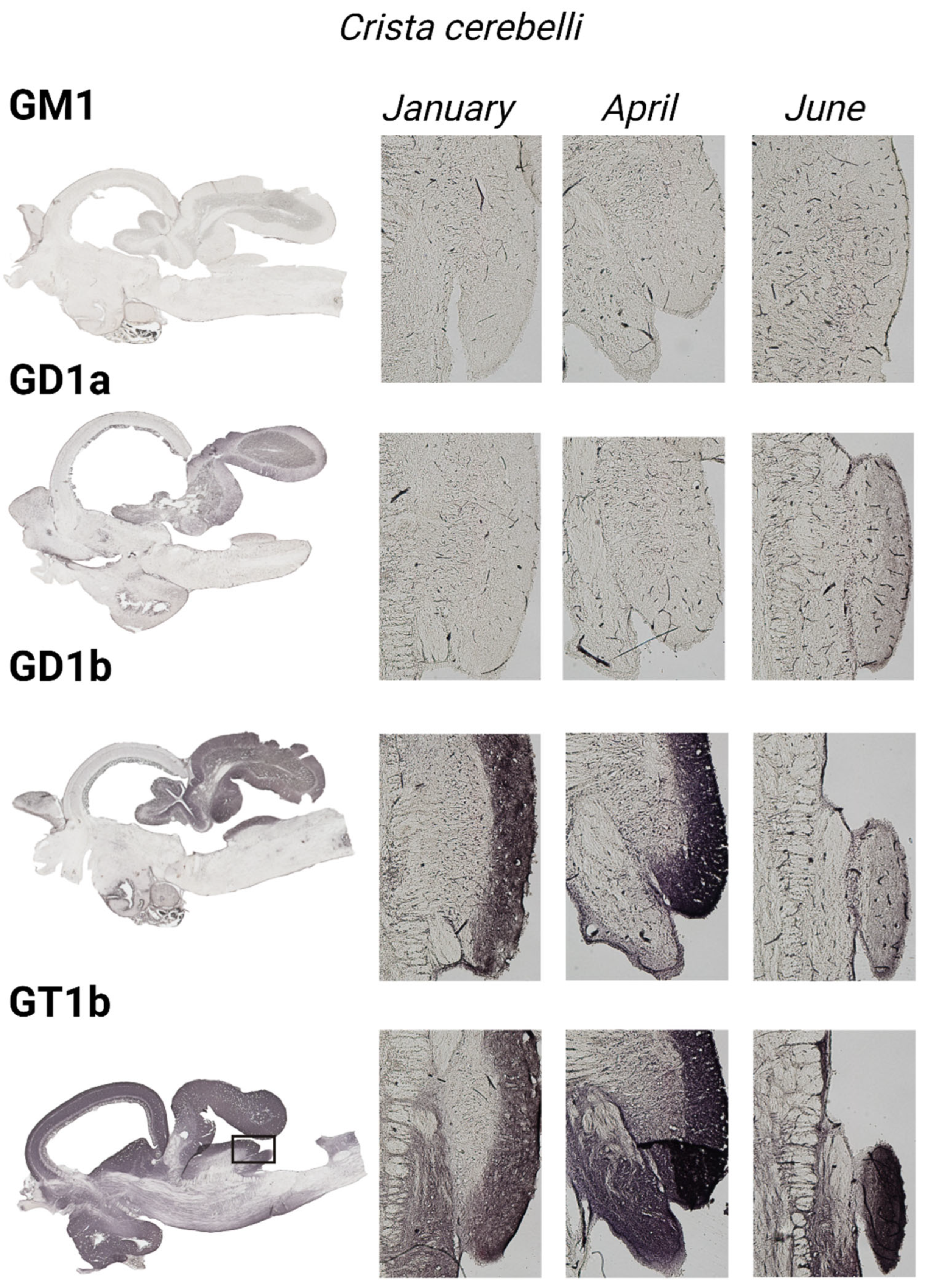
2.2.2. Immunohistochemical Analysis
2.2.3. Statistical Analysis
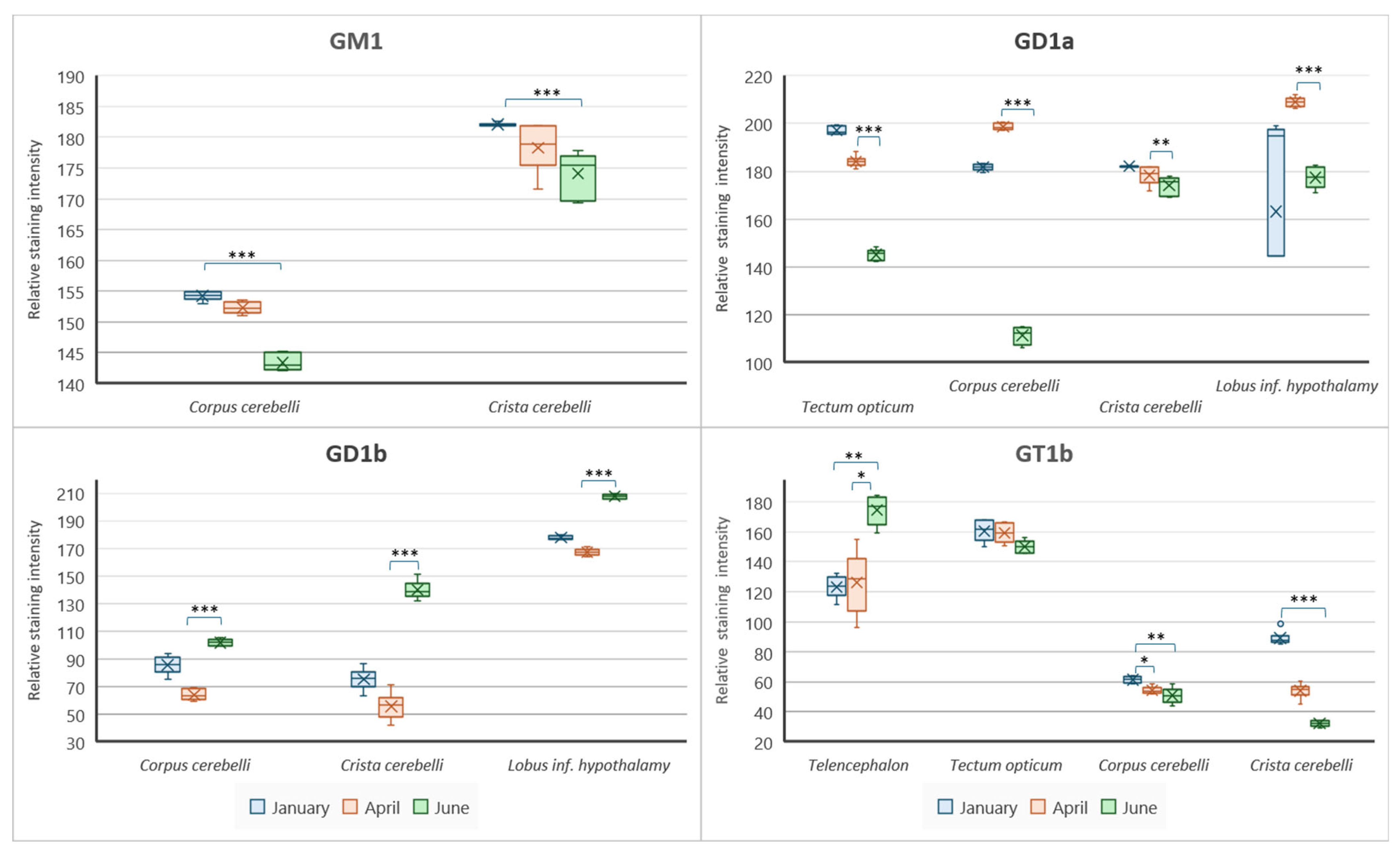
3. Results
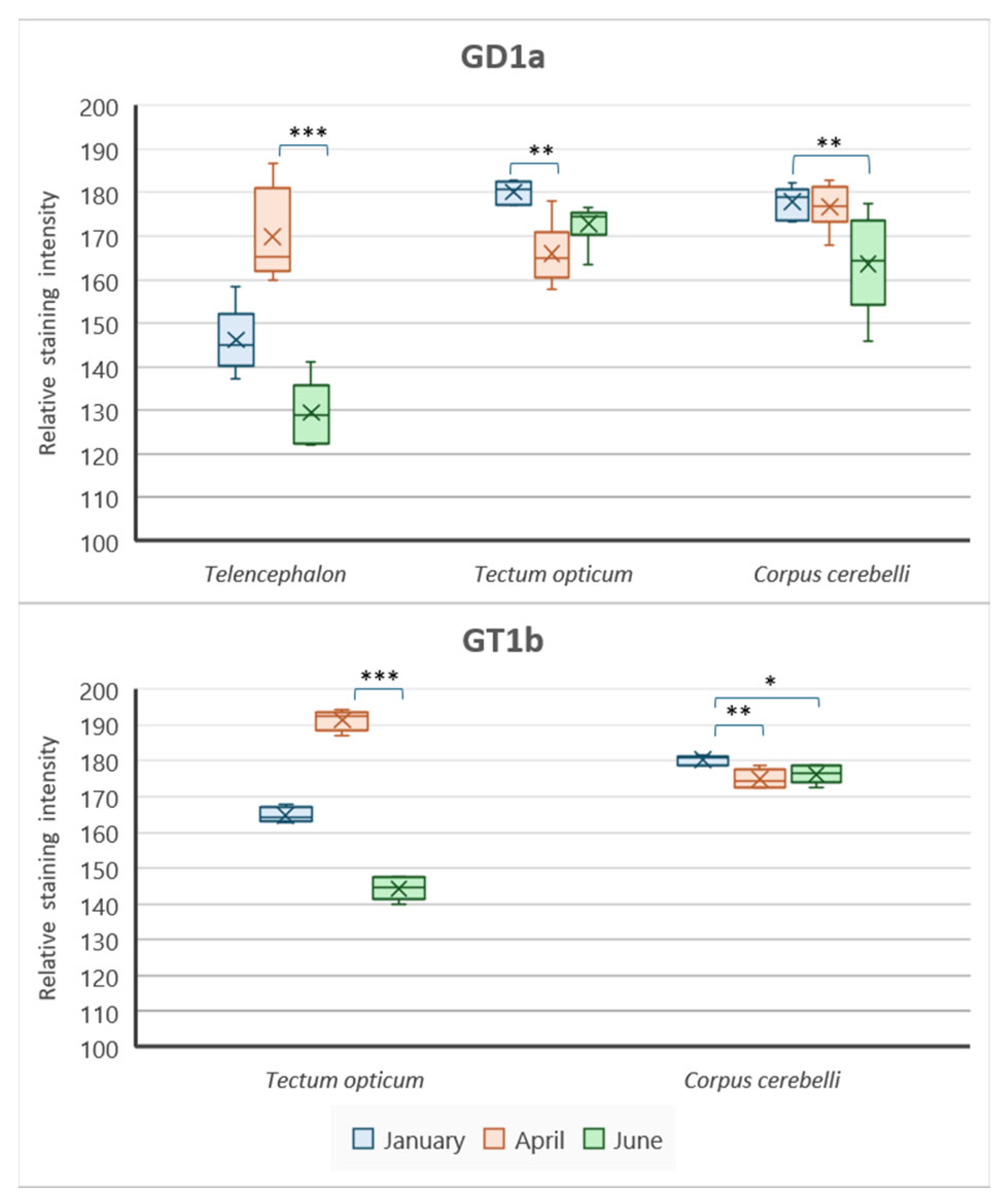
3.1. The Forebrain—Telencephalon
3.2. The Optic Tectum—Tectum Opticum
3.3. The Cerebellar Crest—Crista cerebelli
3.4. The Cerebellar Corpus—Corpus cerebelli
3.5. The Inferior Lobe of the Hypothalamus—Lobus inferior hypothalami
4. Discussion
Limitations of the Study and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priya, A.K.; Muruganandam, M.; Rajamanickam, S.; Sivarethinamohan, S.; Gaddam, M.K.R.; Velusamy, P.; Gamathi, R.; Ravindiran, G.; Gurugubelli, T.R.; Muniasamy, S.K. Impact of Climate Change and Anthropogenic Activities on Aquatic Ecosystem—A Review. Environ. Res. 2023, 238, 117233. [Google Scholar] [CrossRef]
- da Silva, J.C.; Soares, C.M.; Bialetzki, A. Effect of an Invasive Fish Species on Nutrient Cycling and on the Community Structure: An Experimental Approach. In Aquatic Ecology; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Guo, T.; Wang, Y.; Li, J.; Guo, X.; Xu, S.; Han, H.; Yu, J.; Li, J.; Liu, Q. Accumulated CO2 Affects Growth, Acid-Base Regulation and Ion Balance of Turbot (Scophthalmus maximus) in a Recirculating Aquaculture System. Aquaculture 2024, 578, 740070. [Google Scholar] [CrossRef]
- Oehlert, A.M.; Garza, J.; Nixon, S.; Frank, L.; Folkerts, E.J.; Stieglitz, J.D.; Lu, C.; Heuer, R.M.; Benetti, D.D.; del Campo, J.; et al. Implications of Dietary Carbon Incorporation in Fish Carbonates for the Global Carbon Cycle. Sci. Total Environ. 2024, 916, 169895. [Google Scholar] [CrossRef] [PubMed]
- Dosdat, A.; Servais, F.; Métailler, R.; Huelvan, C.; Desbruyères, E. Comparison of Nitrogenous Losses in Five Teleost Fish Species. Aquaculture 1996, 141, 107–127. [Google Scholar] [CrossRef]
- de Carvalho, D.R.; Sparks, J.P.; Flecker, A.S.; Alves, C.B.M.; Moreira, M.Z.; Pompeu, P.S. Nitrogen Pollution Promotes Changes in the Niche Space of Fish Communities. Oecologia 2021, 197, 485–500. [Google Scholar] [CrossRef]
- Little, A.G.; Loughland, I.; Seebacher, F. What Do Warming Waters Mean for Fish Physiology and Fisheries? J. Fish Biol. 2020, 97, 328–340. [Google Scholar] [CrossRef]
- Du, J.; Xie, M.; Wang, Y.; Chen, Z.; Liu, W.; Liao, J.; Chen, B. Connectivity of Fish Assemblages along the Mangrove-Seagrass-Coral Reef Continuum in Wenchang, China. Acta Oceanol. Sin. 2020, 39, 43–52. [Google Scholar] [CrossRef]
- Baladrón, A.; Costa, M.J.; Bejarano, M.D.; Pinheiro, A.; Boavida, I. Can Vegetation Provide Shelter to Cyprinid Species under Hydropeaking? Sci. Total Environ. 2021, 769, 145339. [Google Scholar] [CrossRef]
- Silveira, R.; Leão-Neto, W.M.; Barbosa da Silva, F.H. Small-Sized Fish as Possible Seed Dispersers: Disclosing Novel Fish and Plant Species Interactions in the Pantanal Wetland. Stud. Neotrop. Fauna Environ. 2020, 55, 36–43. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Elliott, M. Fishes as Indicators of Environmental and Ecological Changes within Estuaries: A Review of Progress and Some Suggestions for the Future. J. Fish Biol. 2002, 61, 229–250. [Google Scholar] [CrossRef]
- Seebacher, F.; Narayan, E.; Rummer, J.L.; Tomlinson, S.; Cooke, S.J. How Can Physiology Best Contribute to Wildlife Conservation in a Warming World? Conserv. Physiol. 2023, 11, coad038. [Google Scholar] [CrossRef] [PubMed]
- Chmura, H.E.; Williams, C.T. A Cross-Taxonomic Perspective on the Integration of Temperature Cues in Vertebrate Seasonal Neuroendocrine Pathways. Horm. Behav. 2022, 144, 105215. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature Increase and Its Effects on Fish Stress Physiology in the Context of Global Warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Crawshaw, L.I. Physiological and Behavioral Reactions of Fishes to Temperature Change. J. Fish. Res. Board Can. 1977, 34, 730–734. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of Aquaculture Fish to Climate Change-Induced Extreme Temperatures: A Review. J. World Aquac. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Madeira, D.; Madeira, C.; Costa, P.M.; Vinagre, C.; Pörtner, H.-O.; Diniz, M.S. Different Sensitivity to Heatwaves across the Life Cycle of Fish Reflects Phenotypic Adaptation to Environmental Niche. Mar. Environ. Res. 2020, 162, 105192. [Google Scholar] [CrossRef]
- Brulé, T.; Renán, X.; Colás-Marrufo, T. Potential Impact of Climate Change on Fish Reproductive Phenology: A Case Study in Gonochoric and Hermaphrodite Commercially Important Species from the Southern Gulf of Mexico. Fishes 2022, 7, 156. [Google Scholar] [CrossRef]
- Liu, H.; Yang, R.; Fu, Z.; Yu, G.; Li, M.; Dai, S.; Ma, Z.; Zong, H. Acute Thermal Stress Increased Enzyme Activity and Muscle Energy Distribution of Yellowfin Tuna. PLoS ONE 2023, 18, e0289606. [Google Scholar] [CrossRef]
- Sage, R.F. Global Change Biology: A Primer. Glob. Chang. Biol. 2020, 26, 3–30. [Google Scholar] [CrossRef]
- Madaro, A.; Kristiansen, T.S.; Pavlidis, M.A. How Fish Cope with Stress? In The Welfare of Fish; Kristiansen, T.S., Fernö, A., Pavlidis, M.A., van de Vis, H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 251–281. ISBN 978-3-030-41675-1. [Google Scholar]
- Biederman, A.M.; Kuhn, D.E.; O’Brien, K.M.; Crockett, E.L. Physical, Chemical, and Functional Properties of Neuronal Membranes Vary between Species of Antarctic Notothenioids Differing in Thermal Tolerance. J. Comp. Physiol. B 2019, 189, 213–222. [Google Scholar] [CrossRef]
- Vindas, M.A.; Fokos, S.; Pavlidis, M.; Höglund, E.; Dionysopoulou, S.; Ebbesson, L.O.E.; Papandroulakis, N.; Dermon, C.R. Early Life Stress Induces Long-Term Changes in Limbic Areas of a Teleost Fish: The Role of Catecholamine Systems in Stress Coping. Sci. Rep. 2018, 8, 5638. [Google Scholar] [CrossRef] [PubMed]
- Gesto, M.; López-Patiño, M.A.; Hernández, J.; Soengas, J.L.; Míguez, J.M. The Response of Brain Serotonergic and Dopaminergic Systems to an Acute Stressor in Rainbow Trout: A Time Course Study. J. Exp. Biol. 2013, 216, 4435–4442. [Google Scholar] [CrossRef] [PubMed]
- Sandblom, E.; Clark, T.D.; Gräns, A.; Ekström, A.; Brijs, J.; Sundström, L.F.; Odelström, A.; Adill, A.; Aho, T.; Jutfelt, F. Physiological Constraints to Climate Warming in Fish Follow Principles of Plastic Floors and Concrete Ceilings. Nat. Commun. 2016, 7, 11447. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S. Thermal Preference of Arctic Charr, Salvelinus Alpinus, and Brown Trout, Salmo Trutta—Implications for Their Niche Segregation. Environ. Biol. Fish 2005, 73, 89–96. [Google Scholar] [CrossRef]
- Nivelle, R.; Gennotte, V.; Kalala, E.J.K.; Ngoc, N.B.; Muller, M.; Mélard, C.; Rougeot, C. Temperature Preference of Nile Tilapia (Oreochromis niloticus) Juveniles Induces Spontaneous Sex Reversal. PLoS ONE 2019, 14, e0212504. [Google Scholar] [CrossRef]
- Rahmann, H.; Hilbig, R. The Possible Functional Role of Neuronal Gangliosides in Temperature Adaptation of Vertebrates. J. Therm. Biol. 1981, 6, 315–319. [Google Scholar] [CrossRef]
- Logue, J.A.; De Vries, A.L.; Fodor, E.; Cossins, A.R. Lipid Compositional Correlates of Temperature-Adaptive Interspecific Differences in Membrane Physical Structure. J. Exp. Biol. 2000, 203, 2105–2115. [Google Scholar] [CrossRef]
- Ernst, R.; Ejsing, C.S.; Antonny, B. Homeoviscous Adaptation and the Regulation of Membrane Lipids. J. Mol. Biol. 2016, 428, 4776–4791. [Google Scholar] [CrossRef]
- Malekar, V.C.; Morton, J.D.; Hider, R.N.; Cruickshank, R.H.; Hodge, S.; Metcalf, V.J. Effect of Elevated Temperature on Membrane Lipid Saturation in Antarctic Notothenioid Fish. PeerJ 2018, 6, e4765. [Google Scholar] [CrossRef]
- Maffioli, E.; Nonnis, S.; Negri, A.; Fontana, M.; Frabetti, F.; Rossi, A.R.; Tedeschi, G.; Toni, M. Environmental Temperature Variation Affects Brain Lipid Composition in Adult Zebrafish (Danio rerio). Int. J. Mol. Sci. 2024, 25, 9629. [Google Scholar] [CrossRef]
- Rahmann, H.; Jonas, U.; Kappel, T.; Hildebrandt, H. Differential Involvement of Gangliosides versus Phospholipids in the Process of Temperature Adaptation in Vertebrates: A Comparative Phenomenological and Physicochemical Study. Ann. N. Y. Acad. Sci. 1998, 845, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Rahmann, H. Gangliosides as Modulators of Synaptic Transmission and Long-Term Neuronal Adaptation. Trends Glycosci. Glycotechnol. 1990, 2, 450–469. [Google Scholar] [CrossRef]
- Avrova, N.F. Brain Gangliosides and Their Functions as Natural Adaptogens. Neurosci. Behav. Phys. 2021, 51, 245–255. [Google Scholar] [CrossRef]
- Kolter, T.; Proia, R.L.; Sandhoff, K. Combinatorial Ganglioside Biosynthesis. J. Biol. Chem. 2002, 277, 25859–25862. [Google Scholar] [CrossRef] [PubMed]
- Prinetti, A.; Loberto, N.; Chigorno, V.; Sonnino, S. Glycosphingolipid Behaviour in Complex Membranes. Biochim. Biophys. Acta 2009, 1788, 184–193. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid Rafts and Signal Transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Pike, L.J. Rafts Defined: A Report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef]
- Vernier, P. 1.04—The Brains of Teleost Fishes. In Evolution of Nervous Systems, 2nd ed.; Kaas, J.H., Ed.; Academic Press: Oxford, UK, 2017; pp. 59–75. ISBN 978-0-12-804096-6. [Google Scholar]
- Nieuwenhuys, R.; Donkelaar, H.J.; Nicholson, C. The Central Nervous System of Vertebrates; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-540-56013-5. [Google Scholar]
- Becker, K.; Wöhrmann, A.P.A.; Rahmann, H. Brain Gangliosides and Cold-Adaptation in High-Antarctic Fish. Biochem. Syst. Ecol. 1995, 23, 695–707. [Google Scholar] [CrossRef]
- Behan-Martin, M.K.; Jones, G.R.; Bowler, K.; Cossins, A.R. A near Perfect Temperature Adaptation of Bilayer Order in Vertebrate Brain Membranes. Biochim. Biophys. Acta 1993, 1151, 216–222. [Google Scholar] [CrossRef]
- Gagneux, P.; Panin, V.; Hennet, T.; Aebi, M.; Varki, A. Evolution of Glycan Diversity. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; ISBN 978-1-62182-421-3. [Google Scholar]
- Rahmann, H.; Hilbig, R. Phylogenetical Aspects of Brain Gangliosides in Vertebrates. J. Comp. Physiol. B 1983, 151, 215–224. [Google Scholar] [CrossRef]
- Hilbig, R.; Rahmann, H.; Rösner, H. Brain Gangliosides and Temperature Adaptation in Eury- and Stenothermic Teleost Fish (Carp and Rainbow Trout). J. Therm. Biol. 1979, 4, 29–34. [Google Scholar] [CrossRef]
- Kappel, T.; Anken, R.H.; Hanke, W.; Rahmann, H. Gangliosides Affect Membrane-Channel Activities Dependent on Ambient Temperature. Cell. Mol. Neurobiol. 2000, 20, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Zehmer, J.K.; Hazel, J.R. Thermally Induced Changes in Lipid Composition of Raft and Non-Raft Regions of Hepatocyte Plasma Membranes of Rainbow Trout. J. Exp. Biol. 2005, 208, 4283–4290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Echten, G.; Sandhoff, K. Modulation of Ganglioside Biosynthesis in Primary Cultured Neurons. J. Neurochem. 1989, 52, 207–214. [Google Scholar] [CrossRef]
- Avrova, N.F. Evolutionary Approach to the Analysis of Structure and Function of Gangliosides. Neurochem. Res. 1985, 10, 1547–1554. [Google Scholar] [CrossRef]
- Hilbig, R.; Rahmann, H. Variability in Brain Gangliosides of Fishes. J. Neurochem. 1980, 34, 236–240. [Google Scholar] [CrossRef]
- Viljetić, B.; Labak, I.; Majić, S.; Stambuk, A.; Heffer, M. Distribution of Mono-, Di- and Trisialo Gangliosides in the Brain of Actinopterygian Fishes. Biochim. Biophys. Acta 2012, 1820, 1437–1443. [Google Scholar] [CrossRef]
- The Freshwater Fishes of Europe. Cyprinidae 2, Gasterosteidae; Bǎnǎrescu, P.M., Paepke, H.-J., Eds.; Aula Verlag: Wiebelsheim, Germany, 2001; Volume 5/III. [Google Scholar]
- Stanković, D.; Crivelli, A.J.; Snoj, A. Rainbow Trout in Europe: Introduction, Naturalization, and Impacts. Rev. Fish. Sci. Aquac. 2015, 23, 39–71. [Google Scholar] [CrossRef]
- Schnaar, R.L. Isolation of Glycosphingolipids. In Guide to Techniques in Glycobiology; Academic Press: Cambridge, MA, USA, 1994; Volume 230, pp. 348–370. ISBN 0076-6879. [Google Scholar]
- Svennerholm, L. Quantitive Estimation of Sialic Acids: II. A Colorimetric Resorcinol-Hydrochloric Acid Method. Biochim. Biophys. Acta 1957, 24, 604–611. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Needham, L.K. Thin-Layer Chromatography of Glycosphingolipids. Meth. Enzymol. 1994, 230, 371–389. [Google Scholar]
- BioRender.com. Available online: https://app.biorender.com/illustrations/65fb12682d08c19562fd5ba5 (accessed on 21 March 2024).
- Armstrong, J.B.; Fullerton, A.H.; Jordan, C.E.; Ebersole, J.L.; Bellmore, J.R.; Arismendi, I.; Penaluna, B.; Reeves, G.H. The Importance of Warm Habitat to the Growth Regime of Cold-Water Fishes. Nat. Clim. Chang. 2021, 11, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Paukert, C.P.; Moore, T.L. Effects of Latitude, Season, and Temperature on Lake Sturgeon Movement. N. Am. J. Fish. Manag. 2021, 41, 916–928. [Google Scholar] [CrossRef]
- Popović, J. Značajke Ljuske Ilirskog Klena. Ribarstvo 1994, 52, 1–11. [Google Scholar]
- Raleigh, R.F.; Hickman, T.; Solomon, R.C.; Nelson, P.C. Habitat Suitability Information: Rainbow Trout; Western Energy and Land Use Team, Division of Biological Services, Research and Development, Fish and Wildlife Service, US Department of the Interior: Washington, DC, USA, 1984.
- Balon, E.K. Origin and Domestication of the Wild Carp, Cyprinus carpio: From Roman Gourmets to the Swimming Flowers. Aquaculture 1995, 129, 3–48. [Google Scholar] [CrossRef]
- Topal, A.; Özdemir, S.; Arslan, H.; Çomaklı, S. How Does Elevated Water Temperature Affect Fish Brain? (A Neurophysiological and Experimental Study: Assessment of Brain Derived Neurotrophic Factor, cFOS, Apoptotic Genes, Heat Shock Genes, ER-Stress Genes and Oxidative Stress Genes). Fish Shellfish Immunol. 2021, 115, 198–204. [Google Scholar] [CrossRef]
- Li, Q.; Xiong, L.; Zhu, Y.; Zheng, A.; Zheng, S. Effects of Acute Temperature Stress on the Expression of Related Genes in the Brain of Opsariichthys bidens. Fishes 2024, 9, 248. [Google Scholar] [CrossRef]
- Su, J.; Li, W.; Li, H.; Zhou, Z.; Miao, Y.; Yuan, Y.; Li, Y.; Tao, M.; Zhang, C.; Zhou, Y.; et al. Comparative Transcriptomic Analysis of the Brain-Liver Axis Reveals Molecular Mechanisms Underlying Acute Cold Stress Response in Gynogenetic Mrigal Carp. Aquaculture 2024, 588, 740908. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, H.; Li, H.; Wang, A.; Yu, H. Effect of High Temperature on Immune Response of Grass Carp (Ctenopharyngodon idellus) by Transcriptome Analysis. Fish Shellfish Immunol. 2016, 58, 89–95. [Google Scholar] [CrossRef]
- Haesemeyer, M. Thermoregulation in Fish. Mol. Cell. Endocrinol. 2020, 518, 110986. [Google Scholar] [CrossRef]
- Rahmann, H. Gangliosides and Thermal Adaptation in Vertebrates. Jpn. J. Exp. Med. 1978, 48, 85–96. [Google Scholar] [PubMed]
- Probst, W.; Rösner, H.; Wiegandt, H.; Rahmann, H. The Complexing Ability of Gangliosides for Ca2, I. Influence of Mono- and Divalent Cations and of Acetylcholin (Author’s transl). Hoppe-Seyler’s Z. Physiol. Chem. 1979, 360, 979–986. [Google Scholar] [CrossRef]
- Probst, W.; Rahmann, H. Influence of Temperature Changes on the Ability of Gangliosides to Complex with Ca2+. J. Therm. Biol. 1980, 5, 243–247. [Google Scholar] [CrossRef]
- Rösner, H.; Breer, H.; Hilbig, R.; Rahmann, H. Temperature Effects on the Incorporation of Sialic Acid into Gangliosides and Glycoproteins of Fish Brain. J. Therm. Biol. 1979, 4, 69–73. [Google Scholar] [CrossRef]
- van den Burg, E.H.; Verhoye, M.; Peeters, R.R.; Meek, J.; Flik, G.; Van der Linden, A. Activation of a Sensorimotor Pathway in Response to a Water Temperature Drop in a Teleost Fish. J. Exp. Biol. 2006, 209, 2015–2024. [Google Scholar] [CrossRef]
- van den Burg, E.H.; Peeters, R.R.; Verhoye, M.; Meek, J.; Flik, G.; Van der Linden, A. Brain Responses to Ambient Temperature Fluctuations in Fish: Reduction of Blood Volume and Initiation of a Whole-Body Stress Response. J. Neurophysiol. 2005, 93, 2849–2855. [Google Scholar] [CrossRef]
- Kristal, B.S.; Brown, A.M. Apoptogenic Ganglioside GD3 Directly Induces the Mitochondrial Permeability Transition. J. Biol. Chem. 1999, 274, 23169–23175. [Google Scholar] [CrossRef]
- Dhanushkodi, A.; Xue, Y.; Roguski, E.E.; Ding, Y.; Matta, S.G.; Heck, D.; Fan, G.-H.; McDonald, M.P. Lentiviral-Mediated Knock-down of GD3 Synthase Protects against MPTP-Induced Motor Deficits and Neurodegeneration. Neurosci. Lett. 2019, 692, 53–63. [Google Scholar] [CrossRef]
- Inamori, K.; Inokuchi, J. Roles of Gangliosides in Hypothalamic Control of Energy Balance: New Insights. Int. J. Mol. Sci. 2020, 21, 5349. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Liu, S.-T.; Hu, M.Y.; Chen, R.-D.; Lee, J.-R.; Hwang, P.-P. Brain Functioning under Acute Hypothermic Stress Supported by Dynamic Monocarboxylate Utilization and Transport in Ectothermic Fish. Front. Zool. 2014, 11, 53. [Google Scholar] [CrossRef]
- Bueschke, N.; do Amaral-Silva, L.; Hu, M.; Santin, J.M. Lactate Ions Induce Synaptic Plasticity to Enhance Output from the Central Respiratory Network. J. Physiol. 2021, 599, 5485. [Google Scholar] [CrossRef] [PubMed]
- Soengas, J.L.; Aldegunde, M. Energy Metabolism of Fish Brain. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 131, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wang, H.; Song, H.; Yang, R.; Wang, L.; Xue, X.; Sun, W.; Hu, J. Lactate Is Answerable for Brain Function and Treating Brain Diseases: Energy Substrates and Signal Molecule. Front. Nutr. 2022, 9, 800901. [Google Scholar] [CrossRef]
- Wullimann, M.F.; Meyer, D.L. Phylogeny of Putative Cholinergic Visual Pathways through the Pretectum to the Hypothalamus in Teleost Fish. Brain Behav. Evol. 1990, 36, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, J.S.; Finger, T.E.; Caprio, J. Forebrain Connections of the Gustatory System in Ictalurid Catfishes. J. Comp. Neurol. 1988, 278, 353–376. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, J.P.C. Telencephalon and Behavior in Teleost Fish. In Comparative Neurology of the Telencephalon; Ebbesson, S.O.E., Ed.; Springer: Boston, MA, USA, 1980; pp. 175–201. ISBN 978-1-4613-2988-6. [Google Scholar]
- Wullimann, M.F.; Northcutt, R.G. Connections of the Corpus Cerebelli in the Green Sunfish and the Common Goldfish: A Comparison of Perciform and Cypriniform Teleosts. Brain Behav. Evol. 1988, 32, 293–316. [Google Scholar] [CrossRef]
- Hilbig, R. Regional Differences in Brain Gangliosides of a Teleost Fish Following Thermal Acclimations. J. Therm. Biol. 1983, 8, 469–470. [Google Scholar] [CrossRef]
- Rösner, H. Developmental Expression and Possible Roles of Gangliosides in Brain Development. Prog. Mol. Subcell. Biol. 2003, 32, 49–73. [Google Scholar]
- Yu, R.K.; Macala, L.J.; Taki, T.; Weinfeld, H.M.; Yu, F.S. Developmental Changes in Ganglioside Composition and Synthesis in Embryonic Rat Brain. J. Neurochem. 1988, 50, 1825–1829. [Google Scholar] [CrossRef]
- Qi, Y.; Xue, Q. Ganglioside Levels in Hypoxic Brains from Neonatal and Premature Infants. Mol. Chem. Neuropathol. 1991, 14, 87–97. [Google Scholar] [CrossRef]
- Britton, J.R.; Boar, R.R.; Grey, J.; Foster, J.; Lugonzo, J.; Harper, D.M. From Introduction to Fishery Dominance: The Initial Impacts of the Invasive Carp Cyprinus carpio in Lake Naivasha, Kenya, 1999 to 2006. J. Fish Biol. 2007, 71, 239–257. [Google Scholar] [CrossRef]
- Miller, S.A.; Provenza, F.D. Mechanisms of Resistance of Freshwater Macrophytes to Herbivory by Invasive Juvenile Common Carp. Freshw. Biol. 2007, 52, 39–49. [Google Scholar] [CrossRef]
- Pinto, L.; Chandrasena, N.; Pera, J.; Hawkins, P.; Eccles, D.; Sim, R. Managing Invasive Carp (Cyprinus carpio L.) for Habitat Enhancement at Botany Wetlands, Australia. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 447–462. [Google Scholar] [CrossRef]
- Yeagle, P.L. Lipid Regulation of Cell Membrane Structure and Function. FASEB J. 1989, 3, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Sandhoff, R.; Sandhoff, K. Emerging Concepts of Ganglioside Metabolism. FEBS Lett. 2018, 592, 3835–3864. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Ariga, T.; Yanagisawa, M.; Zeng, G. Gangliosides in the Nervous System: Biosynthesis and Degradation. In Glycoscience: Chemistry and Chemical Biology; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1671–1695. ISBN 978-3-540-30429-6. [Google Scholar]
- Spessott, W.; Crespo, P.M.; Daniotti, J.L.; Maccioni, H.J.F. Glycosyltransferase Complexes Improve Glycolipid Synthesis. FEBS Lett. 2012, 586, 2346–2350. [Google Scholar] [CrossRef]
- Lauc, G.; Dabelic, S.; Supraha, S.; Flögel, M. Glycobiology of Stress. Ann. N. Y. Acad. Sci. 1998, 851, 397–403. [Google Scholar] [CrossRef]
- Nakayama, J.; Fukuda, M.N.; Hirabayashi, Y.; Kanamori, A.; Sasaki, K.; Nishi, T.; Fukuda, M. Expression Cloning of a Human GT3 Synthase. J. Biol. Chem. 1996, 271, 3684–3691. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef]
- McDonald, A.G.; Hayes, J.M.; Davey, G.P. Metabolic Flux Control in Glycosylation. Curr. Opin. Struct. Biol. 2016, 40, 97–103. [Google Scholar] [CrossRef]
- Desplats, P.A.; Denny, C.A.; Kass, K.E.; Gilmartin, T.; Head, S.R.; Sutcliffe, J.G.; Seyfried, T.N.; Thomas, E.A. Glycolipid and Ganglioside Metabolism Imbalances in Huntington’s Disease. Neurobiol. Dis. 2007, 27, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Vigor, C.; Vercauteren, J.; Reversat, G.; Zhou, B.; Surget, A.; Larroquet, L.; Lanuque, A.; Sandres, F.; Terrier, F.; et al. Characterization and Modulation of Brain Lipids Content of Rainbow Trout Fed with 100% Plant Based Diet Rich in Omega-3 Long Chain Polyunsaturated Fatty Acids DHA and EPA. Biochimie 2020, 178, 137–147. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavić, V.; Viljetić, B.; Blažetić, S.; Labak, I.; Has-Schön, E.; Heffer, M. Temperature-Induced Seasonal Dynamics of Brain Gangliosides in Rainbow Trout (Oncorhynchus mykiss Walbaum) and Common Carp (Cyprinus carpio L.). Life 2024, 14, 1273. https://doi.org/10.3390/life14101273
Pavić V, Viljetić B, Blažetić S, Labak I, Has-Schön E, Heffer M. Temperature-Induced Seasonal Dynamics of Brain Gangliosides in Rainbow Trout (Oncorhynchus mykiss Walbaum) and Common Carp (Cyprinus carpio L.). Life. 2024; 14(10):1273. https://doi.org/10.3390/life14101273
Chicago/Turabian StylePavić, Valentina, Barbara Viljetić, Senka Blažetić, Irena Labak, Elizabeta Has-Schön, and Marija Heffer. 2024. "Temperature-Induced Seasonal Dynamics of Brain Gangliosides in Rainbow Trout (Oncorhynchus mykiss Walbaum) and Common Carp (Cyprinus carpio L.)" Life 14, no. 10: 1273. https://doi.org/10.3390/life14101273
APA StylePavić, V., Viljetić, B., Blažetić, S., Labak, I., Has-Schön, E., & Heffer, M. (2024). Temperature-Induced Seasonal Dynamics of Brain Gangliosides in Rainbow Trout (Oncorhynchus mykiss Walbaum) and Common Carp (Cyprinus carpio L.). Life, 14(10), 1273. https://doi.org/10.3390/life14101273







