Influence of the Weak Nuclear Force on Metal-Promoted Autocatalytic Strecker Synthesis of Amino Acids: Formation of a Chiral Pool of Precursors for Prebiotic Peptide and Protein Synthesis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowan, J.A.; Furnstahl, R.J. Origin of Chirality in the Molecules of Life. ACS Earth Space Chem. 2022, 6, 2575–2581. [Google Scholar] [CrossRef]
- Ikehara, K. Possible Steps to the Emergence of Life: The [GADV]-Protein World Hypothesis. Chem. Rec. 2005, 5, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Legnani, L.; Darù, A.; Jones, A.X.; Blackmond, D.G. Mechanistic Insight into the Origin of Stereoselectivity in the Ribose-Mediated Strecker Synthesis of Alanine. J. Am. Chem. Soc. 2021, 143, 7852–7858. [Google Scholar] [CrossRef] [PubMed]
- Famiano, M.A.; Boyd, R.N.; Kajino, T.; Onaka, T. Selection of Amino Acid Chirality via Neutrino Interactions with 14N in Crossed Electric and Magnetic Fields. Astrobiology 2018, 18, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Penghua, Q.; Wencai, L.; Wei, Q.; Wei, Z.; Hui, X. Theoretical Studies on Complexes of Calcium Ion with Amino Acids. Chem. Res. Chin. Univ. 2014, 30, 125–129. [Google Scholar]
- Kazmierczak, J.; Kempe, S.; Kremer, S.B. Calcium in the Early Evolution of Living Systems: A Biohistorical Approach. Curr. Org. Chem. 2013, 17, 1738–1750. [Google Scholar] [CrossRef]
- Haxel, G.B.; Boore, S.; Mayfield, S. Relative Abundance of Elements in the Earth’s Upper Crust, United States Geological Survey, 2003.
- Saravanan, S.; Khan, N.H.; Bera, P.K.; Kureshy, R.I.; Abdi, S.H.R.; Kumari, P.; Bajaj, H.C. Catalysis of Enantioselective Strecker Reaction in the Synthesis of d-Homophenylalanine Using Recyclable, Chiral, Macrocyclic MnIII–Salen Complexes. ChemCatChem 2023, 5, 1374–1385. [Google Scholar] [CrossRef]
- Shibasaki, M.; Matsunaga, S.; Kumagai, N. Lanthanide Lewis Acid. In Acid Catalysis in Modern Organic Synthesis; Yamamoto, H., Ishihara, K., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 635–720. [Google Scholar]
- Peralta, R.A.; Lyu, P.; López-Olvera, A.; Obeso, J.L.; Leyva, N.C.; Jeong, C.; Ibarra, I.A.; Maurin, G. Switchable Metal Sites in Metal–Organic Framework MFM-300(Sc): Lewis Acid Catalysis Driven by Metal–Hemilabile Linker Bond Dynamics. Ang. Chem. Int. Ed. 2022, 61, e202210857. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Solid-Supported Gallium Triflate: An Efficient Catalyst for the Three-Component Ketonic Strecker Reaction. ChemSusChem 2012, 5, 332–338. [Google Scholar] [CrossRef]
- Gandhi, S.; Sharma, V.; Koul, I.S.; Mandal, S.K. Shedding Light on the Lewis Acid Catalysis in Organic Transformations Using a Zn-MOF Microflower and Its ZnO Nanorod. Catal. Letts 2023, 153, 887–902. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Zheng, J.; Chen, X.; Shan, L.; Gao, L.; Wang, L.; Yu, M.; Fan, Y. Lanthanide coordination polymer constructed from 2,20-bipyridyl-4,40-dicarboxylic acid: Structure, catalysis and fluorescence. Inorg. Chim. Acta 2015, 437, 81–86. [Google Scholar] [CrossRef]
- Josephsohn, N.S.; Kuntz, K.W.; Snapper, M.L.; Hoveyda, A.H. Mechanism of Enantioselective Ti-Catalyzed Strecker Reaction: Peptide-Based Metal Complexes as Bifunctional Catalysts. J. Am. Chem. Soc. 2001, 123, 11594–11599. [Google Scholar] [CrossRef] [PubMed]
- Covington, A.K.; Danish, E.Y. Measurement of Magnesium Stability Constants of Biologically Relevant Ligands by Simultaneous Use of pH and Ion-Selective Electrodes. J. Solut. Chem. 2009, 38, 1449–1462. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Calcium Binding to Amino Acids and Small Glycine Peptides in Aqueous Solution: Toward Peptide Design for Better Calcium Bioavailability. J. Agric. Food Chem. 2016, 64, 4376–4389. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Okada, K.; Tsukamoto, Y.; Wakabayashi, K.; Ito, K. Complex Formation of Calcium(ii) with Amino Acids under Physiological Conditions. J. Chem. Soc. Dalton Trans. 1990, 8, 2337–2339. [Google Scholar] [CrossRef]
- Hergstrom, R.A.; Wein, D.W.; Sandars, P.G.H. Calculation of the parity nonconserving energy difference between mirror-image molecules. J. Chem. Phys. 1980, 73, 2329–2341. [Google Scholar] [CrossRef]
- Rein, D.W.; Hegstrom, R.A.; Sandars, P.G.H. Parity non-conserving energy difference between mirror image molecules. Phys. Lett. A 1979, 71, 499–502. [Google Scholar] [CrossRef]
- Bouchiat, M.A.; Bouchiat, C.C. Weak Neutral Currents in Atmic Physics. Phys. Lett. 1974, 48, 111–114. [Google Scholar] [CrossRef]
- Foot, C.J. Atomic Physics; Oxford: Oxford, UK, 2005; pp. 36–37. [Google Scholar]
- Blundell, S.A.; Sapirstein, J.; Johnson, W.R. High-accuracy calculation of parity nonconversation in cesium and implications for particle physics. Phys. Rev. D Part Fields 1992, 45, 1602–1623. [Google Scholar] [CrossRef]
- Soncini, A.; Faglioni, F.; Lazzeretti, P. Parity-violating contributions to nuclear magnetic shielding. Phys. Rev. A At. Mol. Opt. Phys. 2003, 68, 033402. [Google Scholar] [CrossRef]
- Tokunaga, S.K.; Stoeffler, C.; Auguste, F.; Shelkovnikov, A.; Daussy, C.; Amy-Klein, A.; Chardonnet, C.; Darquie, B. Probing weak force-induced parity violation by high-resolution mid-infrared molecular spectroscopy. Mol. Phys. 2013, 111, 2363–2373. [Google Scholar] [CrossRef]
- Weijo, V.; Manninen, P.; Vaara, J. Perturbational calculations of parity-violating effects in nuclear-magnetic-resonance parameters. J. Chem. Phys. 2005, 123, 054501. [Google Scholar] [CrossRef] [PubMed]
- Zanasi, R.; Lazzeretti, P. On the stabilization of natural L-enantiomers of a-amino acids via parity-violating effects. Chem. Phys. Lett. 1998, 286, 240–242. [Google Scholar] [CrossRef]
- Pelloni, S.; Faglioni, F.; Lazzeretti, P. Parity violation energies of C4H4X2 molecules for X = O, S, Se, Te and Po†. Mol. Phys. 2013, 111, 2387–2391. [Google Scholar] [CrossRef]
- Bearden, J.A.; Burr, A.F. Reevaluation of X-ray atomic energy levels. Rev. Mod. Phys. 1967, 39, 125–142. [Google Scholar] [CrossRef]
- Cardona, M.; Ley, L. (Eds.) Topics in Applied Physics, Vol. 26: Photoemission in Solids, 1: General Principles; Springer: Berlin/Heidelberg, Germany, 1978; p. 290. [Google Scholar]
- Fuggle, J.C.; Maartensson, N. Core-level binding energies in metals. J. Electron Spectrosc. Relat. Phenom. 1980, 21, 275–281. [Google Scholar] [CrossRef]
- Staeglich, H.; Weiss, E. Die Kristallstrukturen der Erdalkalimethanolate M(OCH), M = Ca, Sr, Ba. Chem. Ber. 1978, 111, 901–905. [Google Scholar] [CrossRef]
- Taillades, J.; Beuzelin, I.; Garrel, L.; Tabacik, V.; Bied, C.; Commeyras, A. N-Carbamoyl Amino AcidsRather than Free Amino Acid Formation in the Primitive Hydrosphere: A Novel Proposal for the Emergence of Prebiotic Peptides. Orig. Life Evol. Biosph. 1998, 28, 61–77. [Google Scholar] [CrossRef]
- Robert, F.; Chaussidon, M. A palaeotemperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 2006, 443, 969–972. [Google Scholar] [CrossRef]
- Gaucher, E.A.; Thomson, J.M.; Burgan, M.F.; Benner, S.A. Inferring the paleoenvironment of ancient bacteria on the basis of resurrected proteins. Nature 2003, 425, 285–288. [Google Scholar] [CrossRef]
- Gaucher, E.A.; Govindarajan, S.; Ganesh, O.K. Palaeotemperature trend for Precambrian life inferred from resurrected proteins. Nature 2008, 451, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, K.-J.; Luo, C.; Chen, H.; Bao, Z.; Wen, H.; Zhang, X. Magnesium isotope fractionation during alkaline brine evaporation and implications for Precambrian seawater chemistry. Chem. Geol. 2021, 585, 120565. [Google Scholar] [CrossRef]
- Zanasi, R.; Lazzeretti, P.; Ligabue, A.; Soncini, A. On the Stabilization of Natural L-Amino Acids and D-Sugars via Parity-Violating Effects. In Advances in BioChirality; Pfilyi, G., Zucchi, C., Caglioti, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 377–385. [Google Scholar]
- Berger, R.; Quack, M. Electroweak quantum chemistry of alanine: Parity violation in gas and condensed phases. ChemPhysChem 2000, 1, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Blackmond, D.G. Asymmetric autocatalysis and its implications for the origin of homochirality. Proc. Natl Acad. Sci. USA 2004, 101, 5732–5736. [Google Scholar] [CrossRef] [PubMed]
- Faglioni, F.; Cuesta, I.G. Parity-Violation Energy of Biomolecules-IV: Protein Secondary Structure. Orig. Life Evol. Biosph. 2011, 41, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Faglioni, F.; Cuesta, I.G.; Lazzeretti, P. Parity violation energy of biomolecules—III: RNA. Chem. Phys. Lett. 2006, 432, 263–268. [Google Scholar] [CrossRef]
- Faglioni, F.; D’Agostino, P.S.; Cadioli, B.; Lazzeretti, P. Parity violation energy of biomolecules—II: DNA. Chem. Phys. Lett. 2005, 407, 522–526. [Google Scholar] [CrossRef]
- Faglioni, F.; Fracassini, B.; Perrone, L. Parity Violation Energy of Biomolecules—V: Protein Metal Centers. Orig. Life Evol. Biosph. 2020, 50, 145–155. [Google Scholar] [CrossRef]
- Famiano, M.A.; Boyd, R.N.; Oaka, T.; Kajino, T. Chiral selection, isotopic abundance, and autocatlysis of meteoritic amino acids. Phys. Rev. Res. 2021, 3, 033025. [Google Scholar] [CrossRef]
- Betts, H.C.; Putick, M.N.; Clark, J.W.; Williams, T.A.; Donoghue, P.C.J.; Pisani, D. Integrated genomic and fossil evidence illuminates life’s early evolution and eukaryote origin. Nature 2018, 2, 1556–1562. [Google Scholar] [CrossRef]
- Bakasov, A.; Ha, T.-K.; Quack, M. Ab initio calculation of molecular energies including parity violating interactions. J. Chem. Phys. 1998, 109, 7263–7285. [Google Scholar] [CrossRef]
- Mirzaeva, I.V.; Kozlova, S.G. Parity violating energy difference for mirror conformers of DABCO linker between two M2+ cations (M = Zn, Cd, and Hg). J. Chem. Phys. 1998, 149, 214302. [Google Scholar] [CrossRef] [PubMed]


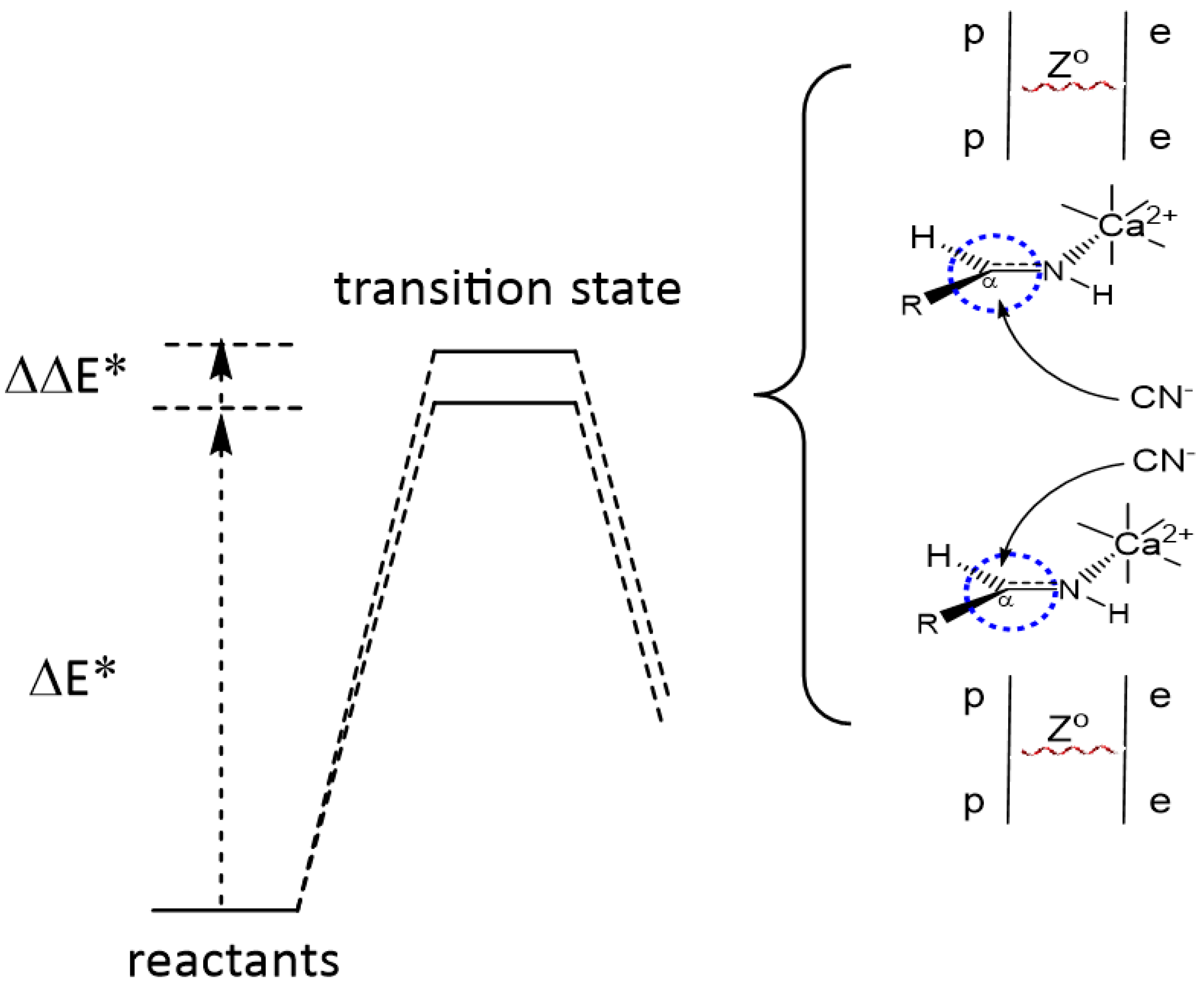
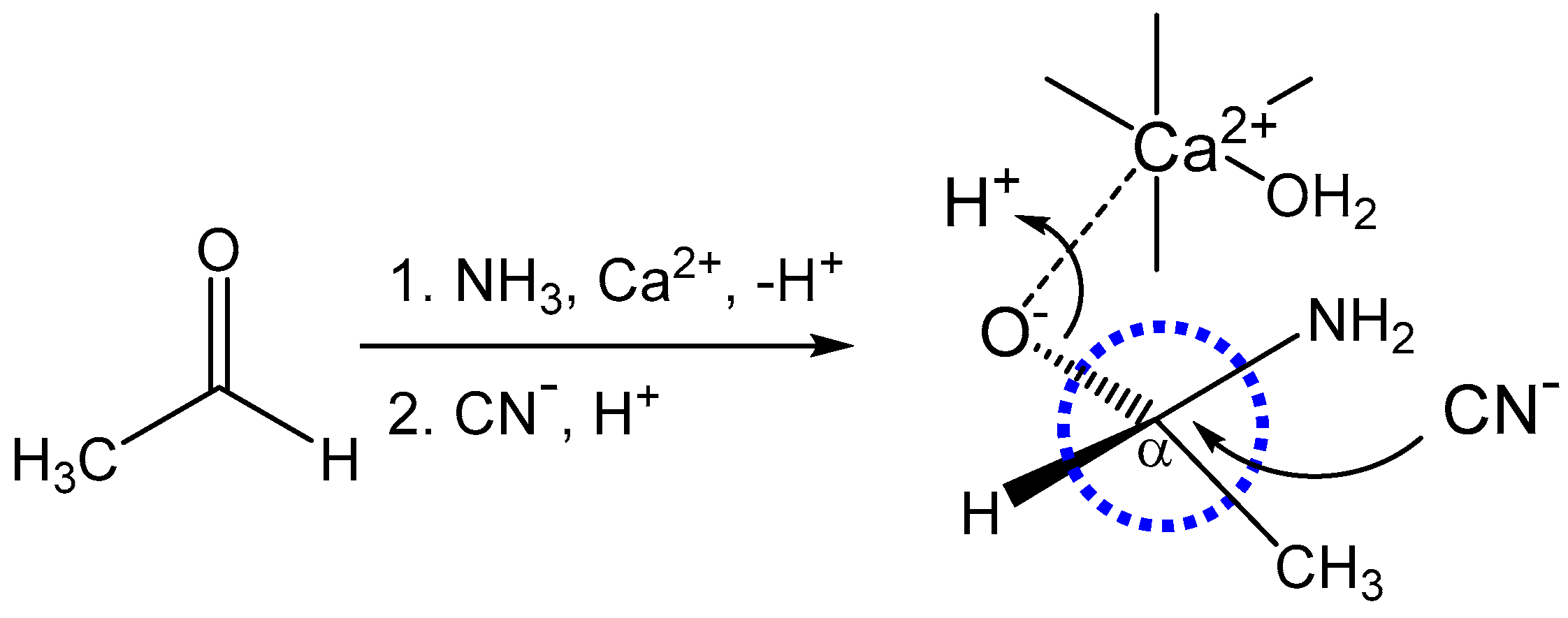
| Cation | Relative Abundance |
|---|---|
| Ca2+ | 8 × 104 |
| Sr2+ | 2 × 102 |
| Ba2+ | 102 |
| Cation | ΔEPNC (au) (Angular) | ΔEPNC (au) (Linear) | ΔEPNC (au) (Linear) ALDOL [1] |
|---|---|---|---|
| 20Ca2+ | 5.4 × 10−12 | 5.8 × 10−13 | 0.3 × 10−20 |
| 38Sr2+ | 1.2 × 10−10 | 1.2 × 10−12 | 0.6 × 10−19 |
| 56Ba2+ | 6.7 × 10−10 | 6.6 × 10−11 | 0.3 × 10−18 |
| ΔEPNC (au) | Selectivity Factor over 104 Years | Selectivity Factor over 103 Years | Selectivity Factor over 102 Years | Selectivity Factor over 10 Years | Selectivity Factor over 1 Year | Selectivity Factor over 0.1 Year |
|---|---|---|---|---|---|---|
| 10−13 | 2.6 × 10 14 | 27.6 | 1.39 | 1.03 | 1 | 1 |
| 10−12 | 1.4 × 10144 | 2.6 × 10 14 | 27.6 | 1.39 | 1.03 | 1 |
| 10−11 | >10300 | 1.4 × 10144 | 2.6 × 1014 | 27.6 | 1.39 | 1.03 |
| 10−10 | >10300 | >10300 | 1.4 × 10144 | 2.6 × 1014 | 27.6 | 1.39 |
| 10−9 | >10300 | >10300 | >10300 | 1.4 × 10144 | 2.6 × 10 14 | 27.6 |
| ΔEPNC (au) | Selectivity Factor over 104 Years | Selectivity Factor over 103 Years | Selectivity Factor over 102 Years | Selectivity Factor over 10 Years | Selectivity Factor over 1 Year | Selectivity Factor over 0.1 Year |
|---|---|---|---|---|---|---|
| 10−13 | 1.74 | 1.06 | 1.01 | 1 | 1 | 1 |
| 10−12 | 253 | 1.74 | 1.06 | 1.01 | 1 | 1 |
| 10−11 | 1.1 × 10 24 | 253 | 1.74 | 1.06 | 1.01 | 1 |
| 10−10 | 1.7 × 10 240 | 1.1 × 10 24 | 253 | 1.74 | 1.06 | 1.01 |
| 10−9 | >10300 | 1.7 × 10 240 | 1.1 × 10 24 | 253 | 1.74 | 1.06 |
| ΔEPNC (au) | Selectivity Factor over 104 Years | Selectivity Factor over 103 Years | Selectivity Factor over 102 Years | Selectivity Factor over 10 Years | Selectivity Factor over 1 Year | Selectivity Factor over 0.1 Year |
|---|---|---|---|---|---|---|
| 10−13 | 1.01 | 1 | 1 | 1 | 1 | 1 |
| 10−12 | 1.10 | 1.01 | 1 | 1 | 1 | 1 |
| 10−11 | 2.51 | 1.10 | 1.01 | 1 | 1 | 1 |
| 10−10 | 1 × 104 | 2.51 | 1.10 | 1.01 | 1 | 1 |
| 10−9 | 1.1 × 1040 | 1 × 104 | 2.51 | 1.10 | 1.01 | 1 |
| Cation | ΔEPNC (au) (Angular) | ΔEPNC (au) (Linear) | ΔEPNC (au) (Linear) ALDOL [1] |
|---|---|---|---|
| 20Ca2+ | 1.4 × 10−12 | 1.1 × 10−14 | 0.3 × 10−20 |
| 38Sr2+ | 3.2 × 10−11 | 7.6 × 10−13 | 0.6 × 10−19 |
| 56Ba2+ | 1.9 × 10−10 | 4.3 × 10−12 | 0.3 × 10−18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cowan, J.A. Influence of the Weak Nuclear Force on Metal-Promoted Autocatalytic Strecker Synthesis of Amino Acids: Formation of a Chiral Pool of Precursors for Prebiotic Peptide and Protein Synthesis. Life 2024, 14, 66. https://doi.org/10.3390/life14010066
Cowan JA. Influence of the Weak Nuclear Force on Metal-Promoted Autocatalytic Strecker Synthesis of Amino Acids: Formation of a Chiral Pool of Precursors for Prebiotic Peptide and Protein Synthesis. Life. 2024; 14(1):66. https://doi.org/10.3390/life14010066
Chicago/Turabian StyleCowan, J. A. 2024. "Influence of the Weak Nuclear Force on Metal-Promoted Autocatalytic Strecker Synthesis of Amino Acids: Formation of a Chiral Pool of Precursors for Prebiotic Peptide and Protein Synthesis" Life 14, no. 1: 66. https://doi.org/10.3390/life14010066
APA StyleCowan, J. A. (2024). Influence of the Weak Nuclear Force on Metal-Promoted Autocatalytic Strecker Synthesis of Amino Acids: Formation of a Chiral Pool of Precursors for Prebiotic Peptide and Protein Synthesis. Life, 14(1), 66. https://doi.org/10.3390/life14010066






