CYP27B1 Enzyme in Psoriasis: A Preliminary Study of Immunohistochemical Observations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Selection
2.2. Clinical Data of the Patients
2.3. Immunohistochemistry, Markers, and Other Variables Measured
2.3.1. Immunohistochemistry Technique for the CYP27B1 Enzyme
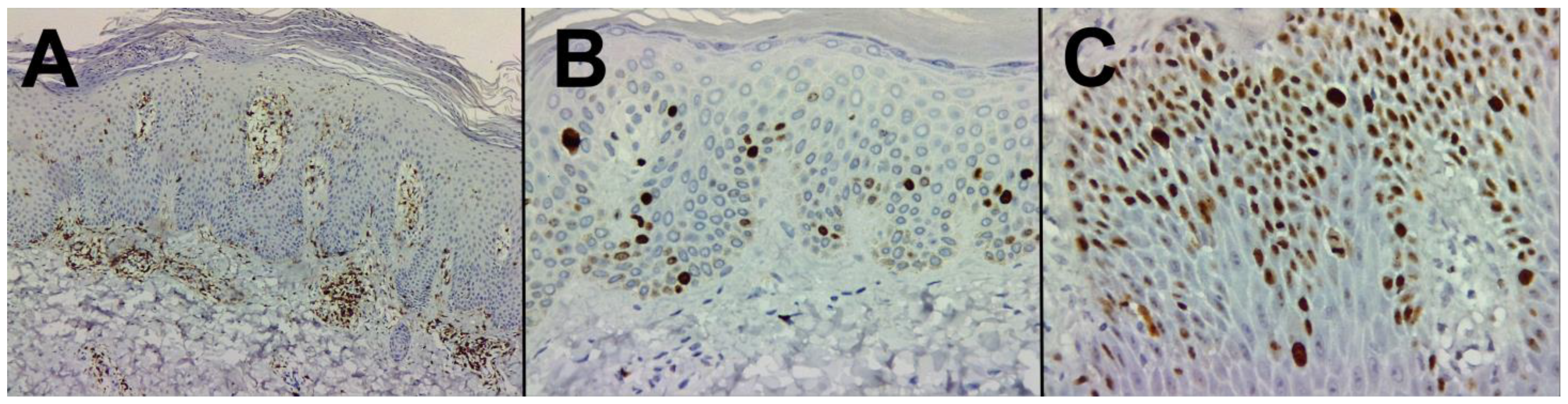
2.3.2. Immunohistochemistry Technique for the Cell Proliferation Antigen Ki67 and the CD45RO+ Memory-Effector T Cells Marker
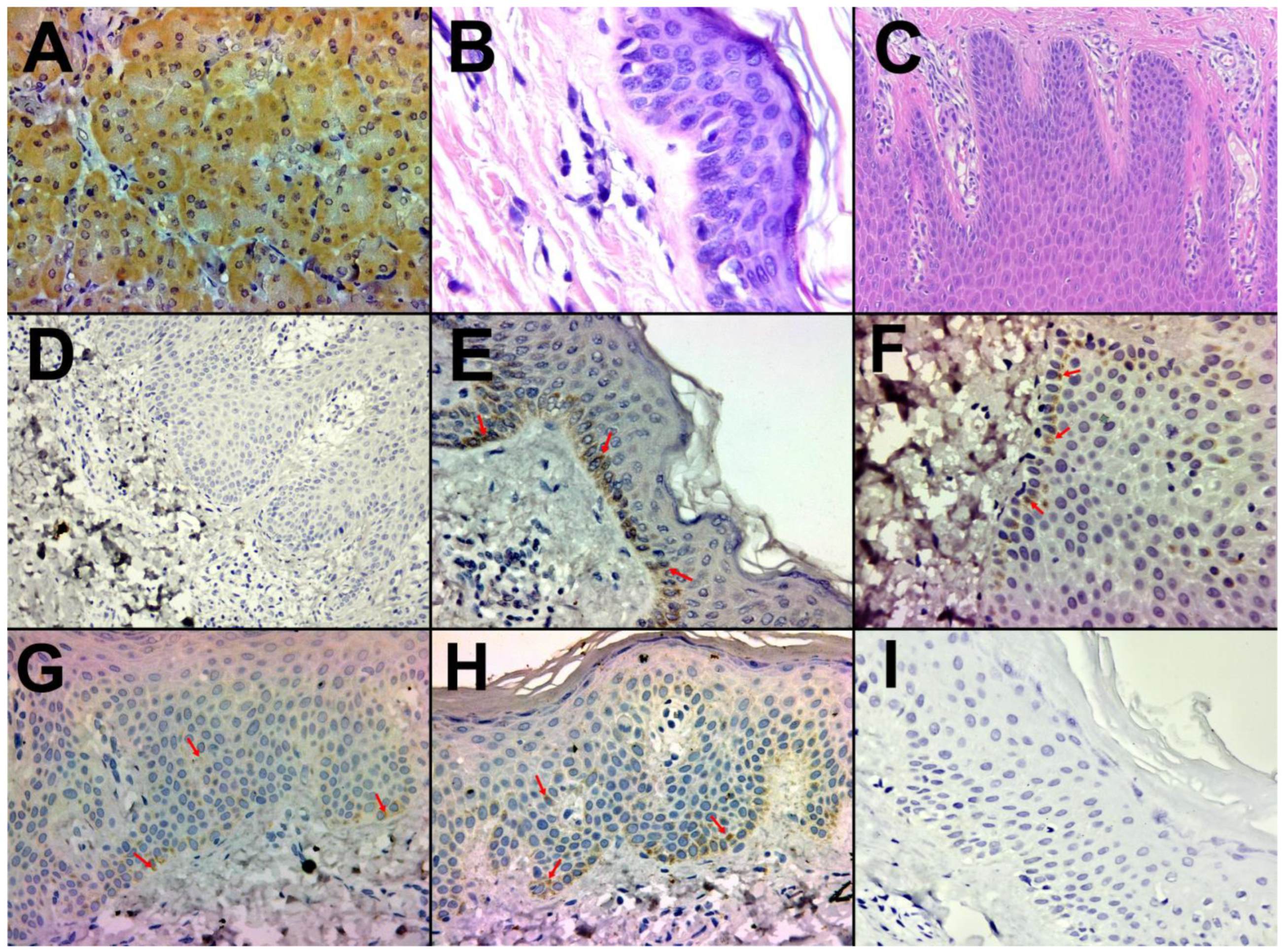
2.3.3. Immunostaining Assessment
- Grade 0: None, when the median count is less than 10.
- Grade 1: Weak, when the median count ranges from 10 to 30.
- Grade 2: Moderate, when the median count ranges from 31 to 50.
- Grade 3: Strong, when the median count exceeds 50.
- Grade 0: No infiltrate when the median count is <10.
- Grade 1: Mild infiltrate when the median count is between 10 and 30.
- Grade 2: Moderate infiltrate when the median count is between 31 and 50.
- Grade 3: Marked infiltrate (severe inflammation) when the median count is >50.
2.4. Statistical Analysis
3. Results
3.1. Analysis of the Disease-Related Aspects and Demographic Characteristics
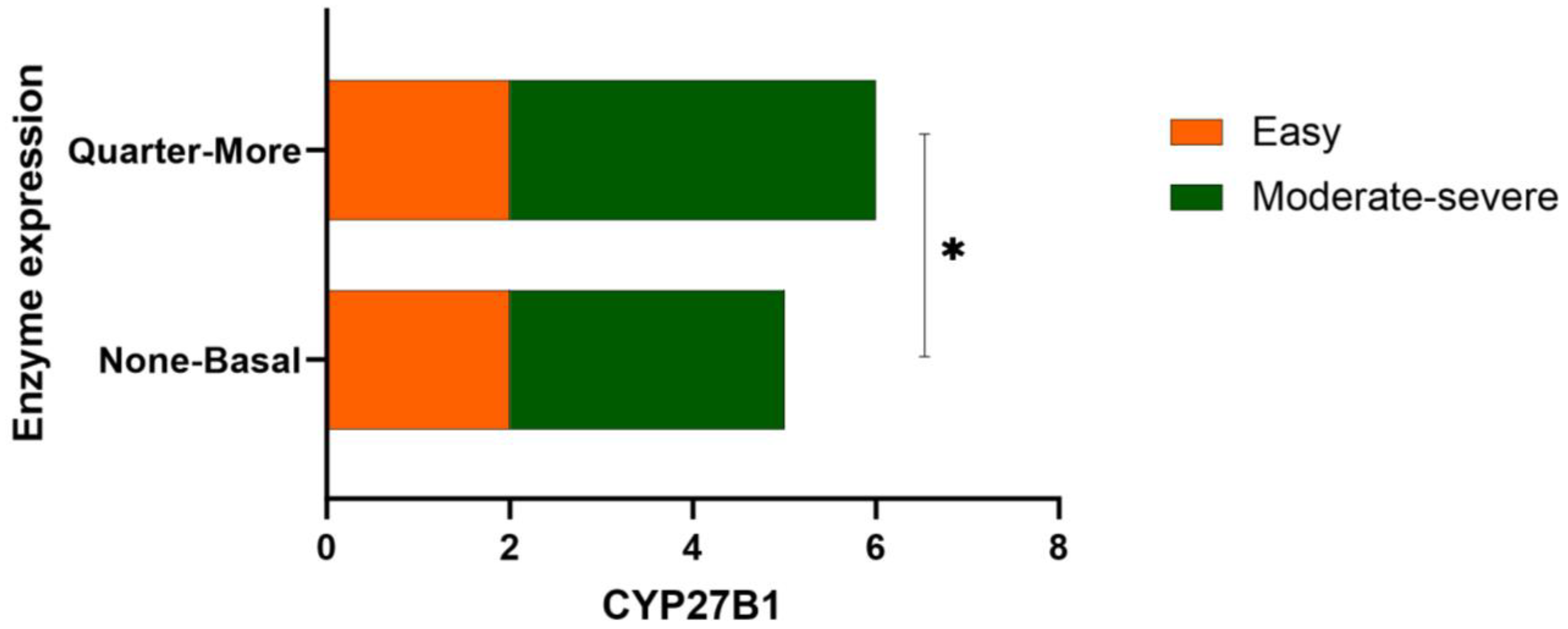
3.2. Results Obtained from Different Severity Types of Psoriasis and Comparison with the Clinical Context
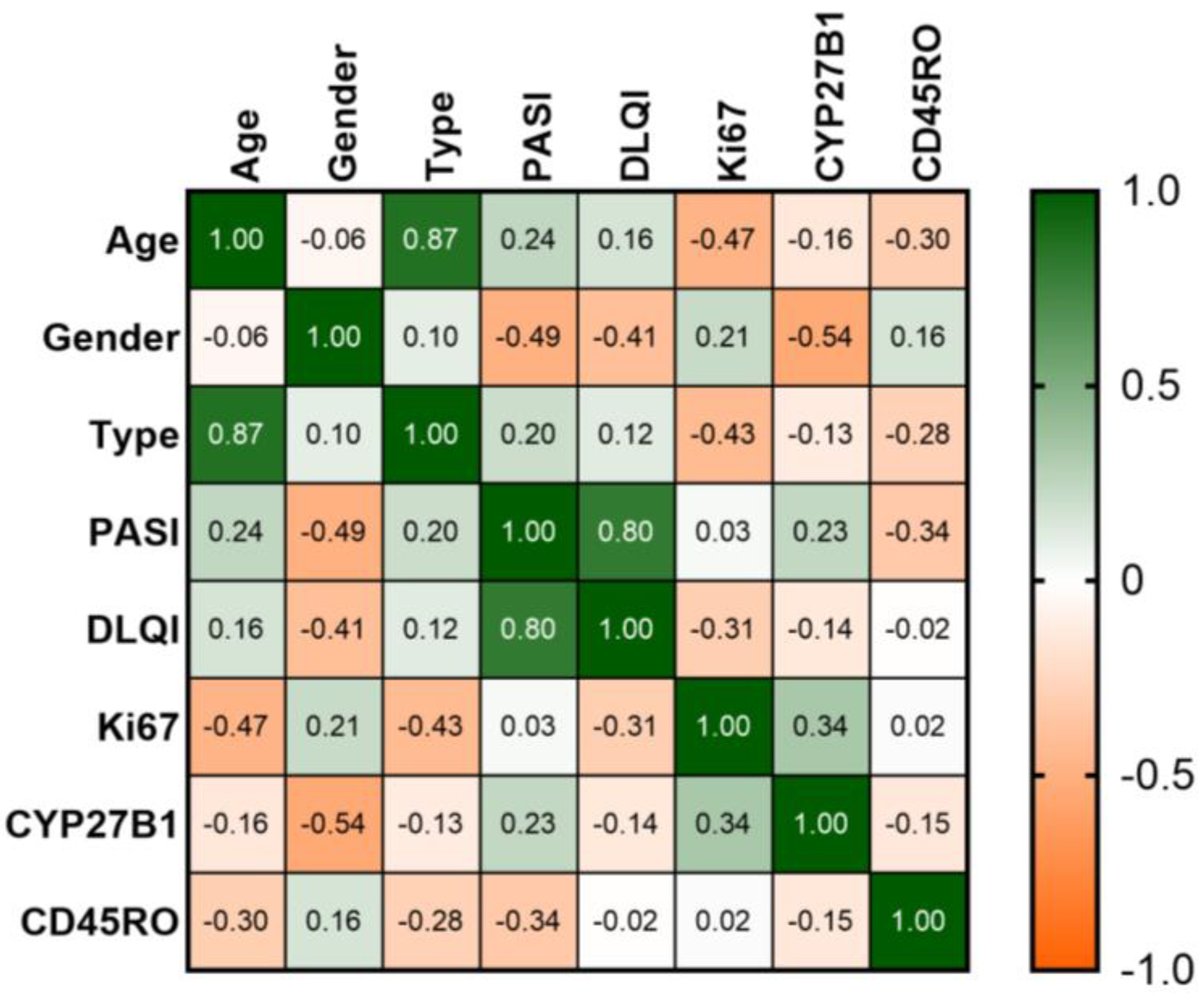
3.3. Immunohistochemical Characterization
3.3.1. Healthy Skin and Control
3.3.2. Psoriasis Skin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA J. Am. Med. Assoc. 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Griffiths, C.E.M. The Immunological Basis of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 1–5. [Google Scholar] [CrossRef]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef]
- Wierzbicka, J.M.; Piotrowska, A.; Purzycka-Bohdan, D.; Olszewska, A.; Nowak, J.I.; Szczerkowska-Dobosz, A.; Nedoszytko, B.; Nowicki, R.J.; Żmijewski, M.A. The Effects of Vitamin d on the Expression of Il-33 and Its Receptor St2 in Skin Cells; Potential Implication for Psoriasis. Int. J. Mol. Sci. 2021, 22, 12907. [Google Scholar] [CrossRef]
- Kittaka, A.; Takano, M.; Saitoh, H. Vitamin D Analogs with Nitrogen Atom at C2 Substitution and Effect on Bone Formation. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2016; Volume 100, pp. 379–394. ISBN 9780128048245. [Google Scholar]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and Its Role in Psoriasis: An Overview of the Dermatologist and Nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef]
- Aschoff, R.; Bewley, A.; Dattola, A.; De Simone, C.; Lahfa, M.; Llamas-Velasco, M.; Martorell, A.; Pavlovic, M.; Sticherling, M. Beyond-Mild Psoriasis: A Consensus Statement on Calcipotriol and Betamethasone Dipropionate Foam for the Topical Treatment of Adult Patients. Dermatol. Ther. 2021, 11, 1791–1804. [Google Scholar] [CrossRef]
- Ponchon, G.; Kennan, A.L.; DeLuca, H.F. “Activation” of Vitamin D by the Liver. J. Clin. Investig. 1969, 48, 2032–2037. [Google Scholar] [CrossRef]
- Cheng, J.B.; Motola, D.L.; Mangelsdorf, D.J.; Russell, D.W. De-Orphanization of Cytochrome P450 2R1: A Microsomal Vitamin D 25-Hydroxylase. J. Biol. Chem. 2003, 278, 38084–38093. [Google Scholar] [CrossRef]
- Fraser, D.R.; Kodicek, E. Unique Biosynthesis by Kidney of a Biologically Active Vitamin D Metabolite. Nature 1970, 228, 764–766. [Google Scholar] [CrossRef]
- Brommage, R.; Deluca, H.F. Evidence That 1,25-Dihydroxyvitamin D3 Is the Physiologically Active Metabolite of Vitamin D3. Endocr. Rev. 1985, 6, 491–511. [Google Scholar] [CrossRef]
- Issa, L.L.; Leong, G.M.; Eisman, J.A. Molecular Mechanism of Vitamin D Receptor Action. Inflamm. Res. 1998, 47, 451–475. [Google Scholar] [CrossRef]
- Brunette, M.G.; Chan, M.; Ferriere, C.; Roberts, K.D. Site of 1,25(OH)2 Vitamin D3 Synthesis in the Kidney. Nature 1978, 276, 287–289. [Google Scholar] [CrossRef]
- Sakaki, T.; Kagawa, N.; Yamamoto, K.; Inouye, K. Metabolism of Vitamin D3 by Cytochromes P450. Front. Biosci. 2005, 10, 119–134. [Google Scholar]
- Ghazarian, J.G.; Jefcoate, C.R.; Knutson, J.C.; Orme-Johnson, W.H.; DeLuca, H.F. Mitochondrial Cytochrome P450—A Component of Chick Kidney 25-Hydroxycholecalciferol-1a-Hydroxylase. J. Biol. Chem. 1974, 249, 3026–3034. [Google Scholar] [CrossRef]
- Matsumoto, K.; Azuma, Y.; Kiyoki, M.; Okumura, H.; Hashimoto, K.; Yoshikawa, K. Involvement of Endogenously Produced 1,25-Dihydroxyvitamin D-3 in the Growth and Differentiation of Human Keratinocytes. BBA-Mol. Cell Res. 1991, 1092, 311–318. [Google Scholar] [CrossRef]
- Kaukinen, A.; Pelkonen, J.; Harvima, I.T. Mast Cells Express CYP27A1 and CYP27B1 in Epithelial Skin Cancers and Psoriasis. Eur. J. Dermatol. 2015, 25, 548–555. [Google Scholar] [CrossRef]
- Mattozzi, C.; Paolino, G.; Richetta, A.G.; Calvieri, S. Psoriasis, Vitamin D and the Importance of the Cutaneous Barrier’s Integrity: An Update. J. Dermatol. 2016, 43, 507–514. [Google Scholar] [CrossRef]
- Viaene, L.; Evenepoel, P.; Meijers, B.; Vanderschueren, D.; Overbergh, L.; Mathieu, C. Uremia Suppresses Immune Signal-Induced CYP27B1 Expression in Human Monocytes. Am. J. Nephrol. 2012, 36, 497–508. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the Systemic Treatment of Psoriasis Vulgaris—Part 1: Treatment and Monitoring Recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef]
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.M.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of Treatment Goals for Moderate to Severe Psoriasis: A European Consensus. Arch. Dermatol. Res. 2011, 303, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morar, I.-I.; Tabăran, F.-A.; Mocan, T.; Jianu, E.-M.; Orăsan, M.-S.; Pop, A.-D.; Orăsan, R.-I. Immunohistochemical Study of Psoriatic Plaques and Perilesional Skin in Psoriasis Vulgaris Patients: A Pilot Study. Exp. Ther. Med. 2019, 18, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Tursen, B.; Kara, T.; Tursen, U.; Apa, D.D.; Gubur, O.; Kaya, T.I. The Changes in Expression of Ki-67, and CD31 in Psoriatic Lesions before and after Etanercept Treatment. Hong Kong J. Dermatol. Venereol. 2013, 21, 5–13. [Google Scholar]
- Brambs, C.E.; Horn, L.C.; Mende, M.; Höckel, M.; Eckey, C.; Hiller, G.G.R.; Höhn, A.K. Epithelial–Mesenchymal Transition (EMT) in Vulvar Cancer with and without Inguinal Lymph Node Involvement. J. Cancer Res. Clin. Oncol. 2022, 148, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Jóźwicki, W.; Janjetovic, Z.; Slominski, A.T. Expression of the Vitamin D-Activating Enzyme 1α-Hydroxylase (CYP27B1) Decreases during Melanoma Progression. Hum. Pathol. 2013, 44, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Barbour, G.L.; Coburn, J.W.; Slatopolsky, E.; Norman, A.W.; Horst, R.L. Hypercalcemia in an Anephric Patient with Sarcoidosis: Evidence for Extrarenal Generation of 1,25-Dihydroxyvitamin D. N. Engl. J. Med. 1981, 305, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Sharma, O.P.; Gacad, M.A.; Singer, F.R. Metabolism of 25-Hydroxyvitamin D3 by Cultured Pulmonary Alveolar Macrophages in Sarcoidosis. J. Clin. Investig. 1983, 72, 1856–1860. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the Extrarenal CYP27B1-Hydroxylase. J. Steroid Biochem. Mol. Biol. 2014, 144, 22–27. [Google Scholar] [CrossRef]
- Smith, S.J.; Rucka, A.K.; Berry, J.L.; Davies, M.; Mylchreest, S.; Paterson, C.R.; Heath, D.A.; Tassabehji, M.; Read, A.P.; Mee, A.P.; et al. Novel Mutations in the 1α-Hydroxylase (P450c1) Gene in Three Families with Pseudovitamin D-Deficiency Rickets Resulting in Loss of Functional Enzyme Activity in Blood-Derived Macrophages. J. Bone Miner. Res. 1999, 14, 730–739. [Google Scholar] [CrossRef]
- Fu, G.K.; Lin, D.; Zhang, M.Y.H.; Bikle, D.D.; Shackleton, C.H.L.; Miller, W.L.; Portale, A.A. Cloning of Human 25-Hydroxyvitamin D-1α-Hydroxylase and Mutations Causing Vitamin D-Dependent Rickets Type 1. Mol. Endocrinol. 1997, 11, 1961–1970. [Google Scholar] [CrossRef][Green Version]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-Mediated Metabolism of Vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Noyola-Martínez, N.; Díaz, L.; Zaga-Clavellina, V.; Avila, E.; Halhali, A.; Larrea, F.; Barrera, D. Regulation of CYP27B1 and CYP24A1 Gene Expression by Recombinant Pro-Inflammatory Cytokines in Cultured Human Trophoblasts. J. Steroid Biochem. Mol. Biol. 2014, 144, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Ren, S.Y.; Arbelle, J.E.; Horiuchi, N.; Gray, R.W.; Clemens, T.L.; Shany, S. Regulated Production and Intracrine Action of 1,25-Dihydroxyvitamin D3 in the Chick Myelomonocytic Cell Line HD-11. Endocrinology 1994, 134, 2567–2573. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.; Silver, J.; Sherwood, L.M. The Effects of Calcium and Vitamin D Metabolites on Cytoplasmic MRNA Coding for Pre-Proparathyroid Hormone in Isolated Parathyroid Cells. Trans. Assoc. Am. Phys. 1984, 97, 296–303. [Google Scholar] [PubMed]
- Halloran, B.P.; DeLuca, H.F. Appearance of the Intestinal Cytosolic Receptor for 1,25-Dihydroxyvitamin D3 during Neonatal Development in the Rat. J. Biol. Chem. 1981, 256, 7338–7342. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.J.; Dardenne, O.; Van Abel, M.; Van Der Kemp, A.W.C.M.; Van Os, C.H.; St.-Arnaud, R.; Bindels, R.M. Modulation of Renal Ca2+ Transport Protein Genes by Dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25hydroxyvitamin D3-1α-hydroxylase Knockout Mice. FASEB J. 2002, 16, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Karaplis, A.C.; Hendy, G.N.; Goltzman, D.; Miao, D. Exogenous 1,25-Dihydroxyvitamin D3 Exerts a Skeletal Anabolic Effect and Improves Mineral Ion Homeostasis in Mice That Are Homozygous for Both the 1α-Hydroxylase and Parathyroid Hormone Null Alleles. Endocrinology 2006, 147, 4801–4810. [Google Scholar] [CrossRef]
- Li, Y.C. Vitamin D Regulation of the Renin-Angiotensin System. J. Cell. Biochem. 2003, 88, 327–331. [Google Scholar] [CrossRef]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef]
- Jensen, S.S.; Madsen, M.W.; Lukas, J.; Binderup, L.; Bartek, J. Inhibitory Effects of 1α,25-Dihydroxyvitamin D3 on the G1–S Phase-Controlling Machinery. Mol. Endocrinol. 2001, 15, 1370–1380. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal Expression of 25-Hydroxyvitamin D3-1α-Hydroxylase 1. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Bikle, D.D.; Elias, P.M. 1,25-Dihydroxyvitamin D Production and Receptor Binding in Human Keratinocytes Varies with Differentiation. J. Biol. Chem. 1988, 263, 5390–5395. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, J.; Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of Terminal Differentiation of Cultured Mouse Epidermal Cells by 1α, 25–Dihydroxyvitamin D3. Endocrinology 1983, 113, 1950–1957. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B.; Ducote, J.; Harmon, C.S. Biphasic Effect of 1,25-dihydroxyvitamin D3 on Primary Mouse Epidermal Keratinocyte Proliferation. J. Cell. Physiol. 1995, 163, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Chang, S.; Crumrine, D.; Elalieh, H.; Man, M.Q.; Dardenne, O.; Xie, Z.; Arnaud, R.S.; Feingold, K.; Elias, P.M. Mice Lacking 25OHD 1α-Hydroxylase Demonstrate Decreased Epidermal Differentiation and Barrier Function. J. Steroid Biochem. Mol. Biol. 2004, 89, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Brozyna, A.A.; Józwicki, W.; Jochymski, C.; Slominski, A.T. Decreased Expression of CYP27B1 Correlates with the Increased Aggressiveness of Ovarian Carcinomas. Oncol. Rep. 2015, 33, 599–606. [Google Scholar] [CrossRef]
- Reichrath, J.; Muller, S.M.; Kerber, A.; Baum, H.P.; Bahmer, F.A. Biologic Effects of Topical Calcipotriol (MC 903) Treatment in Psoriatic Skin. J. Am. Acad. Dermatol. 1997, 36, 19–28. [Google Scholar] [CrossRef]
- Clinckspoor, I.; Hauben, E.; Verlinden, L.; van den Bruel, A.; Vanwalleghem, L.; Vander Poorten, V.; Delaere, P.; Mathieu, C.; Verstuyf, A.; Decallonne, B. Altered Expression of Key Players in Vitamin D Metabolism and Signaling in Malignant and Benign Thyroid Tumors. J. Histochem. Cytochem. 2012, 60, 502–511. [Google Scholar] [CrossRef]
| Patient’s Number | Gender | Age | PASI Score | DLQI Score |
|---|---|---|---|---|
| 1 | Female | 62 | 5.4 | 9 |
| 2 | Female | 37 | 4.4 | 6 |
| 3 | Male | 66 | 9.6 | 9 |
| 4 | Female | 17 | 9.6 | 6 |
| Patient’s Number | Gender | Age | PASI Score | DLQI Score |
|---|---|---|---|---|
| 5 | Male | 59 | 23.1 | 21 |
| 6 | Male | 39 | 44.6 | 27 |
| 7 | Male | 38 | 10.2 | 20 |
| 8 | Female | 76 | 11.8 | 17 |
| 9 | Male | 26 | 11.3 | 16 |
| 10 | Female | 49 | 15.0 | 21 |
| 11 | Male | 65 | 34.1 | 11 |
| Characteristics | Patients (n = 11) | Severity of the Disease | p-Value | |
|---|---|---|---|---|
| Mild (n = 4) | Moderate-sever (n = 7) | |||
| Age | 0.705 | |||
| mean ± SD | 48.55 ± 18.6 | 45.5 ± 22.93 | 50.29 ± 17.43 | |
| median (IQR) | 49 (37–65) | 49.5 (22–65) | 49 (38–65) | |
| Gender, Male (n, %) | 6 (54.5%) | 1 (25%) | 5 (71.4%) | 0.242 |
| PASI | 0.008 *** | |||
| mean ± SD | 16.28 ± 11.3 | 7.25 ± 2.74 | 21.44 ± 13.31 | |
| median (IQR) | 11.3 (9.6–23.1) | 7.5 (4.65–9.6) | 15.0 (11.3–34.1) | |
| DLQI | 0.008 *** | |||
| mean ± SD | 14.82 ± 7.04 | 7.5 ± 1.73 | 19.0 ± 5.0 | |
| median (IQR) | 11.3 (9.6–23.1) | 7.5 (6.0–9.0) | 20.0 (16.0–21.0) | |
| Ki67 | 0.757 | |||
| 1 (n, %) | 4 (36.4%) | 1 (25%) | 3 (42.9%) | |
| 2 (n, %) | 3 (27.3%) | 1 (25%) | 2 (28.6%) | |
| 3 (n, %) | 4 (36.4%) | 2 (50%) | 2 (28.6%) | |
| CD45RO+ | 0.214 | |||
| mean ± SD | 81.82 ± 10.79 | 87.5 ± 5 | 78.57 ± 12.15 | |
| median (IQR) | 90 (70–90) | 90 (82.5–90) | 80 (70–90) | |
| CYP27B1 | 0.073 | |||
| 0 (n, %) | 3 (27.3%) | 0 | 3 (42.9%) | |
| 1 (n, %) | 2 (18.2%) | 2 (50%) | 0 | |
| 2 (n, %) | 6 (54.5%) | 2 (50%) | 4 (57.1%) |
| Patient’s Number | Psoriasis Severity Type | CYP27B1 Staining Score | Ki67 Staining Score | CD45RO+ Staining Score |
|---|---|---|---|---|
| 1 | Mild | 1 | 2 | 90% |
| 2 | Mild | 1 | 3 | 80% |
| 3 | Mild | 2 | 1 | 90% |
| 4 | Mild | 2 | 3 | 90% |
| 5 | Moderate to severe | 2 | 1 | 70% |
| 6 | Moderate to severe | 2 | 3 | 90% |
| 7 | Moderate to severe | 0 | 1 | 90% |
| 8 | Moderate to severe | 2 | 2 | 80% |
| 9 | Moderate to severe | 2 | 3 | 60% |
| 10 | Moderate to severe | 0 | 2 | 90% |
| 11 | Moderate to severe | 0 | 1 | 70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paliu, I.-A.; Olinca, M.-V.; Ianosi, S.-L.; Georgescu, C.-V.; Turcu-Stiolica, A.; Diaconu, M.; Dumitrescu, C.-I.; Tica, A.-A. CYP27B1 Enzyme in Psoriasis: A Preliminary Study of Immunohistochemical Observations. Life 2024, 14, 15. https://doi.org/10.3390/life14010015
Paliu I-A, Olinca M-V, Ianosi S-L, Georgescu C-V, Turcu-Stiolica A, Diaconu M, Dumitrescu C-I, Tica A-A. CYP27B1 Enzyme in Psoriasis: A Preliminary Study of Immunohistochemical Observations. Life. 2024; 14(1):15. https://doi.org/10.3390/life14010015
Chicago/Turabian StylePaliu, Iulia-Alexandra, Maria-Victoria Olinca, Simona-Laura Ianosi, Claudia-Valentina Georgescu, Adina Turcu-Stiolica, Magdalena Diaconu, Cristiana-Iulia Dumitrescu, and Andrei-Adrian Tica. 2024. "CYP27B1 Enzyme in Psoriasis: A Preliminary Study of Immunohistochemical Observations" Life 14, no. 1: 15. https://doi.org/10.3390/life14010015
APA StylePaliu, I.-A., Olinca, M.-V., Ianosi, S.-L., Georgescu, C.-V., Turcu-Stiolica, A., Diaconu, M., Dumitrescu, C.-I., & Tica, A.-A. (2024). CYP27B1 Enzyme in Psoriasis: A Preliminary Study of Immunohistochemical Observations. Life, 14(1), 15. https://doi.org/10.3390/life14010015









