The Impact of Non-Concentrated Storage on the Centrifugation Yield of Microchloropsis gaditana: A Pilot-Scale Study
Abstract
1. Introduction
2. Materials and Methods
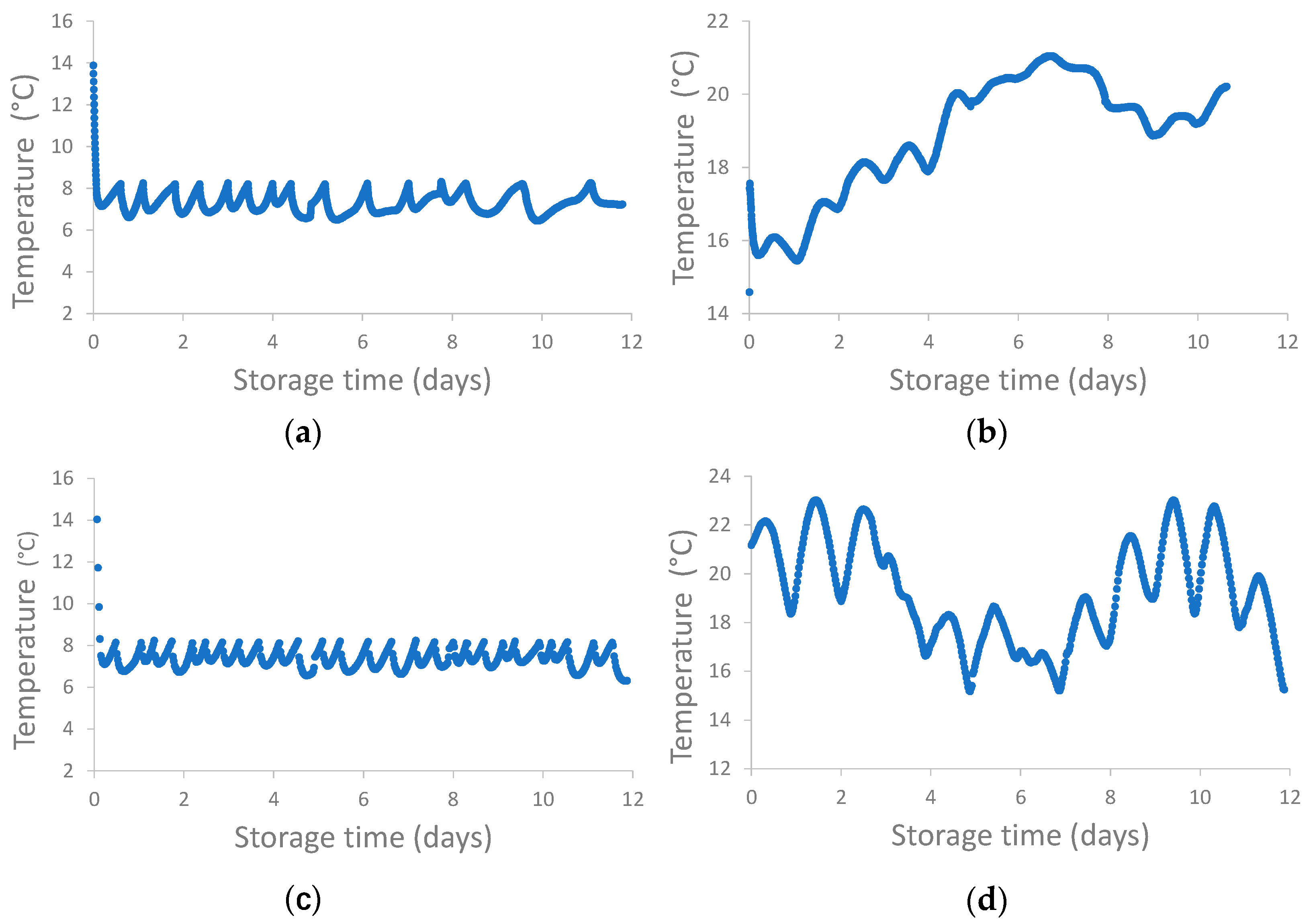
2.1. Pilot-Scale Algae Storage: Algae Cultivation and Storage Conditions
2.2. Biochemical Analyses
2.3. Statistical Analysis
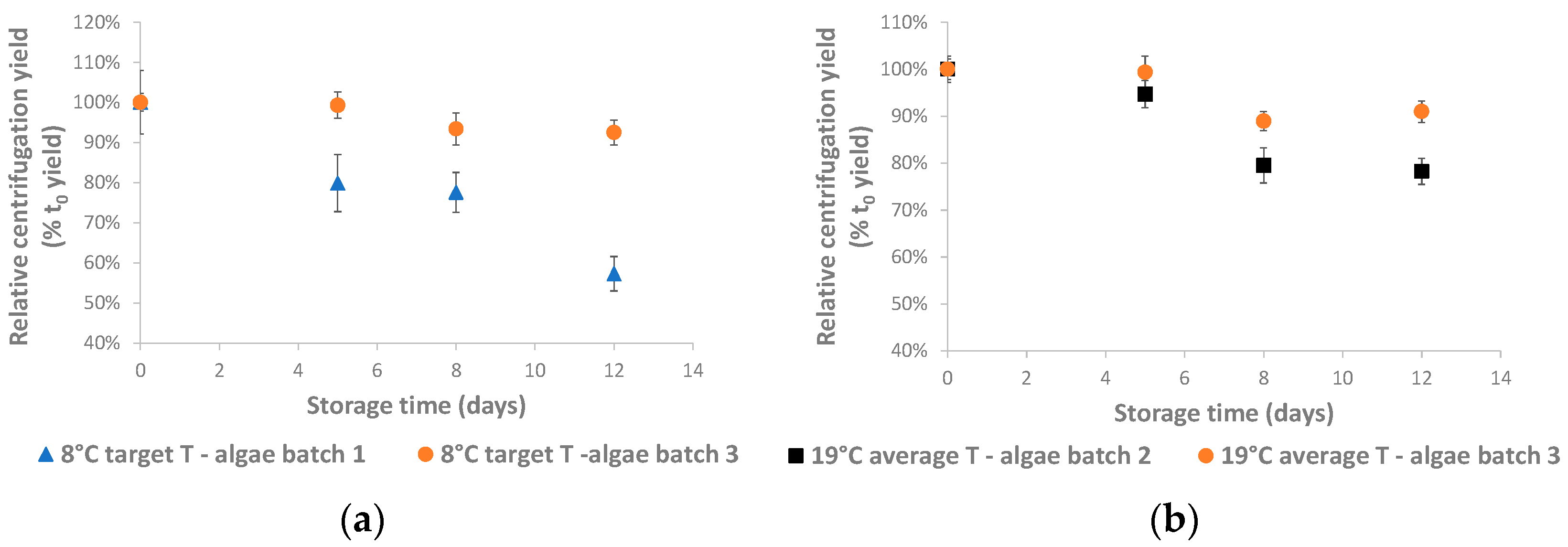
3. Results and Discussion
Pilot-Scale Algae Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, Hopes, and the Way Forward for Microalgal Biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Global Seaweeds and Microalgae Production, 1950–2019; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Ruiz, J.; Olivieri, G.; De Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Towards Industrial Products from Microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Balduyck, L.; Goiris, K.; Bruneel, C.; Muylaert, K.; Foubert, I. Stability of Valuable Components during Wet and Dry Storage; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128011249. [Google Scholar]
- Camacho-Rodríguez, J.; Cerón-García, M.C.; Macías-Sánchez, M.D.; Fernández-Sevilla, J.M.; López-Rosales, L.; Molina-Grima, E. Long-Term Preservation of Concentrated Nannochloropsis Gaditana Cultures for Use in Aquaculture. J. Appl. Phycol. 2016, 28, 299–312. [Google Scholar] [CrossRef]
- Heasman, M.; Diemar, J.; Connor, W.O.; Sushames, T.; Foulkes, L. Development of Extended Shelf-Life Microalgae Concentrate Diets Harvested by Centrifugation for Bivalve Molluscs—A Summary. Aquac. Res. 2000, 31, 637–659. [Google Scholar]
- Montaini, E.; Chini Zittelli, G.; Tredici, M.R.; Molina Grima, E.; Fernández Sevilla, J.M.; Sánchez Pérez, J.A. Long-Term Preservation of Tetraselmis Suecica: Influence of Storage on Viability and Fatty Acid Profile. Aquaculture 1995, 134, 81–90. [Google Scholar] [CrossRef]
- Balduyck, L.; Stock, T.; Bijttebier, S.; Bruneel, C.; Jacobs, G.; Voorspoels, S.; Muylaert, K.; Foubert, I. Integrity of the Microalgal Cell Plays a Major Role in the Lipolytic Stability during Wet Storage. Algal Res. 2017, 25, 516–524. [Google Scholar] [CrossRef]
- Verspreet, J.; Soetemans, L.; Bastiaens, L. Searching for Appropriate Storage Conditions for Short-Term Wet Preservation of Porphyridium Purpureum. Appl. Sci. 2020, 10, 8315. [Google Scholar] [CrossRef]
- Latsos, C.; Wassenaar, E.; Moerdijk, T.; Coleman, B.; Robbens, J.; van Roy, S.; Bastiaens, L.; van Houcke, J.; Timmermans, K.R. Effect of pH on Rhodomonas Salina Growth, Biochemical Composition, and Taste, Produced in Semi-Large Scale under Sunlight Conditions. J. Appl. Phycol. 2022, 34, 1215–1226. [Google Scholar] [CrossRef]
- Wendt, L.M.; Wahlen, B.D.; Li, C.; Kachurin, G.; Ogden, K.L.; Murphy, J.A. Evaluation of a High-Moisture Stabilization Strategy for Harvested Microalgae Blended with Herbaceous Biomass: Part I—Storage Performance. Algal Res. 2017, 25, 567–575. [Google Scholar] [CrossRef]
- Verspreet, J.; Kreps, S.; Bastiaens, L. Evaluation of Microbial Load, Formation of Odorous Metabolites and Lipid Stability during Wet Preservation of Nannochloropsis Gaditana Concentrates. Appl. Sci. 2020, 10, 3419. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Schoeters, F.; Spit, J.; Swinnen, E.; De Cuyper, A.; Vleugels, R.; Noyens, I.; Van Miert, S. Pilot-Scale Cultivation of the Red Alga Porphyridium Purpureum over a Two-Year Period in a Greenhouse. J. Appl. Phycol. 2023, 35, 2095–2109. [Google Scholar] [CrossRef]
- Thoré, E.S.J.; Schoeters, F.; Spit, J.; Van Miert, S. Real-Time Monitoring of Microalgal Biomass in Pilot-Scale Photobioreactors Using Nephelometry. Processes 2021, 9, 1530. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an Analytical Procedure for Extraction of Lipids from Microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Wendt, L.M.; St. Germain, C.C.; Traynor, S.M.; Barboza, C.; Dempster, T.; Gerken, H.; McGowen, J.; You, Y. Effect of Nitrogen Management in Cultivation on the Stability and Microbial Community of Post-Harvest Monoraphidium Sp. Algae Biomass. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad004. [Google Scholar] [CrossRef]
- Ponis, E.; Parisi, G.; Chini Zittelli, G.; Lavista, F.; Robert, R.; Tredici, M.R. Pavlova Lutheri: Production, Preservation and Use as Food for Crassostrea Gigas Larvae. Aquaculture 2008, 282, 97–103. [Google Scholar] [CrossRef]
- Sales, R.; Mélo, R.C.S.; de Moraes, R.M.; da Silva, R.C.S.; Cavalli, R.O.; do Amaral Ferraz Navarro, D.M.; de Souza Santos, L.P. Production and Use of a Flocculated Paste of Nannochloropsis Oculata for Rearing Newborn Seahorse Hippocampus Reidi. Algal Res. 2016, 17, 142–149. [Google Scholar] [CrossRef]
- Brown, M.; Robert, R. Preparation and Assessment of Microalgal Concentrates as Feeds for Larval and Juvenile Pacific Oyster (Crassostrea Gigas). Aquaculture 2002, 207, 289–309. [Google Scholar] [CrossRef]
- Ye, J.; Sha, J.; Liu, Q.; Zhang, X.; Hu, Q.; Chen, Y. Influence of Growth Phase on the Harvesting of Scenedesmus Acuminatus Using Ultrafiltration. Sci. Total Environ. 2019, 660, 25–31. [Google Scholar] [CrossRef]
- Zhang, X.; Amendola, P.; Hewson, J.C.; Sommerfeld, M.; Hu, Q. Influence of Growth Phase on Harvesting of Chlorella Zofingiensis by Dissolved Air Flotation. Bioresour. Technol. 2012, 116, 477–484. [Google Scholar] [CrossRef]
- Xu, Y.; Milledge, J.J.; Abubakar, A.; Swamy, R.A.R.; Bailey, D.; Harvey, P.J. Effects of Centrifugal Stress on Cell Disruption and Glycerol Leakage from Dunaliella Salina. Microalgae Biotechnol. 2015, 1, 20–27. [Google Scholar] [CrossRef]
- Bemejo-Padilla, E.; Kinsou, H.; Filali, R.; Perez-Bibbins, B.; Taidi, B. Rapid Indicators for Monitoring the Health of Chlamydomonas Nivalis Biomass during Preservation. J. Appl. Phycol. 2021, 33, 2723–2732. [Google Scholar] [CrossRef]
- Yue, L.; Lv, H.; Zhen, J.; Jiang, S.; Jia, S.; Shen, S.; Gao, L.; Dai, Y. Bacterial Species and Biochemical Characteristic Investigations of Nostoc Flagelliforme Concentrates during Its Storage. J. Microbiol. Biotechnol. 2016, 26, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of Microalgae by Centrifugation for Biodiesel Production: A Review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Beacham, T.A.; Bradley, C.; White, D.A.; Bond, P.; Ali, S.T. Lipid Productivity and Cell Wall Ultrastructure of Six Strains of Nannochloropsis: Implications for Biofuel Production and Downstream Processing. Algal Res. 2014, 6, 64–69. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The Potential of Microalgae and Their Biopolymers as Structuring Ingredients in Food: A Review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Li, N.; Wang, P.; Wang, S.; Wang, C.; Zhou, H.; Kapur, S.; Zhang, J.; Song, Y. Electrostatic Charges on Microalgae Surface: Mechanism and Applications. J. Environ. Chem. Eng. 2022, 10, 107516. [Google Scholar] [CrossRef]
- Harith, Z.T.; Yusoff, F.M.; Shariff, M.; Ariff, A.B. Effect of Different Separation Techniques and Storage Temperatures on the Viability of Marine Microalgae, Chaetoceros Calcitrans, during Storage. Biotechnology 2010, 9, 387–391. [Google Scholar] [CrossRef][Green Version]
| Storage Time (Days) | Organic Matter (g/L) | Organic Matter Retained (% of t0 Organic Matter) |
|---|---|---|
| 8 °C target T—algae batch 1 * | ||
| 0 | 2.77 ± 0.08 (a) | 100.0% ± 2.8% (a) |
| 5 | 2.84 ± 0.11 (a) | 102.4% ± 4.1% (a) |
| 8 | 2.85 ± 0.08 (a) | 103.0% ± 3.0% (a) |
| 12 | 2.76 ± 0.03 (a) | 99.7% ± 1.2% (a) |
| 8 °C target T—algae batch 3 * | ||
| 0 | 3.18 ± 0.07 (a) | 100.0% ± 2.1% (a) |
| 5 | 3.10 ± 0.08 (ab) | 97.5% ± 2.4% (ab) |
| 8 | 3.05 ± 0.06 (b) | 96.0% ± 1.9% (b) |
| 12 | 3.03 ± 0.02 (b) | 95.4% ± 0.6% (b) |
| 19 °C average T—algae batch 2 * | ||
| 0 | 4.02 ± 0.02 (a) | 100.0% ± 0.6% (a) |
| 5 | 3.99 ± 0.14 (a) | 99.1% ± 3.6% (a) |
| 8 | 3.85 ± 0.05 (a) | 95.7% ± 1.3% (a) |
| 12 | 3.92 ± 0.08 (a) | 97.6% ± 2.1% (a) |
| 19 °C average T—algae batch 3 * | ||
| 0 | 3.18 ± 0.07 (a) | 100.0% ± 2.1% (a) |
| 5 | 3.09 ± 0.04 (ab) | 97.2% ± 1.4% (ab) |
| 8 | 3.03 ± 0.04 (b) | 95.4% ± 1.1% (b) |
| 12 | 3.11 ± 0.04 (ab) | 97.8% ± 1.2% (ab) |
| Storage Time (Days) | Lipid Level in Pellet (% dm) | Carbohydrates * in Pellet (% dm) | |
|---|---|---|---|
| 8 °C target T—algae batch 1 ** | |||
| 0 | 22.1 ± 0.5 (a) | 14.6 ± 0.2 (a) | |
| 5 | 21.9 ± 1.1 (a) | 16.2 ± 2.6 (a) | |
| 8 | 22.1 ± 0.6 (a) | 14.2 ± 0.1 (a) | |
| 12 | 21.5 ± 0.4 (a) | 14.3 ± 0.0 (a) | |
| 8 °C target T—algae batch 3 ** | |||
| 0 | 18.3 ± 0.1 (a) | 23.2 ± 0.4 (a) | |
| 5 | 18.3 ± 0.5 (a) | 24.1 ± 0.7 (ab) | |
| 8 | 19.6 ± 0.3 (a) | 23.2 ± 0.1 (a) | |
| 12 | 18.9 ± 0.5 (a) | 24.8 ± 1.0 (ab) | |
| 19 °C average T—algae batch 2 ** | |||
| 0 | 23.5 ± 0.3 (ab) | 17.8 ± 0.3 (a) | |
| 5 | 22.9 ± 1.7 (b) | 16.7 ± 0.1 (b) | |
| 8 | 24.7 ± 0.1 (ab) | 16.1 ± 0.1 (bc) | |
| 12 | 26.5 ± 0.1 (a) | 15.8 ± 0.3 (c) | |
| 19 °C average T—algae batch 3 ** | |||
| 0 | 18.3 ± 0.1 (a) | 23.1 ± 0.4 (a) | |
| 5 | 20.2 ± 0.0 (a) | 21.9 ± 0.1 (b) | |
| 8 | 20.5 ± 0.4 (a) | 21.8 ± 0.5 (b) | |
| 12 | 22.8 ± 2.4 (a) | 22.6 ± 0.4 (ab) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verspreet, J.; Schoeters, F.; Bastiaens, L. The Impact of Non-Concentrated Storage on the Centrifugation Yield of Microchloropsis gaditana: A Pilot-Scale Study. Life 2024, 14, 131. https://doi.org/10.3390/life14010131
Verspreet J, Schoeters F, Bastiaens L. The Impact of Non-Concentrated Storage on the Centrifugation Yield of Microchloropsis gaditana: A Pilot-Scale Study. Life. 2024; 14(1):131. https://doi.org/10.3390/life14010131
Chicago/Turabian StyleVerspreet, Joran, Floris Schoeters, and Leen Bastiaens. 2024. "The Impact of Non-Concentrated Storage on the Centrifugation Yield of Microchloropsis gaditana: A Pilot-Scale Study" Life 14, no. 1: 131. https://doi.org/10.3390/life14010131
APA StyleVerspreet, J., Schoeters, F., & Bastiaens, L. (2024). The Impact of Non-Concentrated Storage on the Centrifugation Yield of Microchloropsis gaditana: A Pilot-Scale Study. Life, 14(1), 131. https://doi.org/10.3390/life14010131