2. Materials and Methods
The study included 56 eyes from 28 patients who had been diagnosed with Stargardt disease (STGD) (38 eyes of 19 patients), cone dystrophy (CD) (10 eyes of 5 patients), or cone–rod dystrophy (CRD) (8 eyes of 4 patients). One eye, belonging to a female patient diagnosed with CD, was excluded from the analysis of photoreceptor parameters because it was not possible to obtain a good-quality image in any quadrant during the follow-up check. However, this eye was included in the analysis of factors that could potentially lead to incomplete data acquisition.
The examinations occurred in 2015 and were repeated in 2021, conducted at the Department of Ophthalmology, Medical University of Warsaw, in the SPKSO Ophthalmic University Hospital.
Each patient received his/her respective diagnosis of CD, CRD, or STGD through an evaluation that incorporated the clinical appearance of the eye fundus, FAF, AF, perimetry, and electrophysiological testing. Genetic testing was carried out in 20 patients, 13 of whom with STGD tested positive for ABCA4 mutations. In the remaining patients, no mutation causing their conditions was found.
The exclusion criteria for the study encompassed other ocular pathologies such as glaucoma, cataract, previous ocular surgeries, history of uveitis, obesity (body mass index (BMI) > 30 kg/m), and diabetes. Each participant, and parents of those under 18, provided written consent. This study adhered to the tenets of the Declaration of Helsinki and secured approval from the bioethics committee of the Medical University of Warsaw (KB/87/2015).
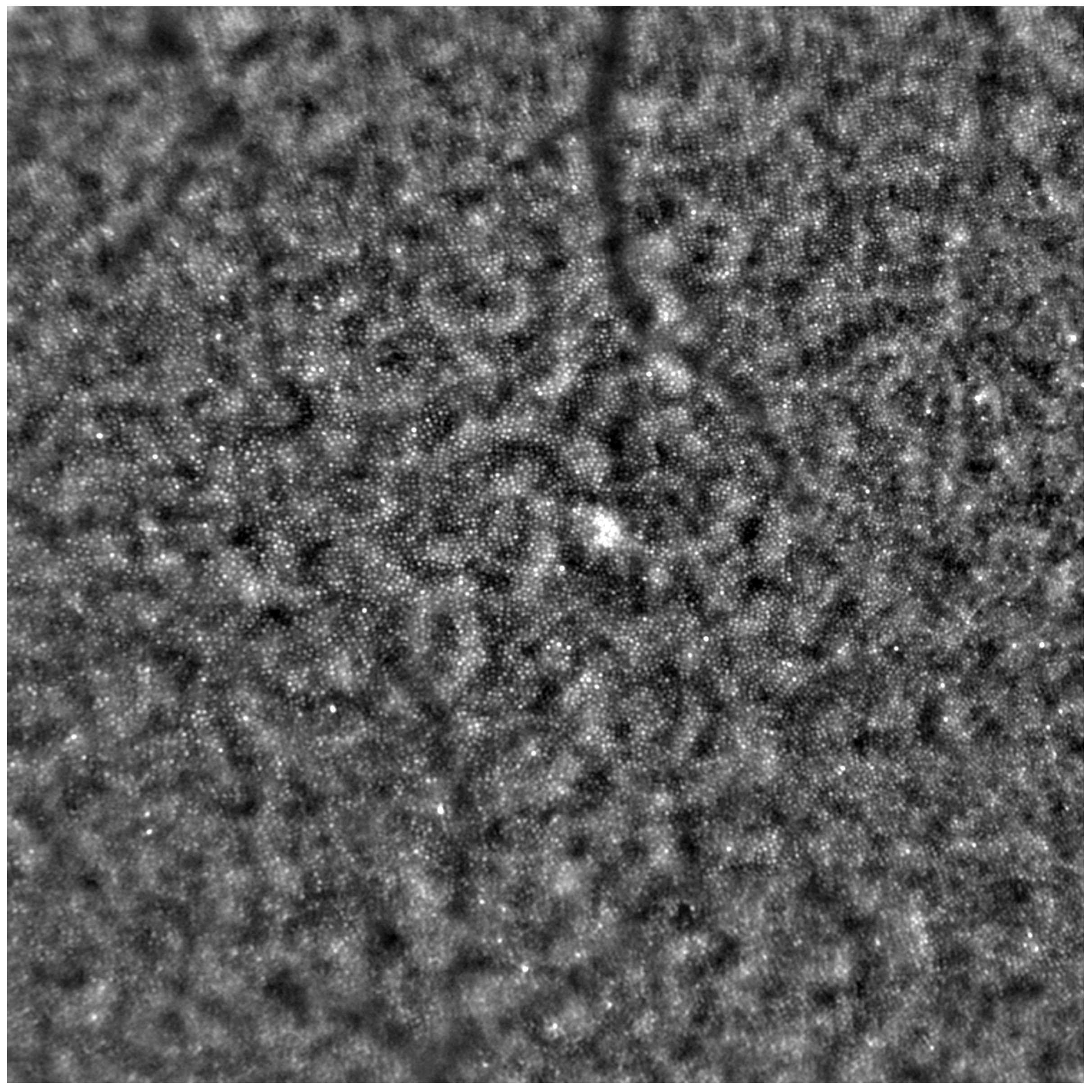
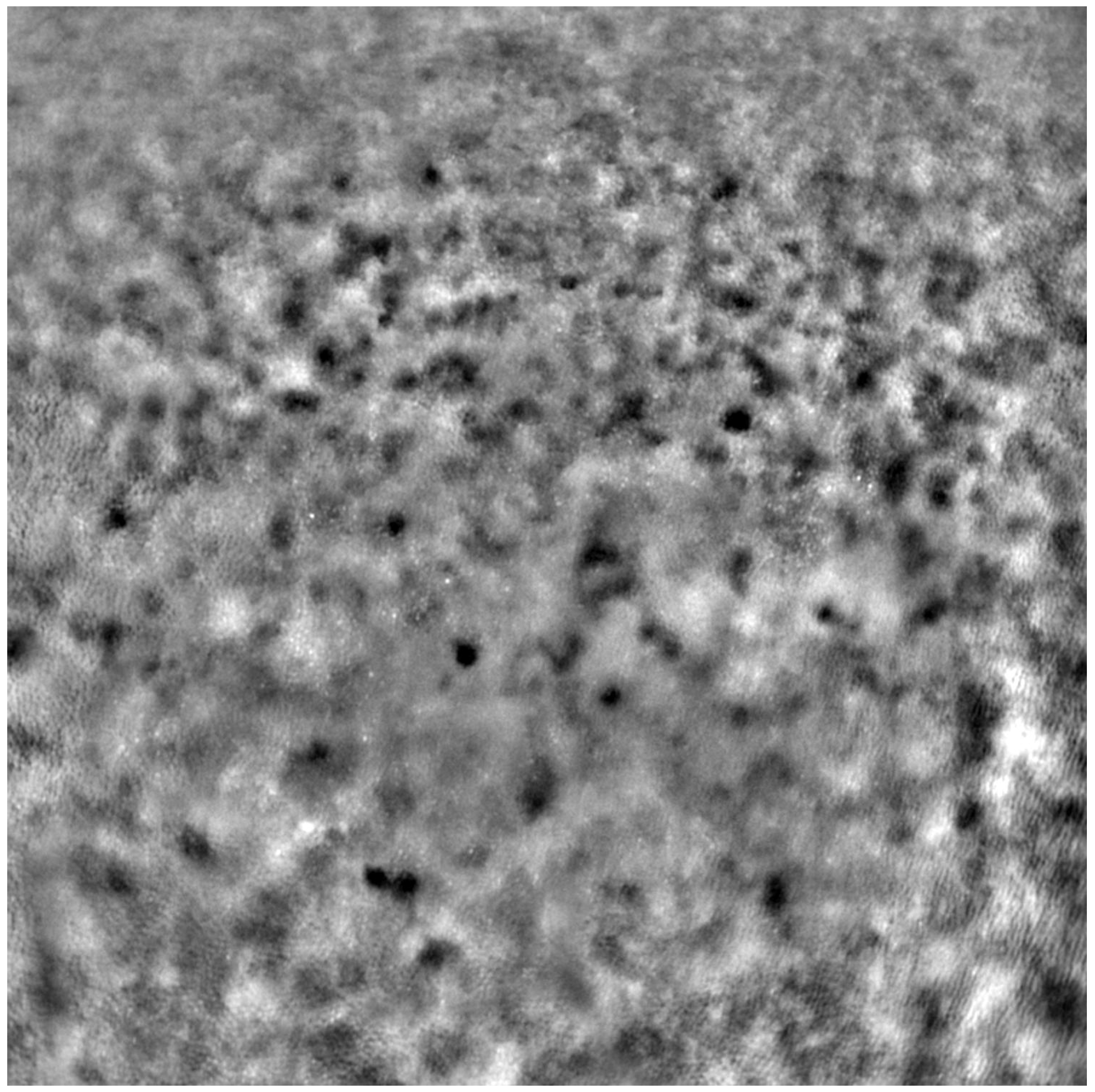
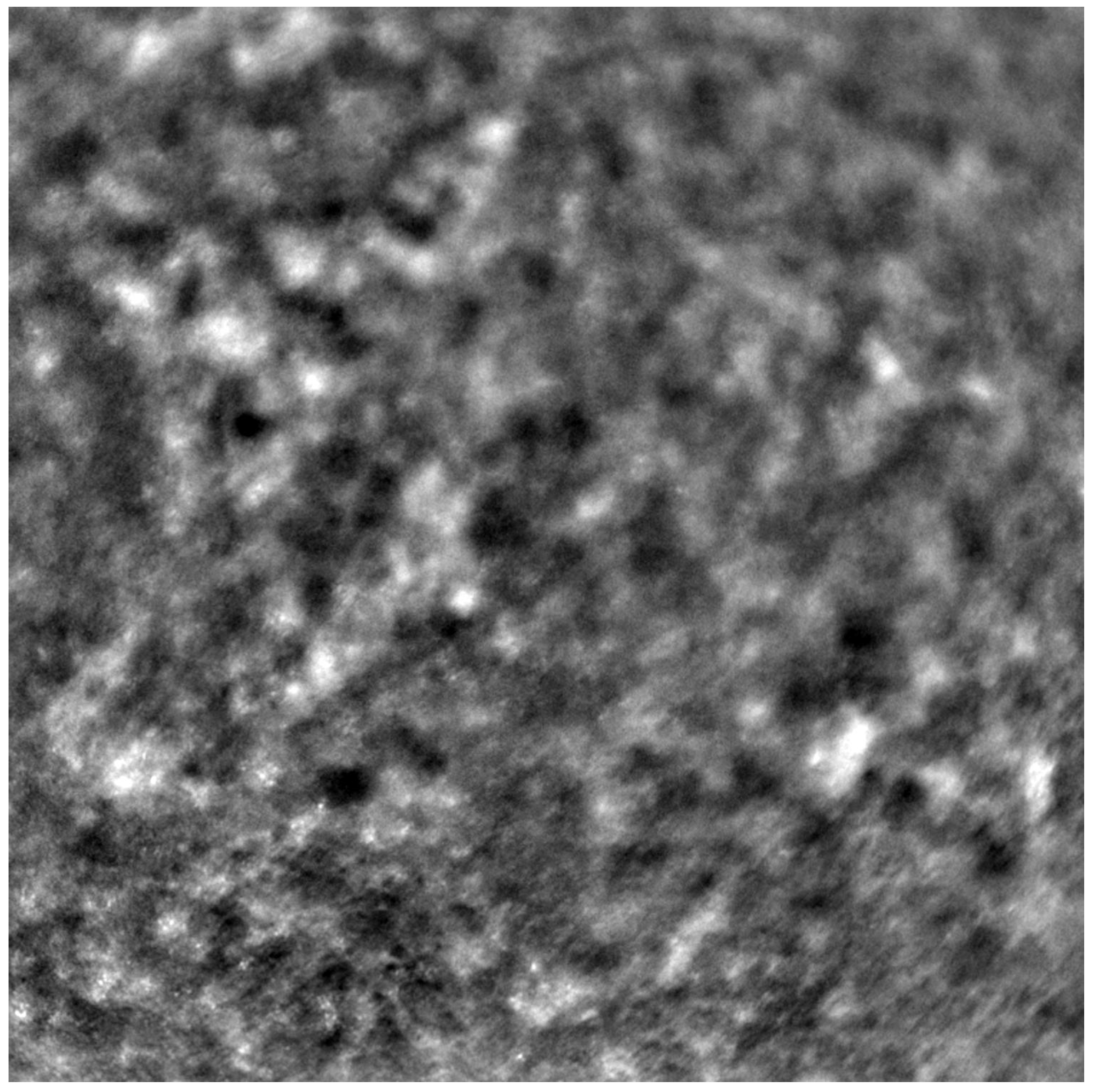
Before each examination, the best corrected visual acuity (BCVA) was checked using a Snellen chart, and the axial length of each eye was measured (LS 900; Haag-Streit; Koeniz, Switzerland). After mydriasis was induced using one drop of 1% tropicamide administered into each eye, the Rtx1™ (Imagine Eyes, France) test was performed. The acquired images were processed with the AO Detect program (version 3.4, also known as AO Image 3.4, Imagine Eyes, Orsay, France), providing numeric values for photoreceptor parameters: DM, cone density; SM, cone spacing; REG, cone regularity.
Each patient’s eyes were examined using Rtx1™ (Imagine Eyes, France), measuring four positions in each eye: 2 away from the fixation point in the superior, inferior, temporal, and nasal quadrants. The area selected for analysis was taken from within the examined frame, specifically in a location where the image quality was adequate for conducting a quantitative analysis.
Due to the poor quality of some scans, the image positions were not considered for subsequent statistical analysis. Instead, we computed the average values from all positions where image acquisition was possible.
Demographic data for patients at initial presentation are detailed in
Table 1. The values of BCVA, DM, SM, and cone regularity are shown in
Table 2 for the initial check and in
Table 3 for the follow-up check.
Data underwent normal distribution testing using the Shapiro–Wilk test. For normal distributions, Student’s t-test was used to compare the mean values of independent variables. If the normality assumption was violated, we employed the non-parametric Mann–Whitney U-test to compare continuous variables between two groups of observations. For comparisons involving more than two groups (as in our case, where three diagnosis types existed), we used one-way ANOVA (for parametric tests) or the Kruskal–Wallis test (for non-parametric tests). These tests were followed by either the HSD Tukey’s post hoc test (ANOVA) or Dunn’s post hoc test (Kruskal–Wallis), with results adjusted using the Bonferroni method. For this analysis, we set the level of statistical significance to p = 0.05. All calculations were carried out in R (Version 4.0.2).
4. Discussion
Our study corroborates that the progression of CD, CRD, and STGD can be accurately tracked based on cone parameters with AO. We observed a deterioration in visual acuity (BCVA), a loss of cone density (DM), and an increase in cone spacing (SM) over a 6-year observation period. However, the changes in cone parameters did not correlate with the loss of BCVA in our study. We hypothesise that, due to the low standard deviation of the functional parameter used in our study (BCVA), the correlation of the morphological and functional parameters cannot be stated. The development of advancements in electrophysiology testing, such as multifocal electroretinography, as well as assessing the patients in early stages of disease, might be crucial for determining the real dependence of anatomical changes in photoreceptors on visual function.
Our goal was to discern whether differences existed in the adaptive optics cone parameters in eyes diagnosed with CD, CRD, and STGD. In both the initial exam of the study described in this article and in our other study [
20], we found differences in DM, SM, and REG among the different diagnosis groups. However, these differences were not confirmed in the follow-up of our current study. We also did not find significant differences among the groups in terms of the DM, SM, and REG changes over time. This observation underscores the need for further longitudinal research on AO visualisation in eyes with IRDs, which would validate our findings.
The correlation between DM change and REG change may suggest a loss of quality in AO data with the progression of photoreceptor loss. However, since REG did not differ significantly between the initial check and the follow-up, we can infer that the quality of the obtained images of the cones was consistent across both examinations. More research is still required to confirm this correlation.
The highest BCVA was noted in the CRD group (0.32 on initial check, 0.28 on follow-up) and the lowest in the STGD group (0.13 on initial check, 0.09 on follow-up). The BCVA in the CD group was 0.17 on the initial check and 0.15 at the follow-up. Other clinical studies have confirmed that vision deterioration proceeds more slowly in CRD than in CD. Furthermore, nyctalopia is less common in patients with CRD [
2,
27]. However, in our study, BCVA deterioration over a 6-year period was not correlated with the diagnosis of CD, CRD, or STGD.
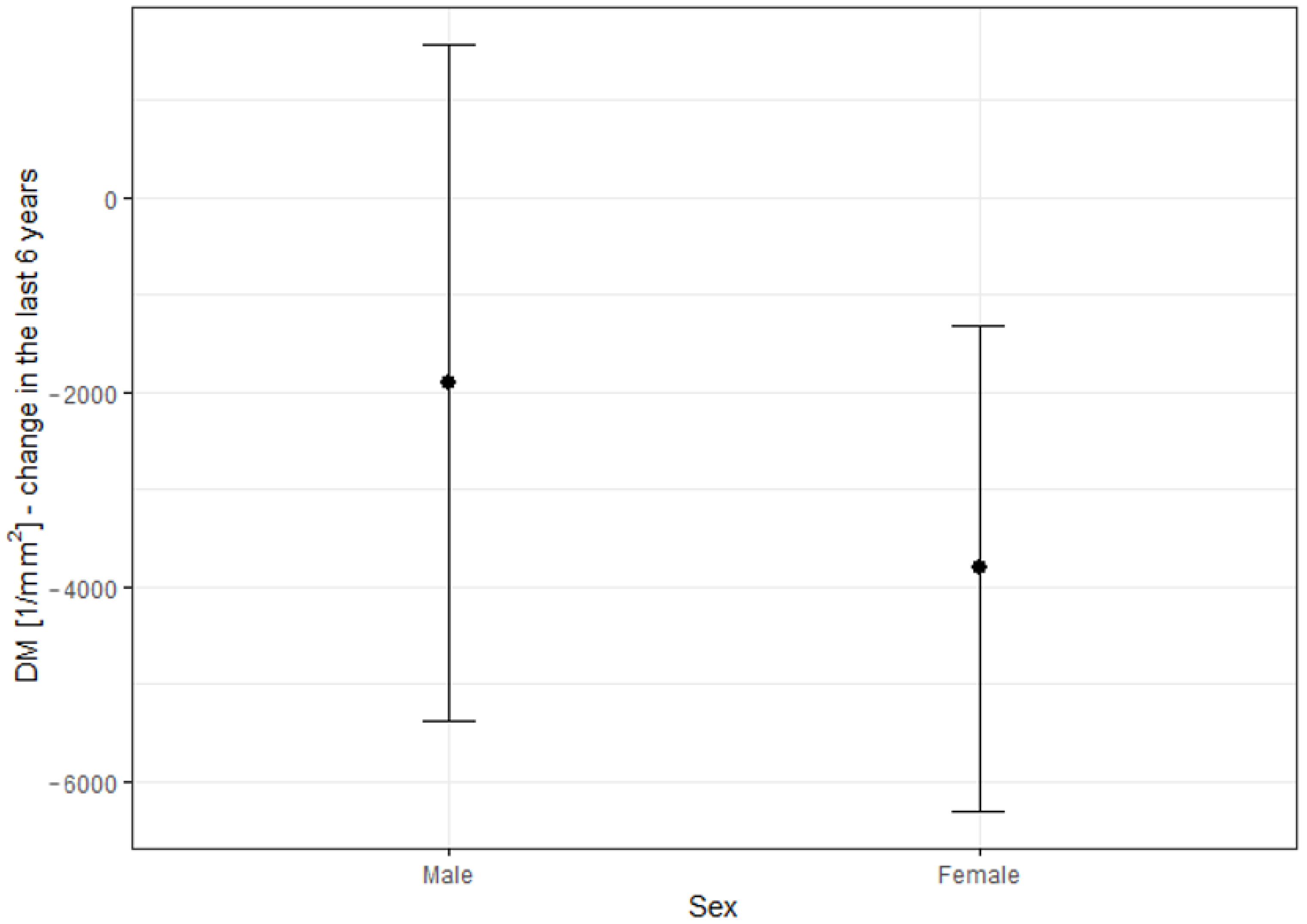
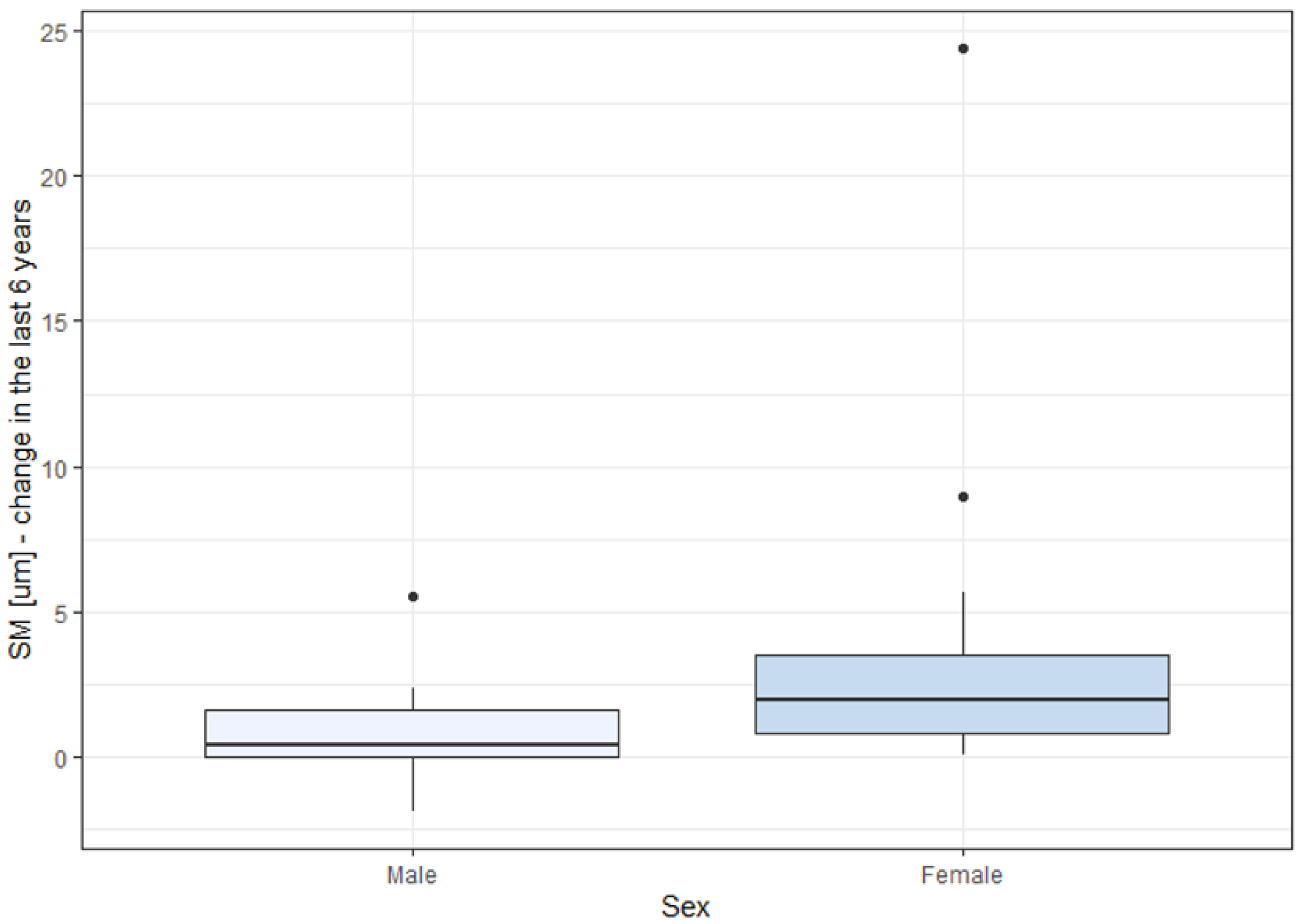
Changes in DM and SM over the six-year observation period were significantly higher in females than in males. This might be due to the higher percentage of females in the STGD group (57.1%, 16 women), compared to other groups. The percentages of females in the CD and CRD groups were 55.6% and 25%, corresponding to 5 and 2 women, respectively, indicating a small group size for reliable statistical analysis. To our knowledge, other studies have not reported a quicker progression of IRDs in females than in males. The observation of the greater prevalence of SM and DM changes in women with IRDs needs to be validated in future studies.
No inter-eye differences were noted in terms of cone density in our study, consistent with our previous study conducted on healthy eyes [
16].
The rarity of inherited retinal dystrophies in the population constrained the number of patients in the study group. Several studies have compared AO imaging outcomes between eyes with IRDs and healthy eyes. For instance, Duncan et al. [
28] contrasted 5 eyes with RP, 3 eyes with CRD, and 8 healthy eyes. Nakatake et al. [
29] analysed 14 eyes with RP alongside 10 healthy controls. Additionally, Giannini et al. [
30] included a range of conditions: 4 eyes with RP, 1 eye with best corrected macular dystrophy, 1 eye with occult macular dystrophy, 2 eyes with macular drusen, 4 eyes with nonproliferative diabetic retinopathy, and 20 healthy subjects. However, none of these studies provided longitudinal observations.
To our knowledge, no study other than ours has longitudinally assessed eyes with CD, CRD, or STGD. However, other reports on various IRDs do exist. For instance, a study on 16 eyes with RCD confirmed the correlation of AO changes with retinal thickness and findings in microperimetry, as well as a significant decline in cone spacing over a 3-year observation in the study group with no significant decline in healthy subjects [
31].
Ueda-Consolvo et al. [
32] carried out a study on 12 eyes of six patients with RP, aged 19–63, confirming the deterioration in cone density over a 2-year follow-up. BCVA was found to deteriorate in only one of six patients. This study did not include healthy controls. An observational case series by Ziccardi et al. [
33] suggested the possibility of monitoring the progression of RP based on three probands over two years. A more-recent study [
34] offered a short-term observation with AO imaging of eight patients with RCD and 10 healthy eyes, confirming the change of DM over a 6-month observation period in rod–cone dystrophy, but did not provide a longitudinal observation of healthy subjects.
Another publication reported the outcomes of imaging the eyes with RCDs [
22], including patients who underwent neparovovec (Luxturna
®) gene therapy. While the study did not report quantitative parameters such as DM and SM, it pointed out a crucial practical aspect of AO imaging in IRDs: the ability to assess the effects of treatments, like gene therapies. Evaluating photoreceptor parameters, along with visual assessment and other auxiliary tests, allows for a quantifiable analysis of the impacts of therapeutic interventions.
The progression of photoreceptor changes due to non-genetic pathology was documented by Potic et al. [
35], where the study group consisted of patients post-retinal detachment repair, and the follow-up time was 3 months.
Differentiation between various types of IRD has been made possible through the use of AO across different genotypes. Mastey et al. [
19] examined 9 subjects with ACHM, 7 of whom had a mutation in the
ATF6 gene, 1 in
CNGA3, and 1 in
CNGB3. In two of the patients, the acquisition of high-quality images was not feasible. Yet, they were able to discern a characteristic clear foveal cone mosaic in
CNGA3 and
CNGB3 patients, in contrast to patients with an
ATF6 gene mutation, where hyporeflective structures, possibly retinal pigment epithelium cells, were identified. The unique characteristics of the photoreceptor mosaic in retinitis-pigmentosa-GTPase-regulator (RPGR)-associated retinopathy and Stargardt disease and the differentiation between them have been reported [
6].
A possible limitation to our study is the broad age range of the patients: 13–61 years, which could potentially impact the consistency of our study group. Nonetheless, in healthy eyes, the correlation between a patient’s age and cone density was not significant, as noted in our previous study [
16]. There is no defined change rate in cone density and cone spacing. Foote et al. [
31] found no change in cone spacing in healthy controls over a 3-year observation. Conversely, according to some authors, photoreceptor density appears to decrease over time. Our other study [
15] describes the changes in AO parameters (mean cone density, cone spacing, cone regularity, and Voronoi analysis) over a two-year observation in healthy eyes and in patients with diabetes.
Another potential limitation of our study is the absence of a control group comprised of healthy eyes. The values of the photoreceptor parameters differed between our study and the study reporting a normative database of cone parameters of healthy eyes [
16]. Longitudinal observations of eyes with IRDs and healthy controls are not commonplace, as outlined above. We believe that longitudinal studies comparing changes in DM and SM over time between eyes with IRDs and healthy eyes should be promoted.
One of the challenges encountered in adaptive optics (AO) imaging of eyes with inherited retinal diseases (IRDs) is the difficulty some eyes have in maintaining central fixation, which compromises the quality of the collected images. Additionally, when patients fixate eccentrically, the precise location of the captured image remains uncertain. Daich-Varela et al. [
10] underscored the challenge of obtaining standardised AO images for each specific IRD.
In a previous study, we identified low cone density as a risk factor for incomplete data acquisition due to the poor quality of the obtained images, rendering quantitative analysis of photoreceptor parameters impossible [
20]. These results align with those presented in the current article, as they are based on a similar cohort of patients. In our current study, the rate of full image acquisition failures ranged from 33.9% to 50%. We describe the correlation of low cone density and high cone spacing with the risk of data collection failure. The impact of low cone regularity as a predictor for incomplete data analysis remains uncertain, as it was not confirmed in a follow-up assessment. Both the current and previous analyses did not establish a correlation between visual acuity, sex, or age and the risk of poor image quality. We suspect that the researchers’ experience with the imaging device may impact the success of data collection, as the failure rate was higher during the initial assessment than during follow-up. Given the high percentage of eyes with IRDs, where obtaining high-quality images is often impossible, we propose considering average values of photoreceptor parameters collected from various quadrants rather than specific locations.
While the quality of imaging can be challenging in certain cases, it offers the ability to monitor disease progression, even in instances that are too advanced for successful standard monitoring with macular OCT, FAF, or electrophysiology testing.