Clinical Outcomes of Patients with Multiple Myeloma after Daratumumab Failure
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Characteristics
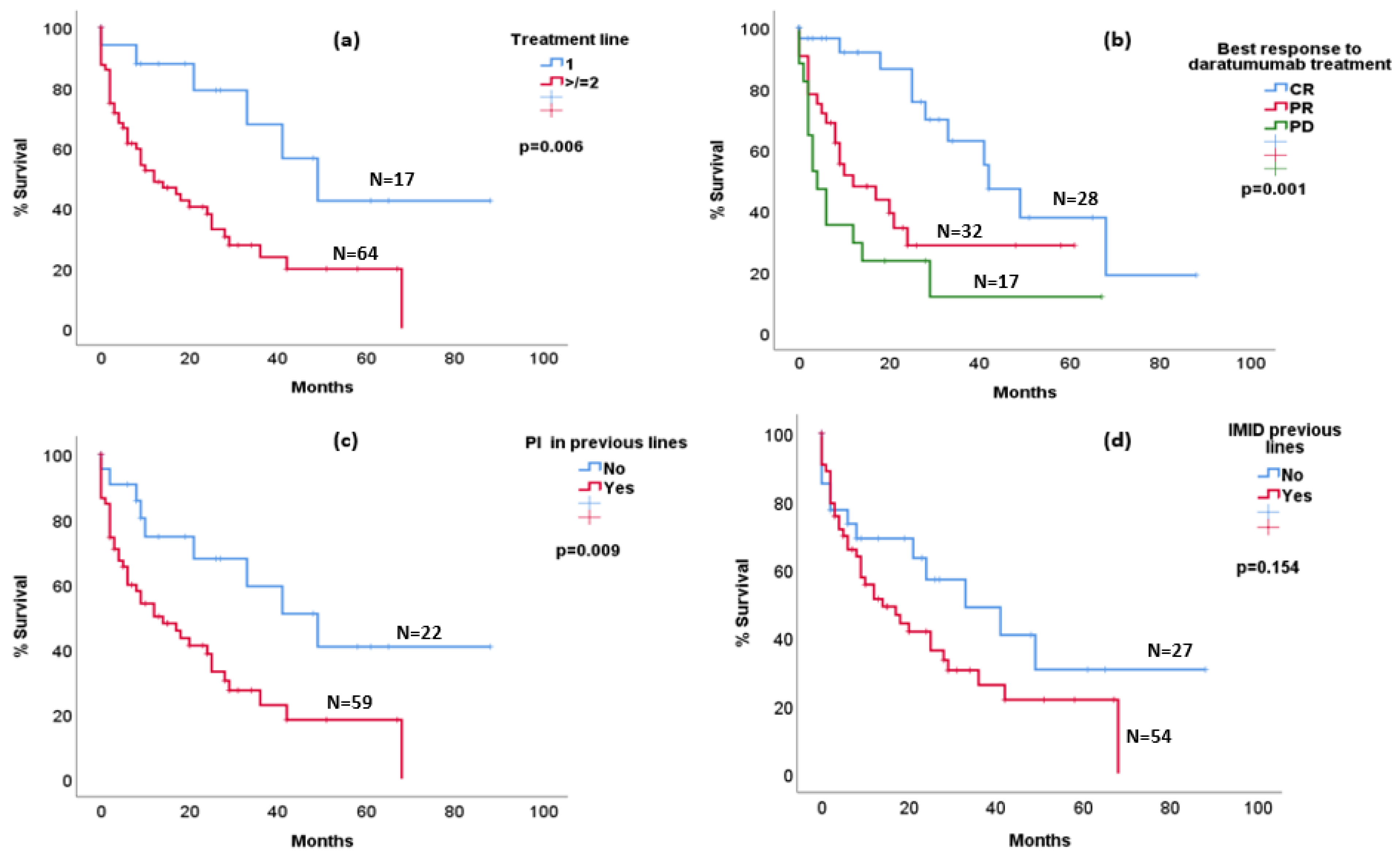
3.2. Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saltarella, I.; Desantis, V.; Melaccio, A.; Solimando, A.G.; Lamanuzzi, A.; Ria, R.; Storlazzi, C.T.; Mariggiò, M.A.; Vacca, A.; Frassanito, M.A. Mechanisms of Resistance to Anti-CD38 Daratumumab in Multiple Myeloma. Cells 2020, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Van de Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 antibodies in multiple myeloma: Back to the future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef]
- Lokhorst, H.M.; Plesner, T.; Laubach, J.P.; Nahi, H.; Gimsing, P.; Hansson, M.; Minnema, M.C.; Lassen, U.; Krejcik, J.; Palumbo, A.; et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N. Engl. J. Med. 2015, 373, 1207–1219. [Google Scholar] [CrossRef]
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.V.; et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 2016, 387, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef] [PubMed]
- Pick, M.; Vainstein, V.; Goldschmidt, N.; Lavie, D.; Libster, D.; Gural, A.; Grisariu, S.; Avni, B.; Ben Yehuda, D.; Gatt, M.E. Daratumumab resistance is frequent in advanced-stage multiple myeloma patients irrespective of CD38 expression and is related to dismal prognosis. Eur. J. Haematol. 2018, 100, 494–501. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Maples, K.T.; Joseph, N.S.; Harvey, R.D. Current developments in the combination therapy of relapsed/refractory multiple myeloma. Expert Rev. Anticancer Ther. 2020, 20, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Harousseau, J.-L.; Durie, B.; Anderson, K.C.; Dimopoulos, M.; Kyle, R.; Blade, J.; Richardson, P.; Orlowski, R.; Siegel, D.; et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 2011, 117, 4691–4695. [Google Scholar] [CrossRef]
- Nooka, A.K.; Joseph, N.S.; Kaufman, J.; Heffner, L.T.; Gupta, V.; Gleason, C.; Boise, L.; Lonial, S. Clinical efficacy of daratumumab, pomalidomide, and dexamethasone in patients with relapsed or refractory myeloma: Utility of re-treatment with daratumumab among refractory patients. Cancer 2019, 125, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Suvannasankha, A.; Fay, J.W.; Arnulf, B.; Kaufman, J.L.; Ifthikharuddin, J.J.; Weiss, B.M.; Krishnan, A.; Lentzsch, S.; Comenzo, R.; et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017, 130, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, A.; Abeykoon, J.P.; Kumar, S.K.; Rajkumar, S.V.; Dingli, D.; Buadi, F.K.; Gonsalves, W.I.; Leung, N.; Dispenzieri, A.; Kourelis, T.V.; et al. Efficacy of daratumumab-based therapies in patients with relapsed, refractory multiple myeloma treated outside of clinical trials. Am. J. Hematol. 2017, 92, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Weiss, B.M.; Plesner, T.; Bahlis, N.J.; Belch, A.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Richardson, P.G.; Chari, A.; et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016, 128, 37–44. [Google Scholar] [CrossRef]
- Yan, X.; Clemens, P.L.; Puchalski, T.; Lonial, S.; Lokhorst, H.; Voorhees, P.M.; Usmani, S.; Richardson, P.G.; Plesner, T.; Liu, K.; et al. Influence of Disease and Patient Characteristics on Daratumumab Exposure and Clinical Outcomes in Relapsed or Refractory Multiple Myeloma. Clin. Pharmacokinet. 2017, 57, 529–538. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All Patients n = 81 (%) | L 1–2 n = 39 (%) | L ≥ 3 n = 42 (%) |
|---|---|---|---|
| Age at diagnosis—median (IQR) | 66 (55.5–73) | 70 (59–75) | 64.5 (50.7–72) |
| Females | 45 (55.6) | 20 (51.3) | 25 (59.5) |
| Extramedullary disease at diagnosis | 18 (22.2) | 9 (23.1) | 9 (21.4) |
| High-risk cytogenetics at diagnosis | 23 (28.3) | 10 (25.6) | 13 (31) |
| IMID previous lines | 54 (66.7) | 15 (38.5) | 39 (92.9) |
| PI previous lines | 59 (72.8) | 18 (46.2) | 41 (97.6) |
| IMID and PI previous lines | 50 (61.7) | 12 (30.8) | 38 (90.5) |
| Previous ASCT | 31 (38.2) | 9 (23.1) | 21 (50) |
| Nº previous treatment lines: median (range) | 2 (0–8) | 1 (0–1) | 3 (2–8) |
| Daratumumab containing regimen: | |||
| Monotherapy with daratumumab | 18 (22.2) | 2 (5.1) | 16 (38) |
| D-VMP | 13 (16) | 13 (33.3) | 0 |
| DKd | 19 (23) | 7 (17.9) | 12 (28.5) |
| DVd | 15 (18.5) | 7 (17.9) | 8 (19) |
| DPd | 5 (6.1) | 2 (5.1) | 3 (7.1) |
| DRd | 5 (6.1) | 4 (10.2) | 1 (2.3) |
| Other | 6 (7.4) | 4 (10.2) | 2 (4.6) |
| Frist Line Therapy (n = 81) | |
|---|---|
| D-VMP: 12 (14.8%) | |
| Regimens with daratumumab | DVd: 4 (5%) |
| DRd: 1 (1.2%) | |
| RD: 8 (9.9%) | |
| Regimens with IMID | VRD: 15 (18.5%) |
| VTD: 13 (16%) | |
| KRD: 1 (1.2%) | |
| VMP: 11 (13.6%) | |
| Regimens with bortezomib | VD: 4 (5%) |
| V-bendamustine-prednisone: 3 (3.7%) | |
| Chemotherapy VBCMP/VBAD: 3 (3.7%) | |
| Others | MP: 1 (1.2%) |
| KMP: 2 (2.4%) | |
| Treatment Regimen (n Patients) | Complete Response | Partial Response | No Response |
|---|---|---|---|
| Pomalidomide-based treatment: | |||
| PomCyDex (18) | 6 (33.3%) | 5 (27.7%) | 7 (38.8%) |
| PomDex (11) | 3 (27.2%) | 2 (18.1%) | 6 (54.5%) |
| PVD (3) | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) |
| Lenalidomide-based treatment: | |||
| Rd (10) | 5 (50%) | 4 (40%) | 1 (10%) |
| KRd (1) | 0 | 1 (100%) | 0 |
| Carfilzomib-based treatment: | |||
| KyCyDex (3) | 0 | 1 (33.3%) | 2 (66.6%) |
| Kd (3) | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) |
| Anti-BCMA: | |||
| Belantamab (2) | 0 | 1 (50%) | 1 (50%) |
| Talquetamab (2) | 0 | 1 (33.3%) | 1 (33.3%) |
| Teclistamab (1) | 0 | 0 | 1 (100%) |
| Not specified (2) | 1 (50%) | 1 (50%) | 0 |
| CAR-T (3) | 2 (66.6%) | 1 (33.3%) | 0 |
| Others (9) | 1 (11.1%) | 3 (33.3%) | 5 (55.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamanillo, I.; Medina de Alba, L.; Gil, R.; de la Puerta, R.; Alonso, R.; Jimenez-Ubieto, A.; Cedena, M.T.; Calbacho, M.; Ayala, R.; Martinez-Lopez, J. Clinical Outcomes of Patients with Multiple Myeloma after Daratumumab Failure. Life 2023, 13, 1841. https://doi.org/10.3390/life13091841
Zamanillo I, Medina de Alba L, Gil R, de la Puerta R, Alonso R, Jimenez-Ubieto A, Cedena MT, Calbacho M, Ayala R, Martinez-Lopez J. Clinical Outcomes of Patients with Multiple Myeloma after Daratumumab Failure. Life. 2023; 13(9):1841. https://doi.org/10.3390/life13091841
Chicago/Turabian StyleZamanillo, Irene, Lucia Medina de Alba, Rodrigo Gil, Rosalia de la Puerta, Rafael Alonso, Ana Jimenez-Ubieto, Maria Teresa Cedena, Maria Calbacho, Rosa Ayala, and Joaquin Martinez-Lopez. 2023. "Clinical Outcomes of Patients with Multiple Myeloma after Daratumumab Failure" Life 13, no. 9: 1841. https://doi.org/10.3390/life13091841
APA StyleZamanillo, I., Medina de Alba, L., Gil, R., de la Puerta, R., Alonso, R., Jimenez-Ubieto, A., Cedena, M. T., Calbacho, M., Ayala, R., & Martinez-Lopez, J. (2023). Clinical Outcomes of Patients with Multiple Myeloma after Daratumumab Failure. Life, 13(9), 1841. https://doi.org/10.3390/life13091841







