Relationship between Cognitive Impairment and Depressive Symptoms with Somatosensory Functions in Diabetic and Non-Diabetic Older Adults and Its Impact on Quality of Life
Abstract
1. Introduction
2. Methods
2.1. Neuropathic Sensory Assessment
2.1.1. Superficial Sensory Pathway
2.1.2. Deep Sensory Pathway
2.2. Mental and Functional Assessment
2.3. Statistical Analysis
3. Results
3.1. Description of the Sample
3.2. Association between Cognitive Functions and Peripheral Sensory Functions
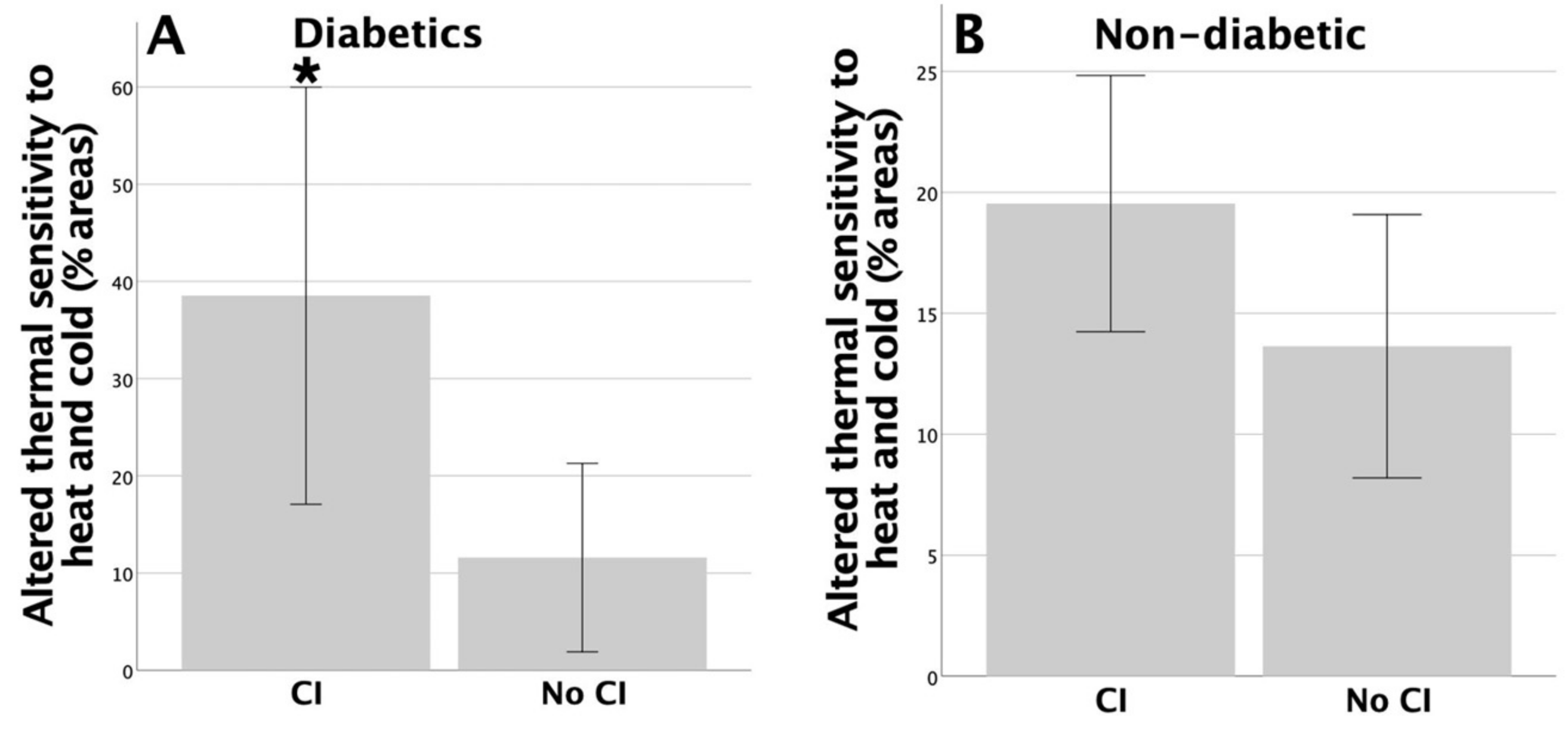
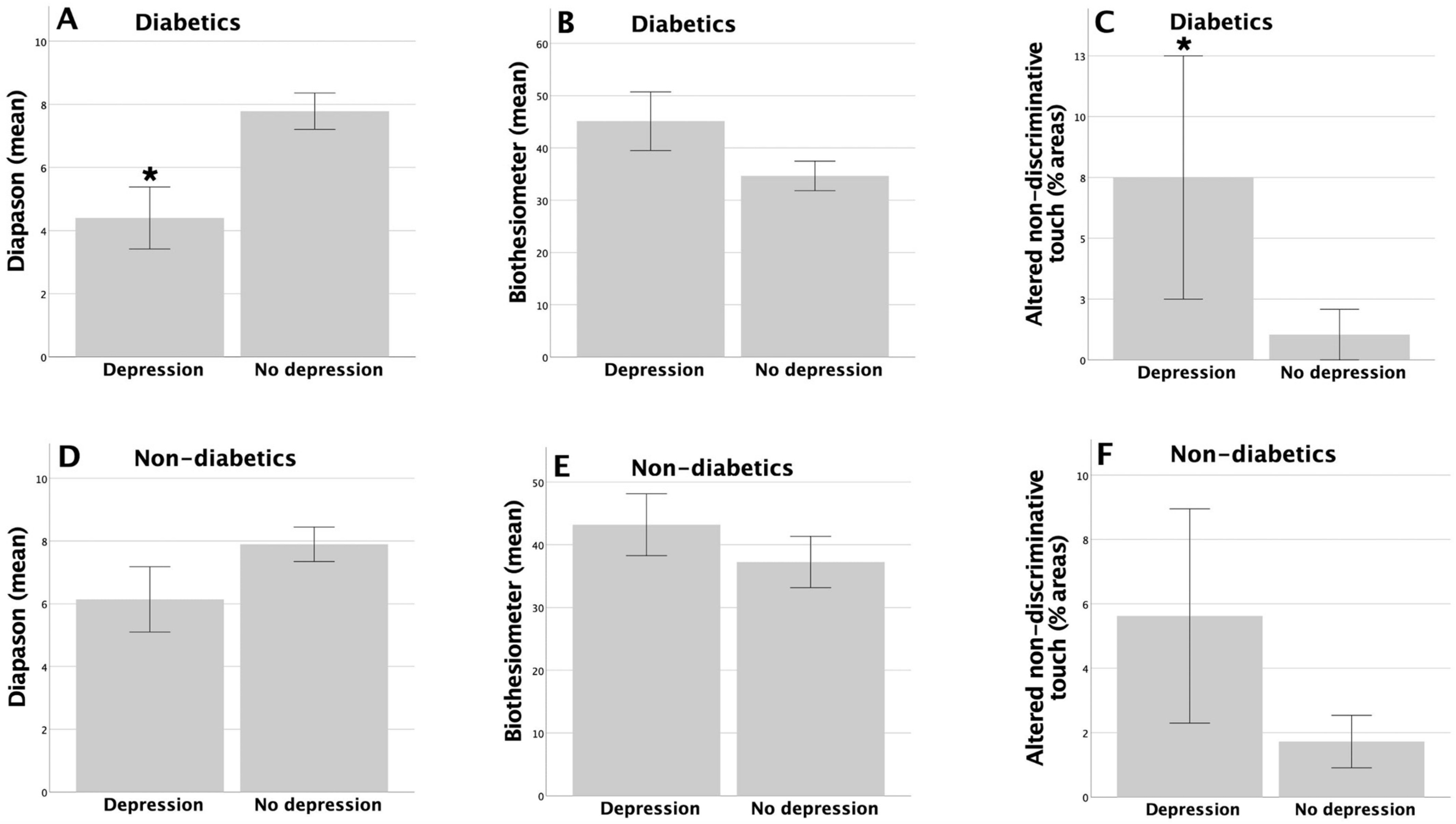
3.3. Association between the Symptoms of Depression and Peripheral Sensory Functions
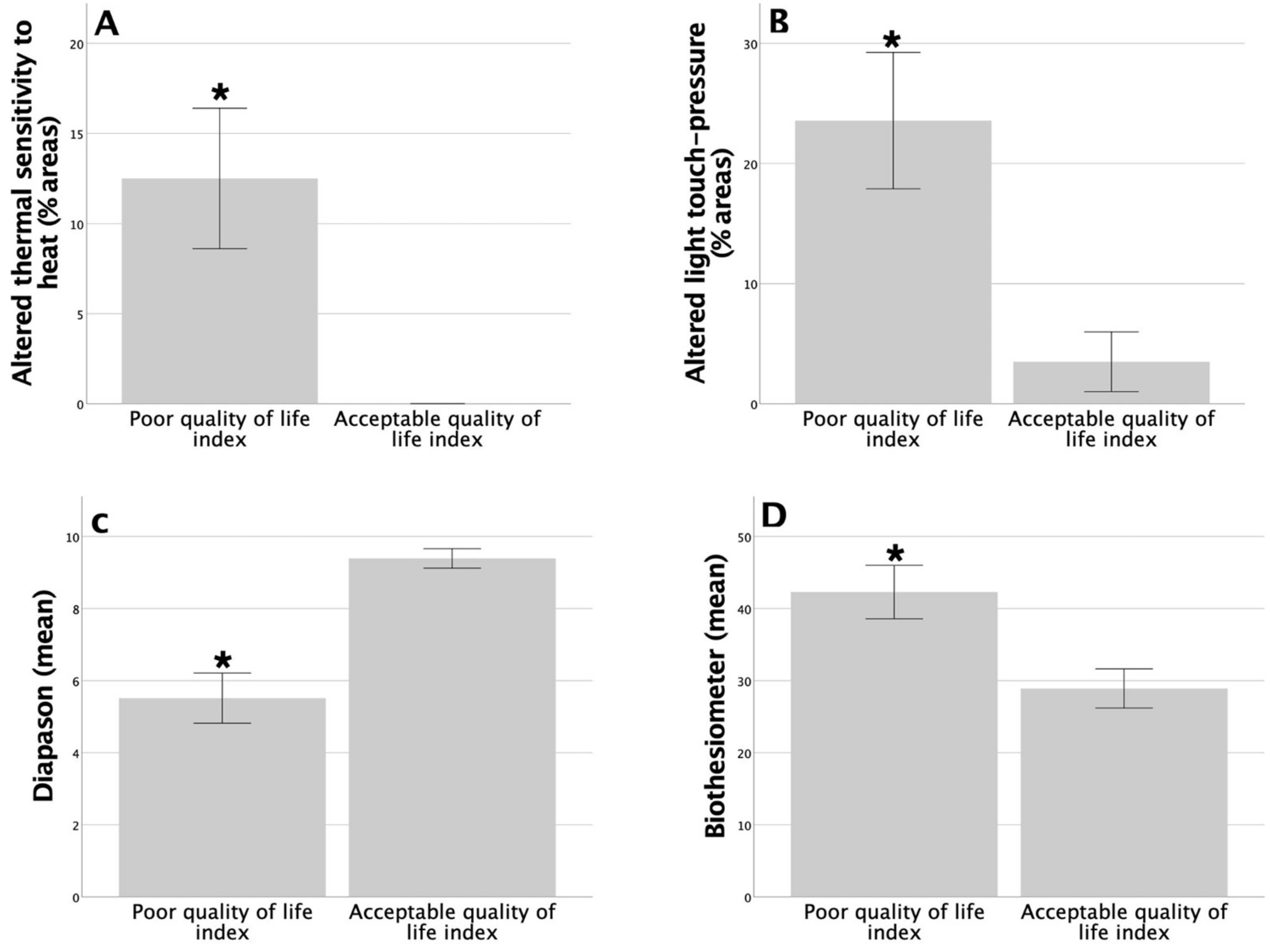
3.4. Association between Functional Status Assessment and Peripheral Sensory Functions
3.5. Logistical Regression Analysis: Cognitive Functions and the Symptoms of Depression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouche, P. Neuropathy of the Elderly. Rev. Neurol. 2020, 176, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Verdú, E.; Ceballos, D.; Vilches, J.J.; Navarro, X. Influence of Aging on Peripheral Nerve Function and Regeneration. J. Peripher. Nerv. Syst. 2000, 5, 191–208. [Google Scholar] [CrossRef]
- García-Piqueras, J.; García-Mesa, Y.; Cárcaba, L.; Feito, J.; Torres-Parejo, I.; Martín-Biedma, B.; Cobo, J.; García-Suárez, O.; Vega, J.A. Ageing of the Somatosensory System at the Periphery: Age-Related Changes in Cutaneous Mechanoreceptors. J. Anat. 2019, 234, 839–852. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.; Nagi, S.S.; McGlone, F.; Olausson, H. The Effects of Ageing on Tactile Function in Humans. Neuroscience 2021, 464, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Delbaere, K.; Sturnieks, D.L. Aging. In Handbook of Clinical Neurology; Day, B.L., Lord, S.R., Eds.; Elsevier: Sydney, Australia, 2018; Volume 159, pp. 157–171. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, Z.; Tang, M.; Wang, L. Age-Related Changes in Plantar Sensation and Ankle Proprioception in Adolescents to Older Adults. Mot. Control 2023, 27, 596–615. [Google Scholar] [CrossRef] [PubMed]
- Skedung, L.; El Rawadi, C.; Arvidsson, M.; Farcet, C.; Luengo, G.S.; Breton, L.; Rutland, M.W. Mechanisms of Tactile Sensory Deterioration amongst the Elderly. Sci. Rep. 2018, 8, 5303. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Hsieh, S.C.; Chao, C.C.; Chang, Y.C.; Hsieh, S.T. Influence of Aging on Thermal and Vibratory Thresholds of Quantitative Sensory Testing. J. Peripher. Nerv. Syst. 2005, 10, 269–281. [Google Scholar] [CrossRef]
- Johnson, C.; Hallemans, A.; Verbecque, E.; De Vestel, C.; Herssens, N.; Vereeck, L. Aging and the Relationship between Balance Performance, Vestibular Function and Somatosensory Thresholds. J. Int. Adv. Otol. 2020, 16, 328. [Google Scholar] [CrossRef]
- Henry, M.; Baudry, S. Control of Movement: Age-Related Changes in Leg Proprioception: Implications for Postural Control. J. Neurophysiol. 2019, 122, 525. [Google Scholar] [CrossRef]
- Guergova, S.; Dufour, A. Thermal Sensitivity in the Elderly: A Review. Ageing Res. Rev. 2011, 10, 80–92. [Google Scholar] [CrossRef]
- Huang, H.W.; Wang, W.C.; Lin, C.C.K. Influence of Age on Thermal Thresholds, Thermal Pain Thresholds, and Reaction Time. J. Clin. Neurosci. 2010, 17, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Daguet, I.; Bergeron-Vezina, K.; Harvey, M.P.; Martel, M.; Coulombe-Leveque, A.; Leonard, G. Decreased Initial Peak Pain Sensation with Aging: A Psychophysical Study. J. Pain. Res. 2020, 13, 2333. [Google Scholar] [CrossRef] [PubMed]
- Culig, L.; Chu, X.; Bohr, V.A. Neurogenesis in Aging and Age-Related Neurodegenerative Diseases. Ageing Res. Rev. 2022, 78, 101636. [Google Scholar] [CrossRef]
- Blinkouskaya, Y.; Caçoilo, A.; Gollamudi, T.; Jalalian, S.; Weickenmeier, J. Brain Aging Mechanisms with Mechanical Manifestations. Mech. Ageing Dev. 2021, 200, 111575. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, E.; Brambilla, M.; Maestri, G.; Nicotra, A.; Cova, I.; Pomati, S.; Pantoni, L. The Clinical Profile of Cerebral Small Vessel Disease: Toward an Evidence-based Identification of Cognitive Markers. Alzheimer’s Dement. 2023, 19, 244–260. [Google Scholar] [CrossRef]
- The LADIS Study Group. 2001–2011: A Decade of the LADIS (Leukoaraiosis And DISability) Study: What Have We Learned about White Matter Changes and Small-Vessel Disease? Cerebrovasc. Dis. 2011, 32, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Bella, R.; Pennisi, G.; Cantone, M.; Palermo, F.; Pennisi, M.; Lanza, G.; Zappia, M.; Paolucci, S. Clinical presentation and outcome of geriatric depression in subcortical ischemic vascular disease. Gerontology 2010, 56, 298–302. [Google Scholar] [CrossRef]
- Juan, S.M.A.; Adlard, P.A. Ageing and Cognition. Subcell. Biochem. 2019, 91, 107–122. [Google Scholar] [CrossRef]
- Turner, G.R.; Spreng, R.N. Executive Functions and Neurocognitive Aging: Dissociable Patterns of Brain Activity. Neurobiol. Aging 2012, 33, 826.e1–826.e13. [Google Scholar] [CrossRef]
- Verkerke, M.; Hol, E.M.; Middeldorp, J. Physiological and Pathological Ageing of Astrocytes in the Human Brain. Neurochem. Res. 2021, 46, 2662–2675. [Google Scholar] [CrossRef]
- Del Pilar Carrera-Gonzalez, M.; Canton-Habas, V.; Rich-Ruiz, M. Aging, Depression and Dementia: The Inflammatory Process. Adv. Clin. Exp. Med. 2022, 31, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.S.; Choi, D.K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Kao, T.W.; Chen, W.L. Relationship between Peripheral Neuropathy and Cognitive Performance in the Elderly Population. Medicine 2021, 100, E26071. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhao, X.; Wu, M.; Li, Z.; Luo, L.; Yang, C.; Yang, F. Prevalence of Depression in Older Adults: A Systematic Review and Meta-Analysis. Psychiatry Res. 2022, 311, 114511. [Google Scholar] [CrossRef] [PubMed]
- Sibille, E. Molecular Aging of the Brain, Neuroplasticity, and Vulnerability to Depression and Other Brain-Related Disorders. Dialogues Clin. Neurosci. 2013, 15, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Prins, N.D.; van Dijk, E.J.; den Heijer, T.; Vermeer, S.E.; Jolles, J.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M.B. Cerebral Small-Vessel Disease and Decline in Information Processing Speed, Executive Function and Memory. Brain 2005, 128, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Hu, X.; Ma, N.; He, H.; Duan, F.; Li, X.; Luo, Y.; Zhang, H. Sleep Quality, Depression, and Cognitive Function in Non-Demented Older Adults. J. Alzheimers Dis. 2020, 76, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Conde, J.A.; Galán-López, J.M. Depression and Cognitive Impairment in Institutionalized Older Adults. Dement. Geriatr. Cogn. Disord. 2020, 49, 107–119. [Google Scholar] [CrossRef]
- Scher, C.; Nepomnyaschy, L.; Amano, T. Comparison of Cognitive and Physical Decline as Predictors of Depression Among Older Adults. J. Appl. Gerontol. 2023, 42, 387–398. [Google Scholar] [CrossRef]
- Dotson, V.M.; McClintock, S.M.; Verhaeghen, P.; Kim, J.U.; Draheim, A.A.; Syzmkowicz, S.M.; Gradone, A.M.; Bogoian, H.R.; De Wit, L. Depression and Cognitive Control across the Lifespan: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2020, 30, 461–476. [Google Scholar] [CrossRef]
- Dominguez, L. Postural Control and Perturbation Response in Aging Populations: Fall Risk Implications. J. Neurophysiol. 2020, 124, 1309–1311. [Google Scholar] [CrossRef] [PubMed]
- Azarpaikan, A.; Hamidreza, T.T. Effect of Somatosensory and Neurofeedback Training on Balance in Older Healthy Adults: A Preliminary Investigation. Aging Clin. Exp. Res. 2018, 30, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Sempere-Bigorra, M.; Brognara, L.; Julian-Rochina, I.; Mazzotti, A.; Cauli, O. Relationship between Deep and Superficial Sensitivity Assessments and Gait Analysis in Diabetic Foot Patients. Int. Wound J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.G.; Marti, C.N.; Dinitto, D.M.; Kunik, M.E.; Pruchno, R. Longitudinal Associations of Falls and Depressive Symptoms in Older Adults. Gerontologist 2019, 59, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Vileikyte, L.; Leventhal, H.; Gonzalez, J.S.; Peyrot, M.; Rubin, R.R.; Ulbrecht, J.S.; Garrow, A.; Waterman, C.; Cavanagh, P.R.; Boulton, A.J.M. Diabetic Peripheral Neuropathy and Depressive SymptomsThe Association Revisited. Diabetes Care 2005, 28, 2378–2383. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, N.; Barbui, C.; Anstey, K.J.; Kivipelto, M.; Barbera, M.; Peters, R.; Zheng, L.; Kulmala, J.; Stephen, R.; Ferri, C.P.; et al. Reducing the Risk of Cognitive Decline and Dementia: WHO Recommendations. Front. Neurol. 2021, 12, 765584. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; An, M.; Zeng, Q. The Risk Factors for Diabetic Peripheral Neuropathy: A Meta-Analysis. PLoS ONE 2019, 14, e0212574. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic Neuropathy. Nat. Rev. Dis. Primers 2019, 5, 42. [Google Scholar] [CrossRef]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar] [CrossRef]
- Kazamel, M.; Dyck, P.J. Sensory Manifestations of Diabetic Neuropathies: Anatomical and Clinical Correlations. Prosthet. Orthot. Int. 2015, 39, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.L.; Beare, R.; Moran, C.; Phan, T.; Wang, W.; Srikanth, V.K. Type 2 Diabetes Mellitus, Brain Atrophy and Cognitive Decline in Older People: A Longitudinal Study. Diabetologia 2019, 62, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Damanik, J.; Yunir, E. Type 2 Diabetes Mellitus and Cognitive Impairment. Acta Med. Indones. 2021, 53, 213–220. [Google Scholar] [PubMed]
- Moran, C.; Beare, R.; Wang, W.; Callisaya, M.; Srikanth, V. Type 2 Diabetes Mellitus, Brain Atrophy, and Cognitive Decline. Neurology 2019, 92, E823–E830. [Google Scholar] [CrossRef] [PubMed]
- Tuligenga, R.H.; Dugravot, A.; Tabák, A.G.; Elbaz, A.; Brunner, E.J.; Kivimäki, M.; Singh-Manoux, A. Midlife Type 2 Diabetes and Poor Glycaemic Control as Risk Factors for Cognitive Decline in Early Old Age: A Post-Hoc Analysis of the Whitehall II Cohort Study. Lancet Diabetes Endocrinol. 2014, 2, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Spauwen, P.J.J.; Köhler, S.; Verhey, F.R.J.; Stehouwer, C.D.A.; Van Boxtel, M.P.J. Effects of Type 2 Diabetes on 12-Year Cognitive ChangeResults from the Maastricht Aging Study. Diabetes Care 2013, 36, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Arvanitakis, Z.; Lynch, E.B.; McAninch, E.A.; Wilson, R.S.; Weuve, J.; Barnes, L.L.; Bianco, A.C.; Evans, D.A. Cognitive Decline Following Incident and Preexisting Diabetes Mellitus in a Population Sample. Neurology 2016, 87, 1681–1687. [Google Scholar] [CrossRef]
- Zhao, L.; Mao, L.; Liu, Q.; Chen, X.; Tang, X.; An, D. Cognitive Impairment in Type 2 Diabetes Patients with and without Diabetic Peripheral Neuropathy: A Mismatch Negativity Study. Neuroreport 2021, 32, 1223–1228. [Google Scholar] [CrossRef]
- Ding, X.; Fang, C.; Li, X.; Cao, Y.J.; Zhang, Q.L.; Huang, Y.; Pan, J.; Zhang, X. Type 1 Diabetes-Associated Cognitive Impairment and Diabetic Peripheral Neuropathy in Chinese Adults: Results from a Prospective Cross-Sectional Study. BMC Endocr. Disord. 2019, 19, 34. [Google Scholar] [CrossRef]
- Hafström, A. Perceived and Functional Balance Control Is Negatively Affected by Diminished Touch and Vibration Sensitivity in Relatively Healthy Older Adults and Elderly. Gerontol. Geriatr. Med. 2018, 4, 2333721418775551. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X.; Zhang, Q.; Zou, R. Diabetes Mellitus and Risk of Falls in Older Adults: A Systematic Review and Meta-Analysis. Age Ageing 2016, 45, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.D.; Orlando, G.; Brown, S.J. Sensory-Motor Mechanisms Increasing Falls Risk in Diabetic Peripheral Neuropathy. Medicina 2021, 57, 457. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Dobson, J. The Contribution of Small and Large Sensory Afferents to Postural Control in Patients with Peripheral Neuropathy. J. Sport. Health Sci. 2019, 8, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Soares Botelhoa, M.C.; Guilherme Conde, M.; Dias Azinheira Rebelo Braz, N.M. Functional Aspects in Ageing Adults with Diabetic Neuropathy. A Review. Curr. Diabetes Rev. 2015, 12, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Camacho, P.; Reddy, S.; Valencia, W.M.; Trence, D.; Matsumoto, A.M.; Morley, J.E. Aging, Diabetes, and Falls. Endocr. Pract. 2017, 23, 1120–1142. [Google Scholar] [CrossRef] [PubMed]
- Brognara, L.; Mazzotti, A.; Di Martino, A.; Faldini, C.; Cauli, O. Wearable Sensor for Assessing Gait and Postural Alterations in Patients with Diabetes: A Scoping Review. Medicina 2021, 57, 1145. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; Baniyas, M.M.; AlDarmaki, R.S.; Tekes, K.; Kalász, H.; Adeghate, E.A. An Update on Therapies for the Treatment of Diabetes-Induced Osteoporosis. Expert. Opin. Biol. Ther. 2019, 19, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Allan, L.M.; Ballard, C.G.; Rowan, E.N.; Kenny, R.A. Incidence and Prediction of Falls in Dementia: A Prospective Study in Older People. PLoS ONE 2009, 4, e5521. [Google Scholar] [CrossRef]
- Chantanachai, T.; Sturnieks, D.L.; Lord, S.R.; Payne, N.; Webster, L.; Taylor, M.E. Risk Factors for Falls in Older People with Cognitive Impairment Living in the Community: Systematic Review and Meta-Analysis. Ageing Res. Rev. 2021, 71, 101452. [Google Scholar] [CrossRef]
- Vaishya, R.; Vaish, A. Falls in Older Adults Are Serious. Indian. J. Orthop. 2020, 54, 69. [Google Scholar] [CrossRef]
- Casey, S.; Lanting, S.; Oldmeadow, C.; Chuter, V. The Reliability of the Ankle Brachial Index: A Systematic Review. J. Foot Ankle Res. 2019, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and Interpretation of the Ankle-Brachial Index: A Scientific Statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, H.; Al Khalili, Y. Physiology, Vibratory Sense; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Warren, S.; Yezierski, R.P.; Capra, N.F. The Somatosensory System II: Nociception, Thermal Sense, and Touch. In Fundamental Neuroscience for Basic and Clinical Applications, 5th ed.; Haines, D.E., Mihailoff, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 258–277.e1. [Google Scholar] [CrossRef]
- Chicharro-Luna, E.; Pomares-Gómez, F.J.; Ortega-Ávila, A.B.; Coheña-Jiménez, M.; Gijon-Nogueron, G. Variability in the Clinical Diagnosis of Diabetic Peripheral Neuropathy. Prim. Care Diabetes 2020, 14, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.M.; Armstrong, D.G.; Albert, S.F.; Frykberg, R.G.; Hellman, R.; Sue Kirkman, M.; Lavery, L.A.; LeMaster, J.W.; Mills, J.L.; Mueller, M.J.; et al. Comprehensive Foot Examination and Risk Assessment: A Report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with Endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008, 31, 1679. [Google Scholar] [CrossRef]
- Bakkers, M.; Faber, C.G.; Reulen, J.P.H.; Hoeijmakers, J.G.J.; Vanhoutte, E.K.; Merkies, I.S.J. Optimizing Temperature Threshold Testing in Small-Fiber Neuropathy. Muscle Nerve 2015, 51, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Bertelsmann, F.W.; Heimans, J.J.; Weber, E.; Van Der Veen, E.A.; Schoutent, J.A. Thermal Discrimination Thresholds in Normal Subjects and in Patients with Diabetic Neuropathy. Neurosurg. Psychiatry 1985, 48, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Abad, F.; Díaz-Gómez, N.M.; Rodríguez, I.; Pérez, R.; Delgado, J.A. Subclinical Pain and Thermal Sensory Dysfunction in Children and Adolescents with Type 1 Diabetes Mellitus. Diabet. Med. 2002, 19, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Jamil, N.K.; Anglin, R.E.S.; Hunt, D.L.; Panju, A. Does This Patient With Diabetes Have Large-Fiber Peripheral Neuropathy? JAMA 2010, 303, 1526–1532. [Google Scholar] [CrossRef]
- Nather, A.; Lin, W.K.; Aziz, Z.; Ong, C.H.J.; Feng, B.M.C.; Lin, C.B. Assessment of Sensory Neuropathy in Patients with Diabetic Foot Problems. Diabet. Foot Ankle 2011, 2, 6367. [Google Scholar] [CrossRef][Green Version]
- Costa, T.; Coelho, L.; Silva, M.F. Automatic Segmentation of Monofilament Testing Sites in Plantar Images for Diabetic Foot Management. Bioengineering 2022, 9, 86. [Google Scholar] [CrossRef]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A. Practical Guidelines on the Prevention and Management of Diabetic Foot Disease (IWGDF 2019 Update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. S1), e3266. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Godínez, S.A.; Zonana-Nacach, A.; Anzaldo-Campos, M.C.; Muñoz-Martínez, J.A. Riesgo de Pie Diabético En Pacientes Con Diabetes Mellitus Tipo 2 En Una Unidad de Medicina de Familia. Semergen 2014, 40, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Conferencia Nacional de Consenso sobre Úlceras de la Extremidad Inferior; Marinello i Roura, J.; Verdú Soriano, J. Conferencia Nacional de Consenso Sobre Las. Úlceras de La. Extremidad Inferior (C.O.N.U.E.I.): Documento de Consenso 2018; Ergon: Flowood, MI, USA, 2018. [Google Scholar]
- Pourhamidi, K.; Dahlin, L.B.; Englund, E.; Rolandsson, O. Evaluation of Clinical Tools and Their Diagnostic Use in Distal Symmetric Polyneuropathy. Prim. Care Diabetes 2014, 8, 77–84. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; McCann, S.M.; Lagan, K.M. Tuning Fork (128 Hz) versus Neurothesiometer: A Comparison of Methods of Assessing Vibration Sensation in Patients with Diabetes Mellitus. Int. J. Clin. Pract. 2006, 60, 174–178. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Bhansali, A.; Bhansali, S.; Dutta, P.; Anantharaman, R.; Shanmugasundar, G.; Ravikiran, M. Validation of Bedside Methods in Evaluation of Diabetic Peripheral Neuropathy. Indian. J. Med. Res. 2011, 133, 645. [Google Scholar]
- Wittenberg, B.; Svendsen, T.K.; Gaist, L.M.; Itani, M.; Gylfadottir, S.S.; Jensen, T.S.; Gaist, D.; Sindrup, S.H.; Krøigård, T. Test-retest and Time Dependent Variation and Diagnostic Values of Vibratory Sensation Determined by Biothesiometer and the Rydel-Seiffer Tuning Fork. Brain Behav. 2021, 11, e2230. [Google Scholar] [CrossRef] [PubMed]
- Temlett, J.A. An Assessment of Vibration Threshold Using a Biothesiometer Compared to a C128-Hz Tuning Fork. J. Clin. Neurosci. 2009, 16, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Christa Maree Stephan, B.; Minett, T.; Pagett, E.; Siervo, M.; Brayne, C.; McKeith, I.G. Diagnosing Mild Cognitive Impairment (MCI) in Clinical Trials: A Systematic Review. BMJ Open 2013, 3, e001909. [Google Scholar] [CrossRef]
- Hayashida, D.Y.; Jacinto, A.F.; Araújo, L.M.Q.; Almada Filho, C.D.M.; Di Tommaso, A.B.; Cendoroglo, M.S. Association between Baseline Mini-Mental State Examination Score and Dementia Incidence in a Cohort of Oldest Old. Arq. Neuropsiquiatr. 2021, 79, 1090–1094. [Google Scholar] [CrossRef]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.E.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the Detection of Dementia in Clinically Unevaluated People Aged 65 and over in Community and Primary Care Populations. Cochrane Database Syst. Rev. 2016, 2016, CD011145. [Google Scholar] [CrossRef]
- Sakurai, R.; Kim, Y.; Inagaki, H.; Tokumaru, A.M.; Sakurai, K.; Shimoji, K.; Kitamura, A.; Watanabe, Y.; Shinkai, S.; Awata, S. MMSE Cutoff Discriminates Hippocampal Atrophy: Neural Evidence for the Cutoff of 24 Points. J. Am. Geriatr. Soc. 2021, 69, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; Mchugh, P.R. “Mini-Mental State” A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- López Miquel, J.; Martí Agustí, G. Mini-Examen Cognoscitivo (MEC). Rev. Esp. Med. Leg. 2011, 37, 122–127. [Google Scholar] [CrossRef]
- Recker Id, L.; Foerster Id, R.M.; Schneider, W.X.; Poth, C.H. Emphasizing Speed or Accuracy in an Eye-Tracking Version of the Trail-Making-Test: Towards Experimental Diagnostics for Decomposing Executive Functions. PLoS ONE 2022, 17, e274579. [Google Scholar] [CrossRef] [PubMed]
- Ashendorf, L.; Jefferson, A.L.; O’Connor, M.K.; Chaisson, C.; Green, R.C.; Stern, R.A. Trail Making Test Errors in Normal Aging, Mild Cognitive Impairment, and Dementia. Arch. Clin. Neuropsychol. 2008, 23, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Martínez de la Iglesia, J.; Vilches Onís, M.C.; Dueñas Herrero, R.; Albert Colomer, C.; Aguado Taberné, C.; Luque Luque, R. The Spanish Version of the Yesavage Abbreviated Questionnaire (GDS) to Screen Depressive Dysfunctions in Patients Older than 65 Years. Medifam 2002, 12, 620–630. [Google Scholar]
- Erazo, M.; Fors, M.; Mullo, S.; González, P.; Viada, C. Internal Consistency of Yesavage Geriatric Depression Scale (GDS 15-Item Version) in Ecuadorian Older Adults. Inquiry 2020, 57, 0046958020971184. [Google Scholar] [CrossRef] [PubMed]
- Keetharuth, A.D.; Hussain, H.; Rowen, D.; Wailoo, A. Assessing the Psychometric Performance of EQ-5D-5L in Dementia: A Systematic Review. Health Qual. Life Outcomes 2022, 20, 139. [Google Scholar] [CrossRef]
- Herdman, M.; Badia, X.; Berra, S. EuroQol-5D: A Simple Alternative for Measuring Health-Related Quality of Life in Primary Care. Aten. Primaria/Soc. Española Med. Fam. Y Comunitaria 2001, 28, 425–430. [Google Scholar] [CrossRef]
- Janssen, M.F.; Pickard, A.S.; Shaw, J.W. General Population Normative Data for the EQ-5D-3L in the Five Largest European Economies. Eur. J. Health Econ. 2021, 22, 1467–1475. [Google Scholar] [CrossRef]
- Cid-Ruzafa, J.; Damián-Moreno, J. Valoración de La Discapacidad Física. El Índice de Barthel. Rev. Esp. Salud Publica 1997, 71, 127–137. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S83–S96. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, J. Digit Symbol Substitution Test: The Case for Sensitivity Over Specificity in Neuropsychological Testing. J. Clin. Psychopharmacol. 2018, 38, 513. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.L.; Thomas, S.J.; Larkin, T. Cortisol, Oxytocin, and Quality of Life in Major Depressive Disorder. Qual. Life Res. 2019, 28, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Sagayadevan, V.; Lee, S.P.; Ong, C.; Abdin, E.; Chong, S.A.; Subramaniam, M. Quality of Life across Mental Disorders in Psychiatric Outpatients. Ann. Acad. Med. Singap. 2018, 47, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Liu, H.; Jiang, Y.; Li, S.; Jin, Y.; Yan, C.; Chen, H. Correlation between Depression and Quality of Life in Patients with Parkinson’s Disease. Clin. Neurol. Neurosurg. 2021, 202, 106523. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Su, M.; Sweet, J.; Calabrese, J.R. Correlation between Depression/Anxiety Symptom Severity and Quality of Life in Patients with Major Depressive Disorder or Bipolar Disorder. J. Affect. Disord. 2019, 244, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.S.; Horwitz, J.L.; Noggle, C.A.; Dean, R.S.; Davis, K.M. Cortical and Subcortical Sensory-Motor Impairment in Patients with Major Depression: A Preliminary Analysis. Int. J. Neurosci. 2010, 120, 352–354. [Google Scholar] [CrossRef]
- Brown, E.C.; Clark, D.L.; Hassel, S.; MacQueen, G.; Ramasubbu, R. Thalamocortical Connectivity in Major Depressive Disorder. J. Affect. Disord. 2017, 217, 125–131. [Google Scholar] [CrossRef]
- Kropf, E.; Syan, S.K.; Minuzzi, L.; Frey, B.N. From Anatomy to Function: The Role of the Somatosensory Cortex in Emotional Regulation. Braz. J. Psychiatry 2019, 41, 261–269. [Google Scholar] [CrossRef]
- Kiely, K.M.; Brady, B.; Byles, J. Gender, Mental Health and Ageing. Maturitas 2019, 129, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Han, G.; Zhao, Y.; Jin, Y.; Ge, T.; Yang, W.; Cui, R.; Xu, S.; Li, B. Gender Differences in Depression: Evidence From Genetics. Front. Genet. 2020, 11, 562316. [Google Scholar] [CrossRef]
- Bora, E.; Harrison, B.J.; Yücel, M.; Pantelis, C. Cognitive Impairment in Euthymic Major Depressive Disorder: A Meta-Analysis. Psychol. Med. 2013, 43, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H.R. Major Depressive Disorder Is Associated with Broad Impairments on Neuropsychological Measures of Executive Function: A Meta-Analysis and Review. Psychol. Bull. 2013, 139, 81–132. [Google Scholar] [CrossRef] [PubMed]
- Nuño, L.; Gómez-Benito, J.; Carmona, V.R.; Pino, O. A Systematic Review of Executive Function and Information Processing Speed in Major Depression Disorder. Brain Sci. 2021, 11, 147. [Google Scholar] [CrossRef]
- De Zihang, P.; Park, C.; Brietzke, E.; Zuckerman, H.; Rong, C.; Mansur, R.B.; Fus, D.; Subramaniapillai, M.; Lee, Y.; McIntyre, R.S. Cognitive Impairment in Major Depressive Disorder. CNS Spectr. 2019, 24, 22–29. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, A.; Sun, N.; Liu, P.; Yang, C.; Li, G.; Liu, Z.; Wang, Y.; Zhang, K. Functional Connectivity between the Thalamus and the Primary Somatosensory Cortex in Major Depressive Disorder: A Resting-State FMRI Study. BMC Psychiatry 2018, 18, 339. [Google Scholar] [CrossRef]
- Polak, T.; Dresler, T.; Zeller, J.B.M.; Warrings, B.; Scheuerpflug, P.; Fallgatter, A.J.; Deckert, J.; Metzger, F.G. Vagus Somatosensory Evoked Potentials Are Delayed in Alzheimer’s Disease, but Not in Major Depression. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 263–267. [Google Scholar] [CrossRef]
- Manschot, S.M.; Biessels, G.J.; Rutten, G.E.H.M.; Kessels, R.C.P.; Gispen, W.H.; Kappelle, L.J. Peripheral and Central Neurologic Complications in Type 2 Diabetes Mellitus: No Association in Individual Patients. J. Neurol. Sci. 2008, 264, 157–162. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Tatavarthy, M.; Bennett, D.A. The Relation of Diabetes to Memory Function. Curr. Neurol. Neurosci. Rep. 2020, 20, 64. [Google Scholar] [CrossRef]
- Mayeda, E.R.; Whitmer, R.A.; Yaffe, K. Diabetes and Cognition. Clin. Geriatr. Med. 2015, 31, 101. [Google Scholar] [CrossRef]
- Luna, R.; Manjunatha, R.T.; Bollu, B.; Jhaveri, S.; Avanthika, C.; Reddy, N.; Saha, T.; Gandhi, F. A Comprehensive Review of Neuronal Changes in Diabetics. Cureus 2021, 13, e19142. [Google Scholar] [CrossRef]
- Yoshida, S.; Hirai, M.; Suzuki, S.; Awata, S.; Oka, Y. Neuropathy Is Associated with Depression Independently of Health-Related Quality of Life in Japanese Patients with Diabetes. Psychiatry Clin. Neurosci. 2009, 63, 65–72. [Google Scholar] [CrossRef]
| Age (Years) | Mean ± SEM: 80.24 ± 1.13 (Min: 60, Max: 104) |
| Sex | 38 (40%) male; 57 (60%) female |
| Marital status | Single: 18 (18.9%) Married: 30 (31.6%) Widow/widower: 44 (46.3%) Divorced: 3 (3.2%) |
| Education level | No/incomplete education: 31 (32.6%) Primary school: 54 (56.8%) Secondary school: 7 (7.4%) University: 3 (3.2%) |
| Mini-Mental State Examination (MMSE) | Mean ± SEM: 22.49 ± 0.70 (min: 6, max: 30) |
| Trail Making Test A (TMT-A; seconds) | Mean ± SEM: 122.23 ± 10.38 (min: 22, max: 360) |
| Yesavage Geriatric Depression Scale | Mean ± SEM: 4.34 ± 0.40 (min: 0, max: 15) |
| Barthel Index | Independency: 20 (25.6%) Dependency: 58 (74.4%) |
| n | Type of DM | Drugs | Dosage |
|---|---|---|---|
| 4 | 1 | Insulin glargine + insulin lispro | 38 IU + conditional |
| 1 | 1 | Insulin degludec + insulin lispro | 38 IU + conditional |
| 1 | 2 | Insulin glargine + Insulin aspart | 12 IU + conditional |
| 1 | 2 | Biphasic insulin aspart | 30/70 |
| 13 | 2 | Metformin | 850 mg–1 g |
| 2 | 2 | Repaglinide | 0.5 mg–1 g |
| 5 | 2 | Metformin/vildagliptin | 850 mg/60 mg |
| 2 | 2 | Metformin/vildagliptin | 1000 mg/50 mg |
| 1 | 2 | Repaglinide/linagliptin | 1 mg/5 mg |
| 1 | 2 | Insulin detemir + repaglinide | 28 IU + 2 mg |
| 1 | 2 | Insulin glargine + dapagliflozin + sitagliptin | 40 IU + 10 mg + 50 mg |
| 2 | 2 | Sitagliptin/metformin | 50 mg/1000 mg |
| 1 | 2 | Saxagliptin/metformin | 2.5 mg/850 mg |
| 2 | 2 | Dapagliflozin/metformin | 10 mg/850 mg |
| 1 | 2 | Alogliptin/metformin | 12.5 mg/850 mg |
| 1 | 2 | Canagliflozin + sitagliptin/metformin | 100 mg + 50 mg/1000 mg |
| 1 | 2 | Dapagliflozin + vildagliptin/metformin | 10 mg + 50 mg/850 mg |
| 1 | 2 | Sitagliptin + glimepiride | 50 mg + 2 mg |
| Alterations | Total Population | Diabetic | Non-Diabetic |
|---|---|---|---|
| Arterial Hypertension | AHT: 73.5% No AHT: 26.5% | AHT: 76.9% No AHT: 23.1% | AHT: 70.5% No AHT: 29.5% |
| Hypercholesterolemia | HCH: 57.8% No HCH: 42.2% | HCH: 64.1% No HCH: 35.9% | HCH: 52.3% No HCH: 47.7% |
| Atrial Fibrillation | AF: 12.1% No AF: 87.9% | AF: 6.3% No AF: 93.8% | AF: 17.6% No AF: 82.4% |
| Peripheral Arterial Disease | PAD: 50% No PAD: 50% | PAD: 56.8% No PAD: 43.3% | PAD: 45.3% No PAD: 54.7% |
| Renal Insufficiency | RI: 76.8% No RI: 23.2% | RI: 75% No RI: 25% | RI: 82.1% No RI: 17.9% |
| Test | Total Population | Diabetic | Non-Diabetic |
|---|---|---|---|
| MMSE | Normal: 55 (59.8%) CI: 37 (40.2%) | Normal: 24 (58.5%) CI: 17 (41.5%) | Normal: 23 (42.6%) CI: 31 (57.4%) |
| TMT-A | Normal: 22 (34.4%) Deficient: 42 (65.6%) | Normal: 12 (40%) Deficient: 18 (60%) | Normal: 10 (29.4%) Deficient: 24 (70.6%) |
| Yesavage Geriatric Depression Scale | Normal: 55 (59.8%) Depression: 37 (40.2%) | Normal: 25 (61%) Depression: 16 (39%) | Normal: 30 (58.8%) Depression: 21 (41.2%) |
| Type of Sensitivity | Type of Assessment |
|---|---|
| Superficial Sensory Pathway (% of altered areas) | Pain (%): mean ± SEM: 11.4 ± 2.1 (min: 0, max: 100) Thermal sensation (%): Heat and cold sensation: mean ± SEM: 19.4 ± 3.2 (min: 0, max: 100) Heat sensation: mean ± SEM: 7.0 ± 1.6 (min: 0, max: 66.7) Cold sensation: mean ± SEM: 11.1 ± 2.1 (min: 0, max: 100) Non-discriminative touch (%): mean ± SEM: 4.4 ± 1.6 (min: 0, max: 100) |
| Deep Sensory Pathway (% of altered areas) | Light touch pressure (%): mean ± SEM: 16.9 ± 2.6 (min: 0, max: 100) Vibratory sensation (TF): mean ± SEM: 6.9 ± 0.4 (min: 0, max: 11.2) Vibratory sensation (BTM): mean ± SEM: 39.0 ± 2.1 (min: 0.8, max: 71.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sempere-Bigorra, M.; Julián-Rochina, I.; Pérez-Ros, P.; Navarro-Flores, E.; Martínez-Arnau, F.M.; Cauli, O. Relationship between Cognitive Impairment and Depressive Symptoms with Somatosensory Functions in Diabetic and Non-Diabetic Older Adults and Its Impact on Quality of Life. Life 2023, 13, 1790. https://doi.org/10.3390/life13091790
Sempere-Bigorra M, Julián-Rochina I, Pérez-Ros P, Navarro-Flores E, Martínez-Arnau FM, Cauli O. Relationship between Cognitive Impairment and Depressive Symptoms with Somatosensory Functions in Diabetic and Non-Diabetic Older Adults and Its Impact on Quality of Life. Life. 2023; 13(9):1790. https://doi.org/10.3390/life13091790
Chicago/Turabian StyleSempere-Bigorra, Mar, Iván Julián-Rochina, Pilar Pérez-Ros, Emmanuel Navarro-Flores, Francisco Miguel Martínez-Arnau, and Omar Cauli. 2023. "Relationship between Cognitive Impairment and Depressive Symptoms with Somatosensory Functions in Diabetic and Non-Diabetic Older Adults and Its Impact on Quality of Life" Life 13, no. 9: 1790. https://doi.org/10.3390/life13091790
APA StyleSempere-Bigorra, M., Julián-Rochina, I., Pérez-Ros, P., Navarro-Flores, E., Martínez-Arnau, F. M., & Cauli, O. (2023). Relationship between Cognitive Impairment and Depressive Symptoms with Somatosensory Functions in Diabetic and Non-Diabetic Older Adults and Its Impact on Quality of Life. Life, 13(9), 1790. https://doi.org/10.3390/life13091790










