Abstract
Basal cell carcinoma (BCC) is a malignant tumor with a rising incidence and is the beneficiary of several innovative evaluation techniques. Histopathology remains the gold standard for assessment, having the possibility of addressing multiple high-risk factors such as perineural invasion (PNI). The current study included a number of 244 BCC patients and targeted the identification of positive PNI and its suggestive signs, and whether they correlated or not with other high-risk tumor signs. PNI was found in 20.1% of patients, with 30.7% of patients having perineural chronic inflammation (PCI), which is a suggestive sign of PNI. PNI was also found in larger tumors, with deeper Clark levels, in high-risk BCCs and high-grade tumors. PNI and PCI are both important for pathology reporting, aiding in treatment choice and further patient management, with possibly positive outcomes concerning morbidity and mortality.
1. Introduction
Basal cell carcinoma (BCC), a malignant tumor with a keratinocyte origin, is a non-melanoma skin cancer (NMSC) with increasing incidence worldwide and has the capacity to develop from a benign lesion (verruca vulgaris) or an inflammatory dermatopathology (such as systemic sclerosis) by develops from the basal cells of the hair follicle or from those found at the level of the inter-follicular epidermis [1,2,3,4]. In the United States of America (USA), BCC has reached top incidences (an increasing trend paralleling that of skin infections) of as high as 50% of all types of cancers, but with low mortality and high morbidity rates (if it is left untreated for a long period of time) [5,6]; it is one of the most frequent malignant tumors found among the white population [7], with a lifetime risk of developing such a tumor of approximately 28% for women and as high as 39% for men [8].
The clinical aspects of BCCs are highly varied, with increased difficulty in making a positive diagnosis due to its myriad facets. When dealing with a BCC clinical lesion, the differential diagnosis sets a wide net of possibilities, spanning from inflammatory pathologies to benign tumors, and even to malignant ones. BCC can be clinically differentiated from either inflammatory (psoriasis or other types of dermatitis) or benign lesions (such as fibroepithelial polyp, seborrheic keratosis, or follicular processes), or from Spitz-Reed nevi, to malignant ones such as melanoma or SCC [1,5,9]. The accuracy of a BCC’s clinical diagnosis has been drastically improved with the help of newer investigative tools such as dermoscopy, reflectance confocal microscopy, optical coherence tomography [5,10], Raman spectroscopy, high-resolution ultrasonography and terahertz pulse imaging, which have been found to yield better results with regard to the tumor’s margins and its depth of invasion, as important clues for its future successful surgical excision [1].
Histopathology evaluation has long been considered as being the gold-standard for a BCC’s positive diagnosis, while Mohs micrographic surgery (MMS) is the gold-standard with regard to the therapeutic approach (especially in tumors arising in the head and neck region, and more specifically the face, which also benefits from this procedure’s cosmetic approach, without wide excision and extensive local impact) [11,12]. A surgical approach, be it classical excisional or MMS, offers, on the one hand, an important view on the tumor via the possibility of evaluating residual nests, and on the other hand a low rate of tumor recurrence after excision. This being said, treatment is patient-oriented and established according to the individual’s characteristics (general health condition, location of tumor, recurrence risk) [13]. Tumors that are diagnosed at an early stage or superficial basal cell carcinomas may benefit from local treatments such as laser, cryotherapy, photodynamic therapy, retinoids or topical agents (5-Fluorouracil or imiquimod cream) [8]; cases of superficial BCCs (a low-risk tumor) include the single BCC subtype approved for treatment with 5-fluorouracil, imiquimod and ingenol mebutate [14,15,16,17]. There are some general indications regarding BCC topical treatment, being indicated for superficial tumors, small and/or multiple lesions, the elderly, immune suppressed patients, those with surgery phobia and those with cosmetic sequelae [17]. Large, inoperable tumors might benefit from radiotherapy as an alternative treatment. Alternatively, tumors that are not suitable for radiotherapy might receive vismodegib, an inhibitor of an intracellular signaling pathway which has been approved since 2014 [14].
PNI was simply defined in 1985 by Batsakis as tumor cells being present inside, around and through a nerve [18]. In the past, the theory of nerve sheath angiolymphatic invasion was taken into consideration as an explanation for such phenomena, but it was later disregarded due to the fact that in such a location there is no lymphatic circulation [19]. PNI represents tumor extension along the nerve fiber through the path of least resistance of the tissue planes, by direct growth. Once the space found in-between the nerve sheath and the nerve fiber itself is reached by the neoplastic malignant process, it can extend freely, without finding any resistance, from the smallest nerve fibers to the central subarachnoid space. The nerve fibers themselves are not affected by the growth that takes place, due to the increased elasticity of the perineural and endoneural spaces (this characteristic also explains the lack of symptoms which accompany PNI, until the late stages); nerve degeneration due to the pressure exerted by the malignancy takes place only in confined areas, which restricts growth. This elasticity is the reason why medicine has its malevolent “surprises”, including such reports sometimes occurring as the one of a tumor extending 14 cm without it being symptomatic [20].
Perineural invasion (PNI) is a tumor feature which indicates a poor patient prognosis, being a mechanism for tumor dissemination; it is a feature of high-risk tumors (due to the decreased chances of tumor eradication that PNI implies) which needs to be detected as early as possible in order to avoid the onset of symptoms and the possible involvement of multiple nerve fascicles with deeper tumor extension [21,22,23]. As well as SCC, another keratinocytic malignancy, BCC, can be considered a neurotropic type of cancer, but with much less frequency [23]. There is a difference between clinical PNI, which implies the radiological involvement and/or clinical symptoms of PNI, and incidental PNI, found in histopathology reporting (which might precede the onset of clinical involvement, and reporting it could lead to early treatment) [24]. Chronic perineural inflammation is considered to be a positive sign for PNI [21,25,26]; it indicates that the particular nerve branch is pathologically involved. Our study on 244 BCC patients tried to identify the positive PNI and the suggestive signs thereof, and whether they might be correlated or not with other high-risk tumor signs.
2. Materials and Methods
Design: The current study is a retrospective one which was carried out in the Pathology Laboratory of “Sfântul Apostol Andrei” Emergency Clinical Hospital of Galati, Romania, and included 244 patients with a positive histopathological diagnosis of BCC over the span of a two-year period, from 2019 (165 patients) to 2020 (79 patients). We used the hospital’s electronic database, the pathology laboratory’s registers and the histopathology slides in order to select the patients eligible for study inclusion.
Inclusion criteria: All patients with skin tumors diagnosed from 2019 to 2020 with a positive histopathological BCC diagnosis were included, after revising the slides and confirming the diagnosis.
Exclusion criteria: This study deals with BCC cases, and all patients without this pathology were excluded from this study in the two years taken into consideration. At the same time, histopathologically confirmed cases of BCC which, during revision, did not meet the criteria for a positive diagnosis, were also excluded.
Main variables collected: Perineural invasion was considered according to the definition of Liebig et al. 2009 (also valid in 2018, Schmidt et al.): “tumor in close proximity to nerve and involving at least 33% of its circumference or tumor cells within any of the 3 layers of the nerve sheath” [23,27]. Maximum tumor dimension: the tumor’s largest dimension on the slide was measured (in micrometers), with the help of a microscope fitted with camera and software. The tumor’s Clark levels were considered to be those that are already in use for melanoma cases: level I—confined to epidermis, II—superficial dermis invasion, III—superficial-deep dermis interface invasion, IV—deep dermis invasion, V—hypodermis invasion [28].
Ethics: All patients included in the study have given their informed consent, which was registered in their medical charts. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the “Sfantul Apostol Andrei” Emergency Clinical Hospital of Galati, Romania, with the approval number 2810 from 2 February 2023.
Statistics: The selected patient data were introduced in an Excel table, then imported and processed with the help of SPSS 27.0. The data were sorted into categories and then the frequency distribution was carried out. The possible influences between the data were investigated with the help of the Fisher and Chi-squared tests. Descriptive statistics were used for the quantitative variables and the possible differences were investigated using parametric and non-parametric tests. The following tests for sample comparison were used: the Kolmogorov–Smirnov test, the t-Student test, the ANOVA test, the Mann–Whitney and the Kruskal–Wallis tests. Statistically significant values were those of p < 0.05, while values of p < 0.01 were highly significant statistically. Multivariate analysis was carried out by using a binary logistical regression test—Forward LR—and the following variables were used: largest tumor dimension, Clark level, BCC subtype, BCC grade, the cleft’s corresponding tumor nest’s width and the largest tumor nest’s width; significant differences were found between the patients with PNI and/or PCI and those without.
3. Results
3.1. Patient Group Characteristics
The current study included 244 patients’ cases from the “Sfântul Apostol Andrei” Emergency Clinical Hospital of Galati, Romania. The group was relatively balanced, with almost equal ratios of male and female patients: 51.8% and, respectively, 48.2%. The total of 244 BCC cases included various BCC subtypes, such as superficial, superficial multicentric, nodular, micronodular, infiltrative, morpheaform, basosquamous BCC and Pinkus tumor, as summarized in Table 1. These tumors were located mostly on the head and neck regions (most of them being found on the forehead and the nasal pyramid), but other locations included abdomen (anterior and posterior), thorax (anterior and posterior), shoulder and thigh (Table 2); each subtype’s histopathology characterization is highlighted in Table 3.

Table 1.
Gender distribution of the BCC subtype.

Table 2.
BCC tumor location by gender distribution.

Table 3.
Summary of BCC subtype histology traits.
3.2. Findings
Table 4 highlights the statistical analysis carried out in the current study, with all of the parameters taken into consideration for evaluation and the obtained results. From the patients’ group, approximately half of the BCC patients enrolled in this study did not present with perineural invasion (120), while 49 were positive for PNI, and the rest of them (75) had suggestive signs of PNI (meaning chronic inflammation located perineurally).

Table 4.
Study parameters’ evaluation and statistical analysis results.
BCC patients showing PNI or with suggestive signs of PNI had larger tumors (with median values of 13,683.965 ± 8791.612 µm/13.68 ± 8.79 mm or 11,418.315 ± 10,066.012 µm/11.41 ± 10.06 mm) compared to patients with BCC but without PNI (p = 0.000) (Figure 1).
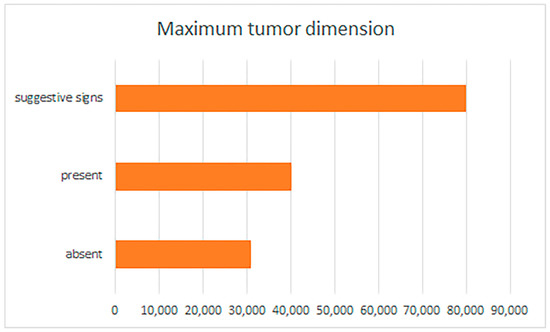
Figure 1.
Perineural invasion and suggestive signs, reported by maximum tumor dimension.
Concerning the Clark level, tumors with Clark level II were found to not have PNI, a trait which was rather found instead in tumors with other Clark levels (III, IV and V) (Figure 2). In those tumors having Clark level III, the number of patients with PNI was reduced (as opposed to deeper Clark levels), to only 1, but this number increased significantly for tumors with Clark level IV; seven cases were PNI positive, while fifty-one cases had suggestive signs. For Clark level V, more than ½ of cases showed PNI—41—and another 22 cases presented suggestive signs. All of these differences found were, again, statistically significant (p = 0.000). Again, there was a parallel increase in PCI with that of the PNI, showing a strong correlation.
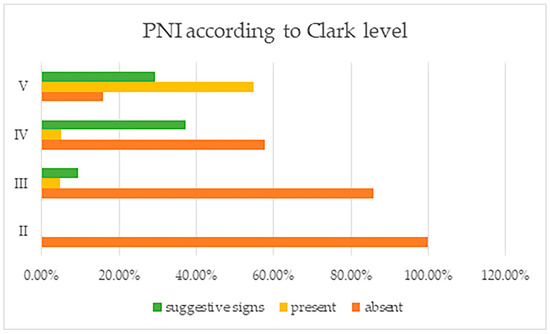
Figure 2.
Perineural invasion and suggestive signs, reported by tumor Clark levels.
PNI was found with high frequencies in BCC subtypes that are considered as having an aggressive behavior, being high-risk (Figure 3). PNI was positively identified in all basosquamous BCCs and morpheaform tumors, in a very large amount of infiltrative BCC subtypes and also in micronodular tumors, and in 29 of the nodular ones, being absent, however, in Pinkus/fibroepithelial BCCs or superficial tumors. These differences between tumor subtypes were also found to be statistically significant.
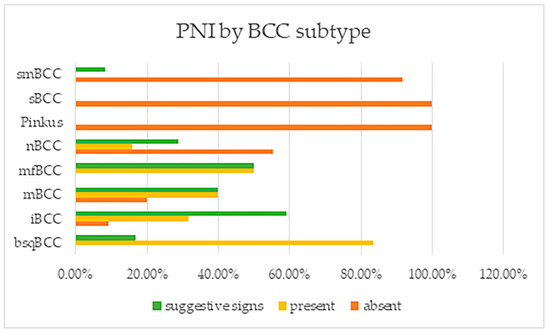
Figure 3.
Perineural invasion and suggestive signs reported by BCC subtype. Note: smBCC—superficial multicentric BCC; sBCC—superficial BCC; nBCC—nodular BCC; mfBCC—morpheaform BCC; mBCC—micronodular BCC; iBCC—infiltrative BCC; and bsqBCC—basosquamous BCC.
The finding that PNI was mostly found in high grade tumors (with or without a low grade tumor component) was also statistically significant (p = 0.000) (Figure 4). This tumor grade concordance with PNI presence was supported by the fact that patients having low grade tumors did not have PNI (in 96 of cases, PNI was absent), while 41 of those having high grade tumors had PNI (18 patients) or PCI (23 patients).
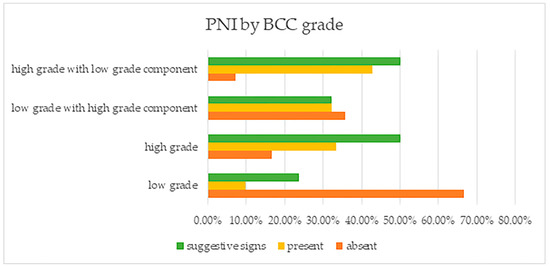
Figure 4.
Perineural invasion and suggestive signs, reported by BCC grade.
The multivariate analysis of the PNI prognosis factors needed a three-step constructed model, which had statistical significance (p < 0.001), with a sensitivity of 63.3% and a specificity of 92.7%. Of the predictor values used, one in particular had statistical significance; BCC grade, an obvious risk factor. Patients having low-grade BCC, but with a high-grade component, had 3.139 times higher chances of having PNI than those with only low-grade tumors; patients with high grade tumors with a low-grade component had 3.977 times higher chances (than those with low-grade tumors) of PNI. This being said, those with high-grade tumors had 6.512 times the chance of having PNI than those with low-grade ones (Table 5).

Table 5.
PNI multivariate analysis by BCC grade.
The multivariate analysis of the PCI prognosis factors needed a three-step constructed model, which had statistical significance (p < 0.001), with a low sensitivity of 5.4% and a high specificity of 97.6%. The model identified two variables as being statistically significant for PCI—the largest tumor dimension and the largest tumor nest (Table 6)—but the associated risks were neutral (OR = 1.000).

Table 6.
PCI multivariate analysis.
4. Discussion
PNI is a tumor trait that is (or should be) part of the pathology report when dealing with a BCC. As mentioned earlier, pathologists may not find PNI on the examined slides of a tumor, but they might see suggestive signs for PNI, which consist of PCI (indicating that something is indeed going on with that specific nerve bundle and should be further investigated/reported) [25,26]. What is worrisome for PNI in such cases is that it might be occult, and signs and symptoms can develop even seven and a half years after the initial diagnosis (and after surgical treatment has been implemented) [30].
The current retrospective study took into consideration 244 BCC-suffering patients during the span of 2 years (of 2019 and 2020), with a significantly larger number of patients addressing medical services in 2019, at the beginning of the Coronavirus disease 2019 (COVID-19) pandemic (for which the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) was responsible). Patient medical care addressability was significantly lower in 2020 due to the measures taken in order to stop COVID-19 from further spreading with morbidity and mortality-related consequences [31,32,33].
This study reported the presence of PNI in 20.1% of cases, and PCI in 30.2% of cases, the remaining numbers not having PNI or PCI. These findings are significantly increased, with the current literature identifying PNI in approximately 1% of cases [34], and some reporting higher incidences of up to 23.1% (closer to our data), a finding dependent on the technique used, conventional pathology or Mohs procedure, and also in connection to larger tumors and to the morpheaform subtype [35,36,37]. Although such ratios have been reported, PNI still remains an underdiagnosed and under-recognized feature of BCCs [38]. The percentages of PNI and PCI parallel one another, and the question of whether such patients should benefit from a more careful follow-up prior to excision is raised. We believe that PCI needs to be considered as a suggestive sign for PNI due to the fact that inflammation in such a location cannot be explained by other processes in the setting of a BCC patient. PCI implies the presence of a local growth; something is developing in this particular place, and the body is reacting to it. The fact that such high numbers of BCC cases recorded the presence of PCI is worrisome and indicative of a much-needed closer patient follow-up, or possibly added treatment options.
The current study found that PNI and PCI in BCC patients were frequently found in cases of larger tumors with more advanced, deeper Clark levels. Larger tumors tend to have deeper invasion, affecting various skin and soft tissue structures (including nerve fibers) and yielding a deeper Clark level in the pathology report. There was a steady increase in PNI and PCI concerning the BCC cases having large tumor maximum dimension, with the largest tumor recorded as having PCI. With regard to Clark levels, starting from Clark level III, a steady increase in frequency can be noticed, with the highest ratios found in BCC cases having Clark levels IV and V. Larger tumor dimensions and higher Clark levels indicate more aggressive behaviors, a fact also supported by PNI and PCI, inclusively.
Ratner et al. (2000) and Brown and Perry (2000) reported their findings of PCI and highlighted that tumors with such findings had a more aggressive histologic growth pattern (morfeaform, infiltrative, sclerosing) [20,39], in support of our study. Low grade tumors exhibited PCI and PNI less frequently, as was to be expected from non-aggressive subtypes. The histological subtype is important when dealing with PNI; nodular BCC, although one of the most frequent BCC subtypes, has a low incidence of PNI, while the superficial subtype most frequently has no PNI.
PNI can be found both in larger nerve bundles and small fibers, but even though there might be histological PNI, there are no clinical symptoms present when small ones are involved. The gross involvement of a nerve might determine the development of pain, paresthesia and hypoesthesia [19,40] or other nerve deficits [41]. A BCC in the head and neck region can affect the trigeminal, facial or even mental nerves [41,42]. Ratner et al. (2000) highlighted that attention must be paid to all nerves affected and that a nerve fiber is not continuously affected by tumor spread, and skip areas exist, with this discontinuous involvement of nerve fibers being explained through an artifactual change during tissue processing by twisting and turning the surgical piece. Finding these discontinuous involvements of nerve fibers and PCI might serve as indicators for a more proximal nerve involvement by the malignant process. As in PNI-positive SCC cases, the presence of PNI requires a more aggressive management (treatment) approach. Whether PNI is present or not has already been regarded as an important prognostic factor, but what about the position of the affected nerve? Lin et al. (2012) have stated that PNI found at the periphery of the tumor might play a prognostic factor role, but insufficient data were evaluated in order to make a concise affirmation. Additionally, the type of PNI might also be important, as a diffuse nerve involvement might indicate a worse prognosis than that of a focal infiltration [43].
Naturally, PNI needs to be differentiated by false-positive tumor aspects. Hassanein et al. (2005) described peritumoral fibrosis with a concentric layering of the fibrous tissue as being a tumor’s stromal trait, which needs to have careful consideration due to the deceiving aspect that it can present with, which mimics PNI. At the same time, they have found this specific peritumoral concentric fibrosis to be a suggestive sign for PNI [44]. By using Mohs micrographic surgery, PNI has been found to be mistaken for other structures such as vessels, erector pili muscles, SCC eddies or granulomatous inflammation [45].
Massey et al. (2020) stated that PNI alone is not a factor of outcome influence, being of relatively low importance, especially in comparison with other parameters that indicate high-risk such as increased tumor diameter, aggressive or high-risk BCC subtype, face location and deep invasion [46], parameters which were reported in our study as being linked to PNI and PCI. This being said, we believe that PNI and PCI are indicators for a more aggressive tumor which can extend beyond the surgical margins. Careful follow-up should be ensured in such patients, and these two parameters should be mentioned in every pathology report, being of help in the patient’s evolution and outcome; a positive PNI diagnosis may help improve treatment interventions, and help with the patient’s morbidity and mortality rates [47]. PNI is indeed an indicator for BCC’s increased morbidity [48]. PNI has been found in larger tumors, and in malignancies which have a higher subclinical extension and higher rates of recurrence, with a significantly increased risk of metastasis. PNI is a tumor trait which was frequently reported as being found in re-excision specimens (Bechert and Stern, 2010) [48,49]. This statement might, in fact, not refer to a real PNI, but a misdiagnosis due to an increased bias towards PNI and due to the nature of the process, which might be a reactive one. The reactive epithelia from adjacent eccrine sweat glands might insinuate into the perineural space and give a false indication of PNI. Although the latter statements disagree with the probability of finding higher incidences of PNI in re-excision specimens, the latter might also be explained by a multifactorial array of other factors: re-excision specimens which might offer an increased amount of tissue for the pathologist to evaluate; the sample might involve deeper skin levels; and pathologists might evaluate with more scrutiny the re-excision specimens for PNI, as opposed to the initial biopsies/excision specimens taken; re-excision specimens might also be a sample taken from a recurrent tumor, rather than a completion of a previous surgical procedure [50]. PNI is a clue for possible future neurological deficits [49], and also for a possible tumor extension with orbital invasion (when dealing with tumors that are site-specific) [51]. A positive PNI diagnosis in a BCC patient is a powerful indicator for long-term patient monitoring, along with obtaining a 5-year local control by combining two treatment options—the surgical one (excision with clear surgical margins) and radiotherapy [52]. PNI in non-melanoma skin cancers such as BCC is a sign for increased rates of local and regional recurrences, and also for diminished disease-free survival periods [53]. Radiotherapy is indicated in BCC cases with PNI (another beneficial aspect of PNI reporting), indicating the possibility of a residual tumor, but the probability of a total cure is reduced as the tumor advances towards the central nervous system [54,55]. An early and accurate PNI diagnosis, with an appropriate risk stratification and a concise and clear patient management strategy, is needed for improving outcomes.
A retrospective study carried out by Adams et al. in 2020 on two databases from Australia regarding the treatments and outcomes in keratinocyte cancers with PNI has found that, in cases of BCCs with incidental PNI, the surgical approach is a suitable treatment option, on the condition that it has tumor-free margins of at least 3 mm (peripheral tumor margin) and perineural tumor margins of at least 5 mm. BCC lesions having PNI that involved nerve bundles of under 0.1 mm in diameter were more frequently treated using the surgical approach, while those tumors involving nerves of at least 0.1 mm in diameter were also associated with adjuvant radiotherapy treatment. These treatment approaches allowed physicians to obtain disease-free intervals of 5 years; after 5 years of the initial treatment, no recurrences were found in the BCC patients enrolled in the study [56].
A possible important future research pathway could stem from the possible inhibition of BCC development and/or progression by using non-steroidal anti-inflammatory drugs (NSAIDs), namely cyclooxygenase-2 (COX-2) inhibitors, which the current literature data report as having chemoprotective properties in patients with such pathology [57]. Muranushi et al. (2016) are in support of this statement, with their study reporting a significant risk reduction in BCC development in patients using such drugs, aiding in those high-risk populations that might be at risk for developing BCC [58]. However, conflicting data emerge, as Yen et al. highlighted, in their 2022 study, that the use of COX-2 inhibitors did not diminish the risk of developing skin cancer, and, even more so, it increased the BCC risk [59]. The exact influence that COX-2 inhibitors exert on the BCC’s development and/or progression is not yet fully understood [60,61], but careful consideration needs to be carried out, as such drugs carry the risk of varied, multiple adverse skin reactions (from fixed drug eruptions, exanthema and urticarial, to Stevens-Johnson syndrome, toxic epidermal necrolysis or drug-induced vasculitis) and even systemic ones (hypertension, edema or congestive heart failure) [62,63].
5. Conclusions
PCI is considered to be a positive clue for perineural involvement by a malignant tumor, in particular that a nerve fiber/bundle is being affected by a process; something is going on with it, otherwise inflammation would not be present. PCI and PNI were frequently found in specific patient and tumor settings, such as in aggressive BCC subtypes and large, high-grade tumors with deeper Clark levels, reflecting the literature and adding updated data. PCI and PNI are important clues for patient prognosis, and they should both be reported by pathologists, giving treating clinicians a more exact view on the tumor’s behavior and influencing treatment options to further ensure a positive patient outcome.
Author Contributions
All authors had substantial contributions to the conception or design of the work, in drafting the work or revising it critically for important intellectual content and in the final approval of the version to be published, and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was paid by the “Dunarea de Jos” University of Galati, Romania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the “Sfantul Apostol Andrei” Emergency Clinical Hospital of Galati, Romania, with the approval number 2810 from 2 February 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to acknowledge that the present study was academically supported by the ‘Dunarea de Jos’ University of Galati, Romania, through the research center Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica—CIM-CID).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Niculet, E.; Craescu, M.; Rebegea, L.; Bobeica, C.; Nastase, F.; Lupasteanu, G.; Stan, D.J.; Chioncel, V.; Anghel, L.; Lungu, M.; et al. Basal cell carcinoma: Comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (Review). Exp. Ther. Med. 2022, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Bobeica, C.; Niculet, E.; Halip, A.I.; Gheuca-Solovastru, L.; Draganescu, M.L.; Popescu, I.A.; Onisor, C.; Chirobocea, S.; Lungu, M.; Craescu, M.; et al. Predictive value of immunological markers in systemic sclerosis. Exp. Ther. Med. 2021, 22, 994. [Google Scholar] [CrossRef] [PubMed]
- Ulutaş, F.; Çomut, E.; Çobankara, V. Development of Two Types of Skin Cancer in a Patient with Systemic Sclerosis: A Case Report and Overview of the Literature. Case Rep. Oncol. Med. 2021, 2021, 6628671. [Google Scholar] [CrossRef] [PubMed]
- Bobeica, C.; Niculet, E.; Tatu, A.L.; Craescu, M.; Vata, D.; Statescu, L.; Iancu, A.V.; Musat, C.L.; Draganescu, M.L.; Onisor, C.; et al. Old and new therapeutic strategies in systemic sclerosis (Review). Exp. Ther. Med. 2022, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Fania, L.; Didona, D.; Morese, R.; Campana, I.; Coco, V.; Di Pietro, F.R.; Ricci, F.; Pallotta, S.; Candi, E.; Abeni, D.; et al. Basal Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2020, 8, 449. [Google Scholar] [CrossRef]
- Tiutiuca, C.; Drăgănescu, M.; Iancu, A.V.; Chesaru, B.I.; Arbune, M.; Maftei, N.; Popescu, E. Resistence profile of the isolate Bacterial stems in invasive infections in three hospitals from the south-east of Romania. Rev. Chim. 2017, 68, 1122–1125. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.D.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef]
- Cantisani, C.; Rossi, R.; Nisticò, S.P.; Vitiello, M.; Farnetani, F.; Bennaro, L.; Pellacani, G. Management of patients with giant basal cell carcinoma during SARS-CoV2 outbreak in Italy. Transl. Biophoton. 2022, 4, e202200009. [Google Scholar] [CrossRef] [PubMed]
- Tatu, A.L. Umbilicated Blue-Black Lesion on the Lateral Thorax. J. Cutan. Med. Surg. 2017, 21, 252. [Google Scholar] [CrossRef]
- Niculet, E.; Tatu, A.L. Comment on Correlation of basal cell carcinoma subtype with histologically confirmed subclinical extension during Mohs micrographic surgery: A prospective multicenter study. J. Am. Acad. Dermatol. 2022, 87, e49–e50. [Google Scholar] [CrossRef]
- Heppt, M.; von Braunmühl, T.; Berking, C. Was gibt es Neues zum Basalzellkarzinom? [What is new in basal cell carcinoma?]. Hautarzt 2016, 67, 876–883. [Google Scholar] [CrossRef]
- Niculet, E.; Bobeica, C.; Craescu, M.; Nicolescu, A.C.; Tocu, G.; Onisor, C.; Arbune, M.; Tatu, A.L. Multimodal Considerations Concerning Basal Cell Carcinoma Clefting—Profile of Structural and Aggressive Traits—Perspectives. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2087–2095. [Google Scholar] [CrossRef]
- Tanese, K. Diagnosis and Management of Basal Cell Carcinoma. Curr. Treat. Options Oncol. 2019, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Berking, C.; Hauschild, A.; Kölbl, O.; Mast, G.; Gutzmer, R. Basal cell carcinoma-treatments for the commonest skin cancer. Dtsch. Arztebl. Int. 2014, 111, 389–395. [Google Scholar] [PubMed]
- Shaw, F.M.; Weinstock, M.A. Comparing Topical Treatments for Basal Cell Carcinoma. J. Investig. Dermatol. 2018, 138, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Paoli, J.; Gyllencreutz, J.D.; Fougelberg, J.; Backman, E.J.; Modin, M.; Polesie, S.; Zaar, O. Nonsurgical Options for the Treatment of Basal Cell Carcinoma. Dermatol. Pract. Concept. 2019, 9, 75–81. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Noaimi, A.A. Basal cell carcinoma: Topical therapy versus surgical treatment. J. Saudi Soc. Dermatol. Dermatol. Surg. 2012, 16, 41–51. [Google Scholar] [CrossRef]
- Batsakis, J.G. Nerves and neurotropic carcinomas. Ann. Otol. Rhinol. Laryngol. 1985, 94, 426–427. [Google Scholar] [CrossRef]
- Cernea, C.R.; Ferraz, A.R.; de Castro, I.V.; Sotto, M.N.; Logullo, A.F.; Bacchi, C.E.; Plopper, C.; Wanderlei, F.; de Carlucci, D., Jr.; Hojaij, F.C. Perineural invasion in aggressive skin carcinomas of the head and neck. ORL J. Otorhinolaryngol. Relat. Spec. 2009, 71, 21–26. [Google Scholar] [CrossRef]
- Ratner, D.; Lowe, L.; Johnson, T.M.; Fader, D.J. Perineural spread of basal cell carcinomas treated with Mohs micrographic surgery. Cancer 2000, 88, 1605–1613. [Google Scholar] [CrossRef]
- Abushukur, Y.; Ibrahim, Y.; Cascardo, C.; Keeley, J.; Knackstedt, T. Basal Cell Carcinoma with Perineural Invasion: A Systematic Review and Pooled Survival Analysis. Dermatol. Surg. 2022, 48, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Coates, D.; Pagliaro, J.; Francis, D.; Williamson, R.; Gaspar, Z. Perineural invasion present exclusively in central tissue blocks of Mohs surgical excisions of basal cell carcinoma. Australas. J. Dermatol. 2018, 59, e62–e65. [Google Scholar] [CrossRef] [PubMed]
- Schmitd, L.B.; Beesley, L.J.; Russo, N.; Bellile, E.L.; Inglehart, R.C.; Liu, M.; Romanowicz, G.; Wolf, G.T.; Taylor, J.M.G.; D’Silva, N.J. Redefining Perineural Invasion: Integration of Biology with Clinical Outcome. Neoplasia 2018, 20, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, L.; De’Ambrosis, B.; DeAmbrosis, K.; Warren, T.; Huilgol, S.; Soyer, H.P.; Panizza, B. Defining incidental perineural invasion: The need for a national registry. Australas. J. Dermatol. 2014, 55, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Chronic Inflammation Implies Perineural Invasion. Available online: https://www.mdedge.com/dermatology/article/46867/melanoma/chronic-inflammation-implies-perineural-invasion (accessed on 12 January 2023).
- Abbas, O.; Bhawan, J. Cutaneous perineural inflammation: A review. J. Cutan. Pathol 2010, 37, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Clark’s Level. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/clarks-level (accessed on 22 March 2023).
- McDaniel, B.; Badri, T.; Steele, R.B. Basal Cell Carcinoma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482439/ (accessed on 22 March 2023).
- Ashraf, D.C.; Kalin-Hajdu, E.; Levin, M.H.; Kersten, R.C. Mixed cranial neuropathies due to occult perineural invasion of basal cell carcinoma. Am. J. Ophthalmol. Case Rep. 2018, 13, 136–139. [Google Scholar] [CrossRef]
- Núñez, A.; Sreeganga, S.D.; Ramaprasad, A. Access to Healthcare during COVID-19. Int. J. Environ. Res. Public Health 2021, 18, 2980. [Google Scholar] [CrossRef]
- Gertz, A.H.; Pollack, C.C.; Schultheiss, M.D.; Brownstein, J.S. Delayed medical care and underlying health in the United States during the COVID-19 pandemic: A cross-sectional study. Prev. Med. Rep. 2022, 28, 101882. [Google Scholar] [CrossRef]
- Tatu, A.L.; Nadasdy, T.; Bujoreanu, F.C. Familial clustering of COVID-19 skin manifestations. Dermatol. Ther. 2020, 33, e14181. [Google Scholar] [CrossRef]
- Kratzsch, D.; Kendler, M.; Simon, J.C.; Ziemer, M. Basal cell carcinomas with perineural invasion: A clinical-therapeutic and histological challange. J. Dtsch. Dermatol. Ges. 2016, 14, 173–175. [Google Scholar] [CrossRef]
- Messeguer, F.; Nagore-Enguídanos, E.; Requena, C.; Guillén-Barona, C. Manejo del carcinoma basocelular con infiltración perineural [Management of basal cell carcinoma with perineural invasion]. Actas Dermosifiliogr. 2010, 101, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.J.; Hoegler, K.M.; Zhou, A.E.; Snow, C.R.; Khachemoune, A. A systematic review of the incidence of basal cell carcinoma with perineural invasion: Conventional pathology versus Mohs micrographic surgery. Arch. Dermatol. Res. 2022, 315, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.B.; Andrade, N.M.; Brandão, L.G.; Cernea, C.R. Which features of advanced head and neck basal cell carcinoma are associated with perineural invasion? Braz. J. Otorhinolaryngol. 2017, 83, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Sherwani, Y.; Aldana, P.C.; Khachemoune, A. Squamous and basal cell carcinoma with perineural invasion: Pathophysiology and presentations. Int. J. Dermatol. 2022, 61, 653–659. [Google Scholar] [CrossRef]
- Brown, C.I.; Perry, A.E. Incidence of perineural invasion in histologically aggressive types of basal cell carcinoma. Am. J. Dermatopathol. 2000, 22, 123–125. [Google Scholar] [CrossRef]
- Longeac, M.; Lapeyre, M.; Delbet Dupas, C.; Barthélémy, I.; Pham Dang, N. Radiothérapie exclusive d’un carcinome basocellulaire de la face avec atteinte du ganglion de Gasser [Exclusive radiotherapy for a facial basal cell carcinoma with trigeminal ganglion involvement]. Cancer Radiother. 2016, 20, 308–311. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Ferlito, A.; Takes, R.P.; Bradford, C.R.; Corry, J.; Fagan, J.J.; Rinaldo, A.; Strojan, P.; Rodrigo, J.P. Cutaneous head and neck basal and squamous cell carcinomas with perineural invasion. Oral Oncol. 2012, 48, 918–922. [Google Scholar] [CrossRef]
- Di Gregorio, C.; Florena, A.M.; Gebbia, V.; Moschella, F. Mental nerve invasion by basal cell carcinoma of the chin: A case report. Anticancer Res. 1998, 18, 4723–4726. [Google Scholar]
- Lin, C.; Tripcony, L.; Keller, J.; Poulsen, M.; Martin, J.; Jackson, J.; Dickie, G. Perineural infiltration of cutaneous squamous cell carcinoma and basal cell carcinoma without clinical features. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 334–340. [Google Scholar] [CrossRef]
- Hassanein, A.M.; Proper, S.A.; Depcik-Smith, N.D.; Flowers, F.P. Peritumoral fibrosis in basal cell and squamous cell carcinoma mimicking perineural invasion: Potential pitfall in Mohs micrographic surgery. Dermatol. Surg. 2005, 31, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Thomas, V.D. Evaluation of nerves in Mohs micrographic surgery: Histologic mimickers of perineural invasion and nervous tissue on frozen section. Dermatol. Surg. 2014, 40, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Massey, P.R.; Morgan, F.C.; Ruiz, E.S.; Schmults, C.D. Histologic perineural invasion of unnamed nerves does not affect basal cell carcinoma outcomes. J. Am. Acad. Dermatol. 2020, 82, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.E.; Hoegler, K.M.; Khachemoune, A. Review of Perineural Invasion in Keratinocyte Carcinomas. Am. J. Clin. Dermatol. 2021, 22, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Bechert, C.J.; Stern, J.B. Basal cell carcinoma with perineural invasion: Reexcision perineural invasion? J. Cutan. Pathol. 2010, 37, 376–379. [Google Scholar] [CrossRef]
- Geist, D.E.; Garcia-Moliner, M.; Fitzek, M.M.; Cho, H.; Rogers, G.S. Perineural invasion of cutaneous squamous cell carcinoma and basal cell carcinoma: Raising awareness and optimizing management. Dermatol. Surg. 2008, 34, 1642–1651. [Google Scholar] [CrossRef]
- Beer, T.W.; Drury, P. Perineural invasion in basal cell carcinomas is generally not re-excision perineural invasion. J. Cutan. Pathol. 2012, 39, 1047–1048. [Google Scholar] [CrossRef]
- Sun, M.T.; Wu, A.; Figueira, E.; Huilgol, S.; Selva, D. Management of periorbital basal cell carcinoma with orbital invasion. Future Oncol. 2015, 11, 3003–3010. [Google Scholar] [CrossRef]
- Jackson, J.E.; Dickie, G.J.; Wiltshire, K.L.; Keller, J.; Tripcony, L.; Poulsen, M.G.; Hughes, M.; Allison, R.W.; Martin, J.M. Radiotherapy for perineural invasion in cutaneous head and neck carcinomas: Toward a risk-adapted treatment approach. Head Neck 2009, 31, 604–610. [Google Scholar] [CrossRef]
- Gupta, A.; Veness, M.; De’Ambrosis, B.; Selva, D.; Huilgol, S.C. Management of squamous cell and basal cell carcinomas of the head and neck with perineural invasion. Australas. J. Dermatol. 2016, 57, 3–13. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Amdur, R.J.; Hinerman, R.W.; Cognetta, A.B.; Mendenhall, N.P. Radiotherapy for cutaneous squamous and basal cell carcinomas of the head and neck. Laryngoscope 2009, 119, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Leibovitch, I.; Huilgol, S.C.; Selva, D.; Richards, S.; Paver, R. Basal cell carcinoma treated with Mohs surgery in Australia I. Experience over 10 years. J. Am. Acad. Dermatol. 2005, 53, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; De’Ambrosis, B.; Panizza, B.; Belt, P.; Emmett, J.; Warren, T.; Whiteman, D.C. Keratinocyte cancer with incidental perineural invasion: A registry analysis of management and 5-year outcomes. Australas. J. Dermatol. 2020, 61, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, N.; Olsen, C.M.; Thompson, B.S.; Dusingize, J.C.; Neale, R.E.; Green, A.C.; Whiteman, D.C.; QSkin Study. Aspirin and nonsteroidal anti-inflammatory drug use and keratinocyte cancers: A large population-based cohort study of skin cancer in Australia. Br. J. Dermatol. 2019, 181, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Muranushi, C.; Olsen, C.M.; Green, A.C.; Pandeya, N. Can oral nonsteroidal antiinflammatory drugs play a role in the prevention of basal cell carcinoma? A systematic review and metaanalysis. J. Am. Acad. Dermatol. 2016, 74, 108–119.e1. [Google Scholar] [CrossRef]
- Yen, H.; Yen, H.; Drucker, A.M.; Han, J.; Li, W.Q.; Li, T.; Qureshi, A.; Cho, E. COX-2 inhibitors show no preventive effect in the development of skin cancer. J. Dtsch. Dermatol. Ges. 2022, 20, 157–166. [Google Scholar] [CrossRef]
- Rundle, C.W.; Dellavalle, R.P. Nonsteroidal anti-inflammatory drug associations with nonmelanoma skin cancer—The saga continues. Br. J. Dermatol. 2019, 181, 654–656. [Google Scholar] [CrossRef]
- Reinau, D.; Surber, C.; Jick, S.S.; Meier, C.R. Nonsteroidal anti-inflammatory drugs and the risk of nonmelanoma skin cancer. Int. J. Cancer 2015, 137, 144–153. [Google Scholar] [CrossRef]
- Tatu, A.L.; Nwabudike, L.C. Bullous Reactions Associated with COX-2 Inhibitors. Am. J. Ther. 2017, 24, e477–e480. [Google Scholar] [CrossRef]
- Sharma, J.N.; Jawad, N.M. Adverse effects of COX-2 inhibitors. Sci. World J. 2005, 5, 629–645. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).