Anti-Proliferative Effect of Radiotherapy and Implication of Immunotherapy in Anaplastic Thyroid Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Preparation of Patient-Derived Human Tumor Tissue (PDTT)
2.3. Irradiation
2.4. Compounds Tested
2.5. Measurement of Cell Viability
2.6. Colony Formation Assay
2.7. Quantitative RT-PCR
2.8. Measurement of Apoptosis/Necrosis
2.9. Western Blot Analysis
2.10. Statistical Analysis
2.11. Ethical Approval
3. Results
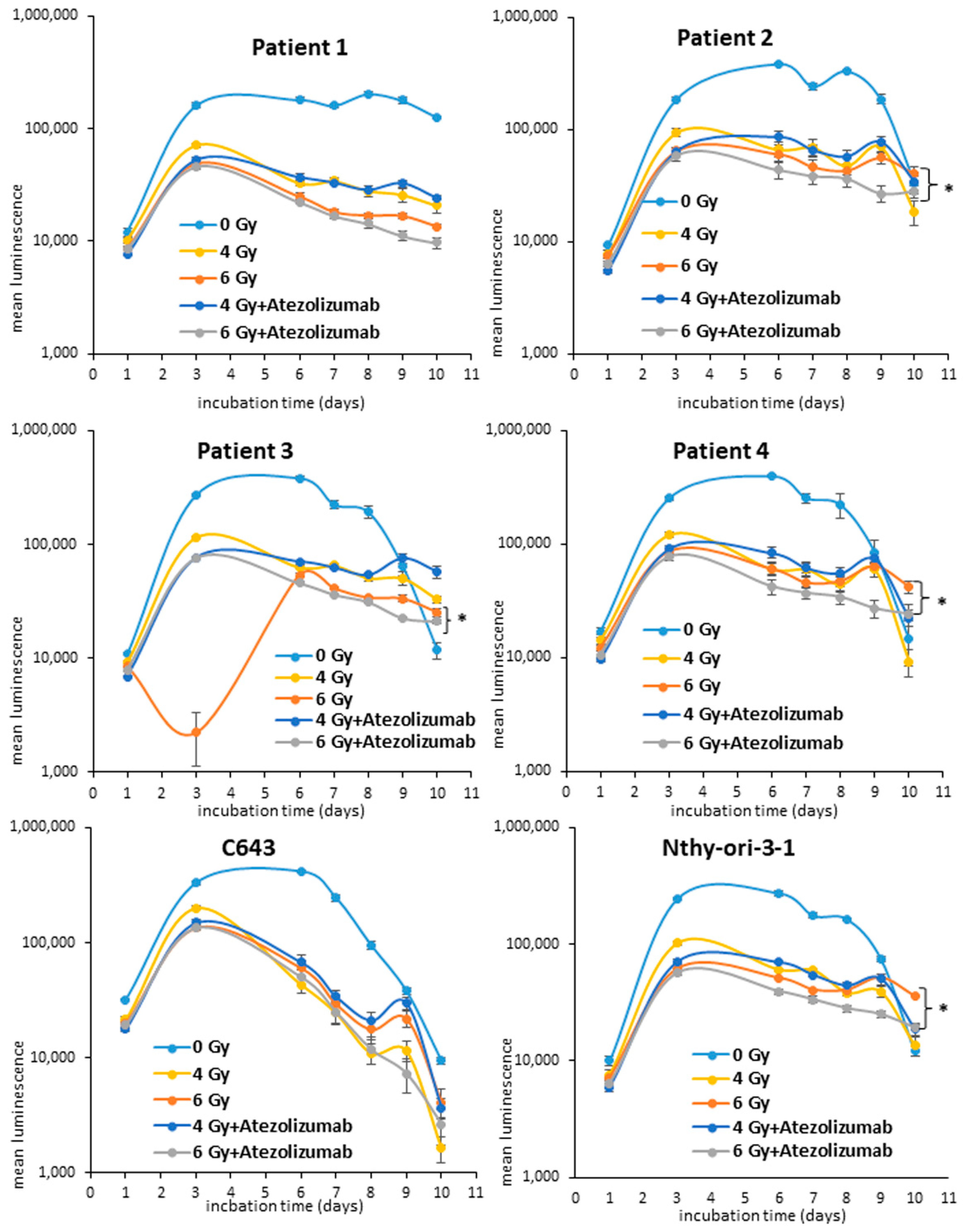
3.1. Effects of Radio- and Immunotherapy on the Cell Proliferation of ATC Cells
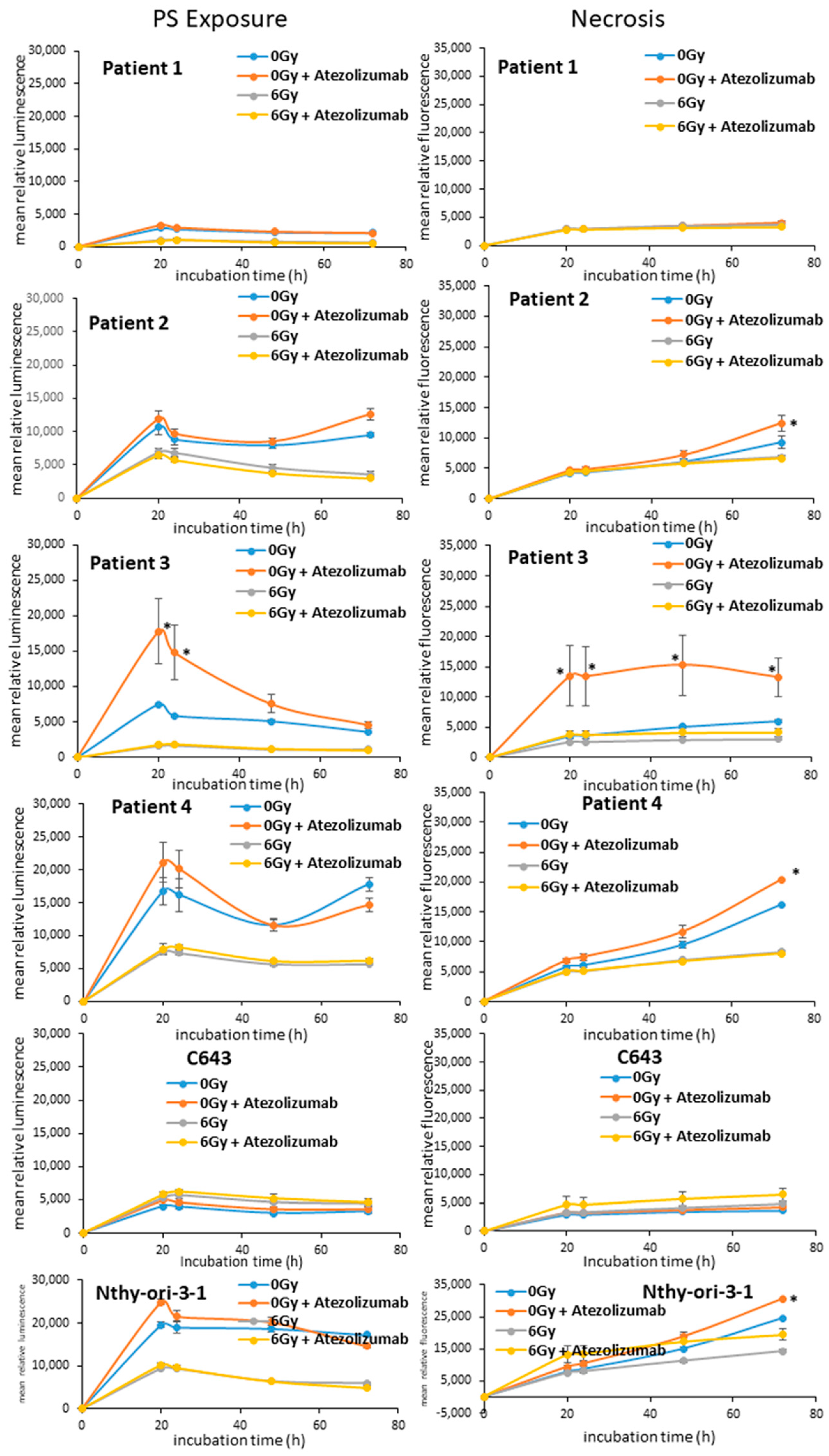
3.2. Apoptotic Effects of Radio- and Immunotherapy in ATC Cells
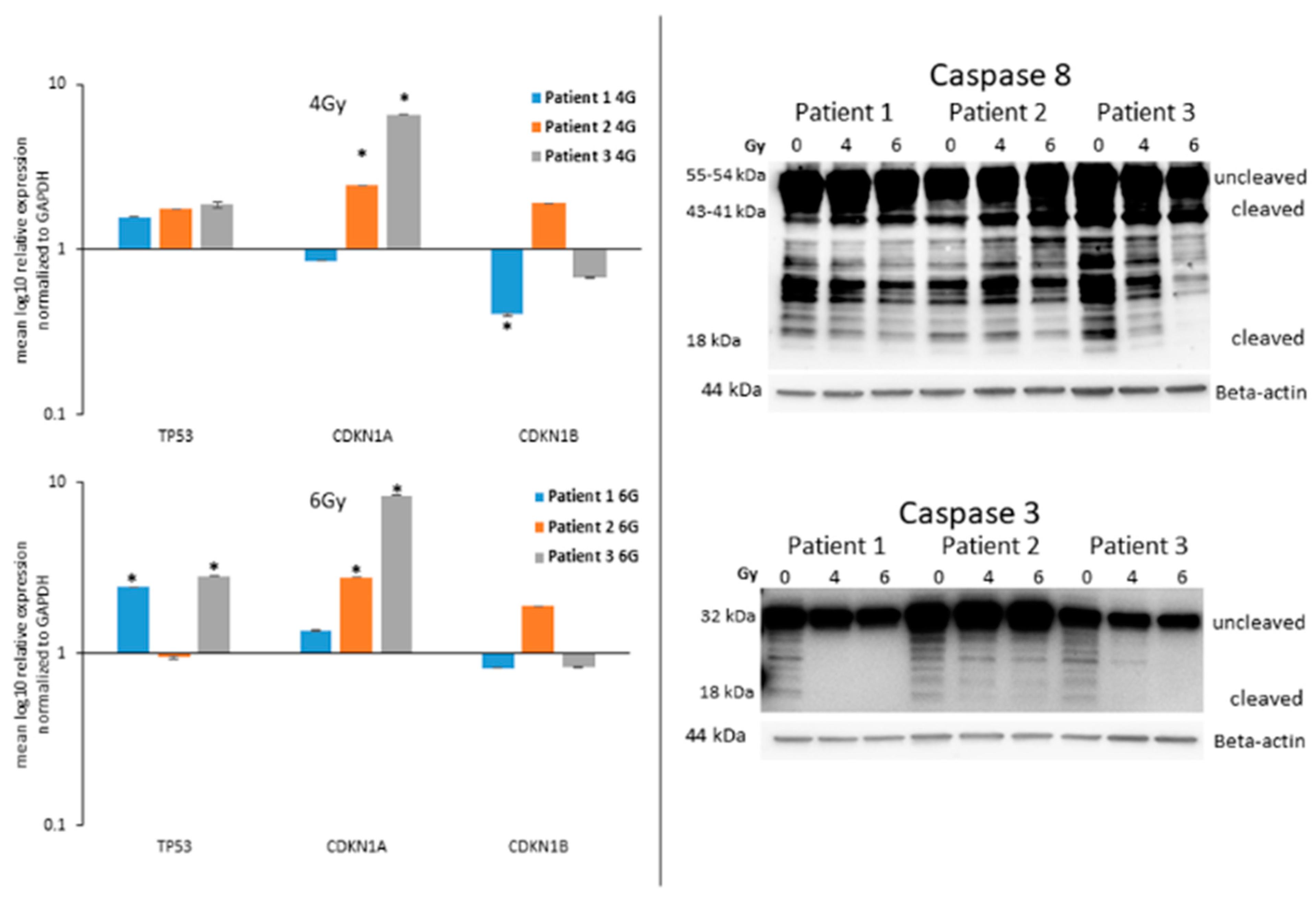
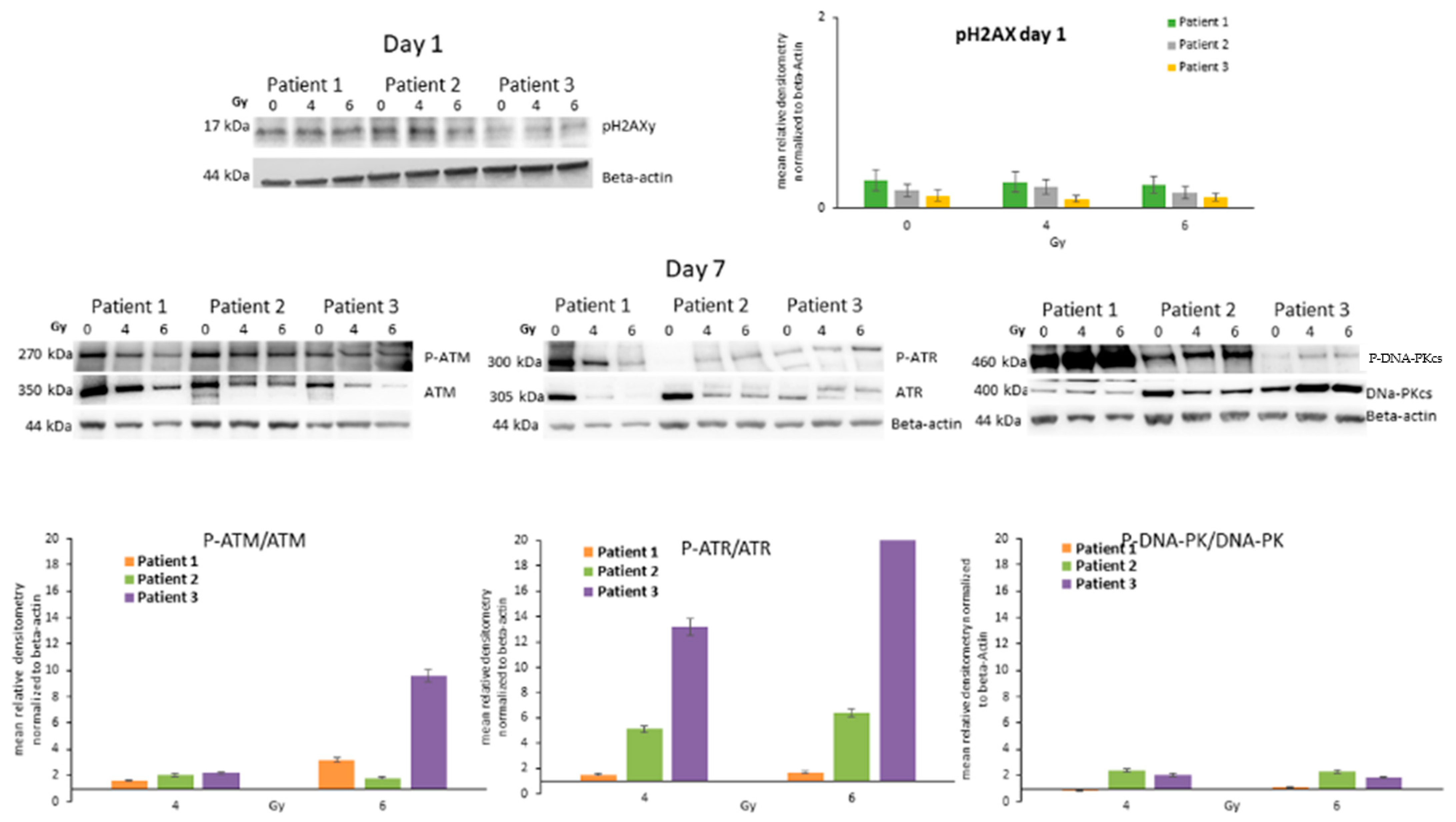
3.3. Analysis of the Guardian of the Cell Destiny after Radiotherapy
3.4. Expression of Caspases in ATC Cells after Radiotherapy
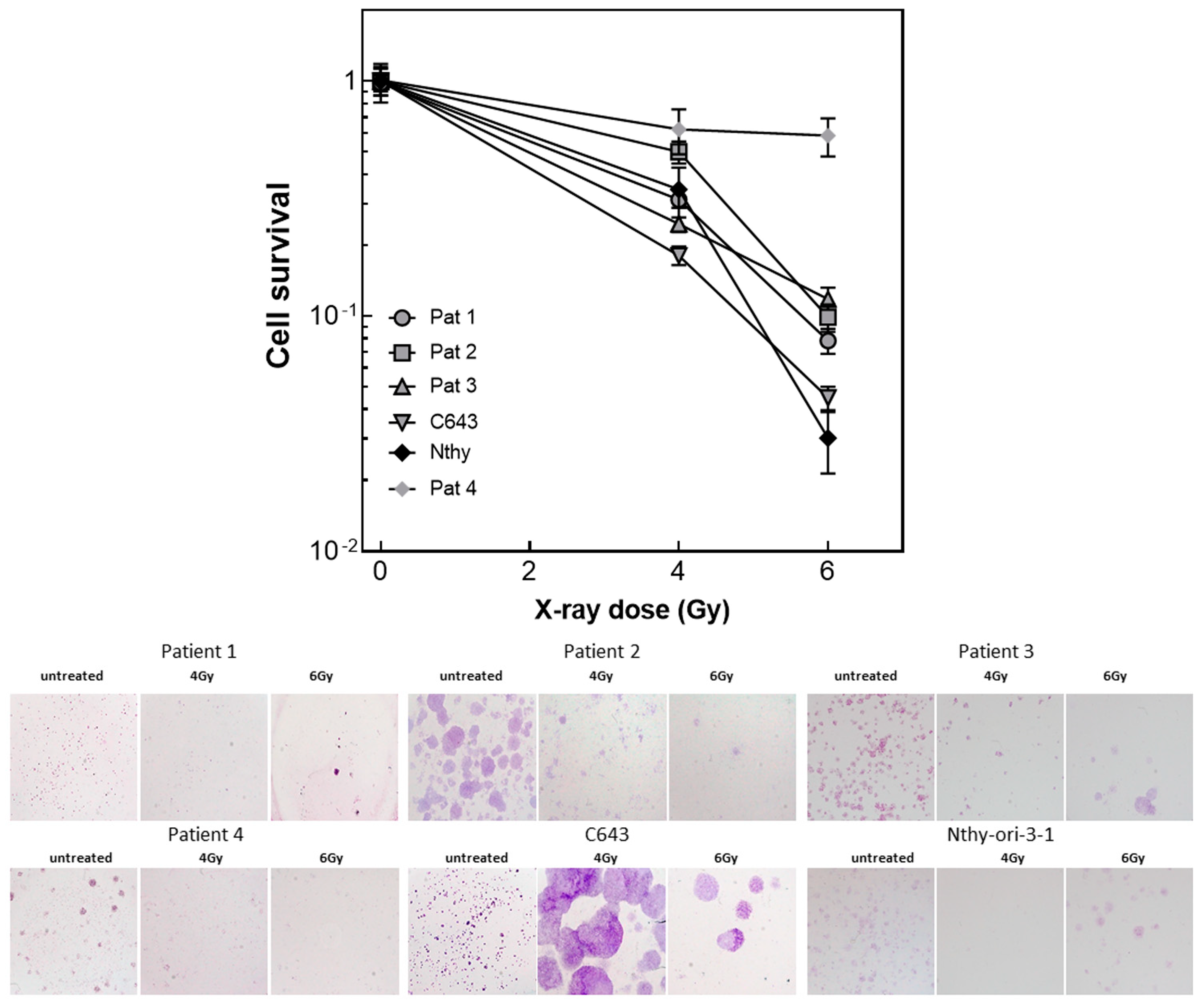
3.5. Radiated ATC Cells Lose Their Ability to Build up Colonies
3.6. ATC Cell DNA Damage/Repair Machinery after Radiotherapy
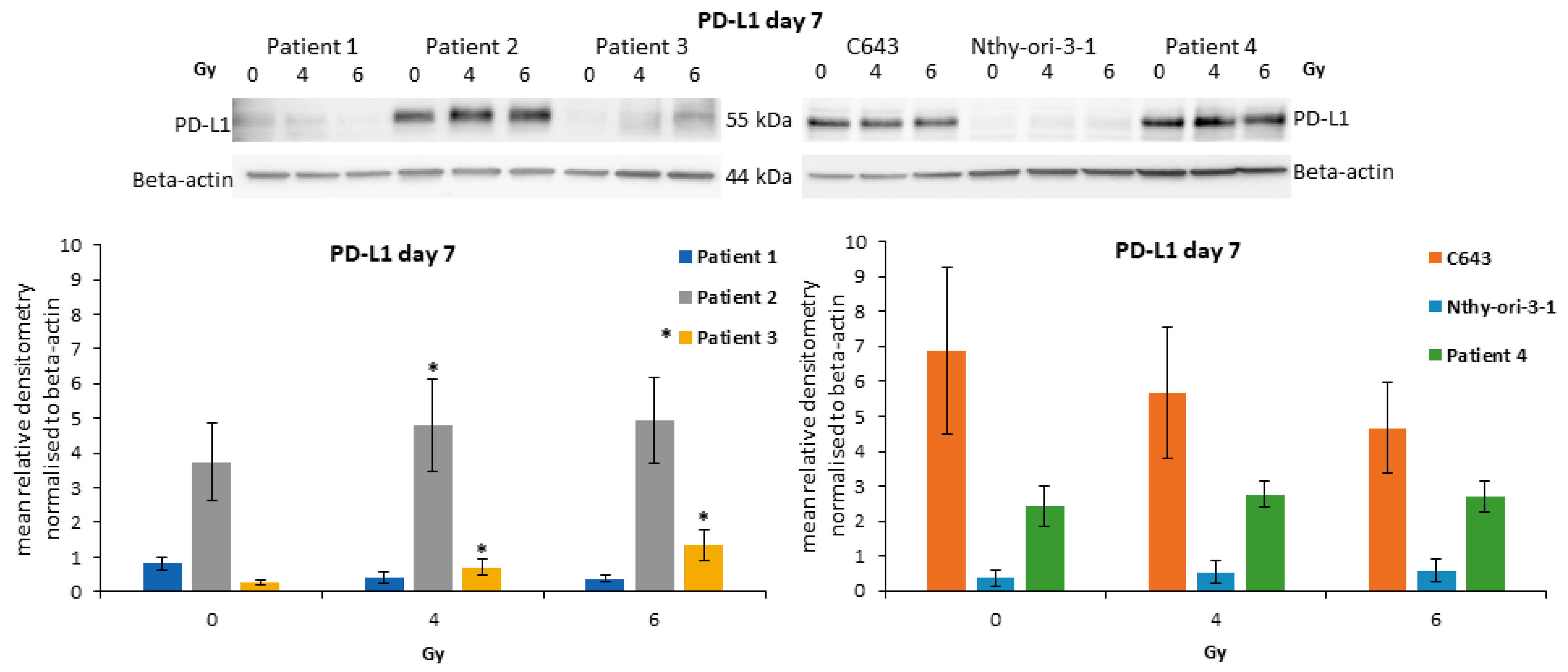
3.7. Modulation of PD-L1 after Irradiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haddad, R.I.; Lydiatt, W.M.; Ball, D.W.; Busaidy, N.L.; Byrd, D.; Callender, G.; Dickson, P.; Duh, Q.Y.; Ehya, H.; Haymart, M.; et al. Anaplastic Thyroid Carcinoma, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1140–1150. [Google Scholar] [CrossRef]
- Passler, C.; Scheuba, C.; Prager, G.; Kaserer, K.; Flores, J.A.; Vierhapper, H.; Niederle, B. Anaplastic (undifferentiated) thyroid carcinoma (ATC). A retrospective analysis. Langenbeck’s Arch. Surg. 1999, 384, 284–293. [Google Scholar] [CrossRef]
- Kebebew, E.; Greenspan, F.S.; Clark, O.H.; Woeber, K.A.; McMillan, A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 2005, 103, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Wächter, S.; Vorländer, C.; Schabram, J.; Mintziras, I.; Fülber, I.; Manoharan, J.; Holzer, K.; Bartsch, D.K.; Maurer, E. Anaplastic thyroid carcinoma: Changing trends of treatment strategies and associated overall survival. Eur. Arch. Oto-Rhino-Laryngology 2020, 277, 1507–1514. [Google Scholar] [CrossRef]
- Savvides, P.; Nagaiah, G.; Lavertu, P.; Fu, P.; Wright, J.J.; Chapman, R.; Wasman, J.; Dowlati, A.; Remick, S.C. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 2013, 23, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Masaki, C.; Ogimi, Y.; Maeda, T.; Osaku, T.; Akaishi, J.; Hames, K.Y.; Tomoda, C.; Matsuzu, K.; Uruno, T.; Ohkuwa, K.; et al. Lenvatinib induces early tumor shrinkage in patients with advanced thyroid carcinoma. Endocr. J. 2017, 64, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Toda, S.; Murayama, D.; Kato, S.; Matsui, A. Relationship between adverse events associated with lenvatinib treatment for thyroid cancer and patient prognosis. Mol. Clin. Oncol. 2021, 14. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.-H.; Shin, J.H.; Hahn, S.Y.; et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr.-Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef]
- Cantara, S.; Bertelli, E.; Occhini, R.; Regoli, M.; Brilli, L.; Pacini, F.; Castagna, M.G.; Toti, P. Blockade of the programmed death ligand 1 (PD-L1) as potential therapy for anaplastic thyroid cancer. Endocrine 2019, 64, 122–129. [Google Scholar] [CrossRef]
- Goodman, A.M.; Piccioni, D.; Kato, S.; Boichard, A.; Wang, H.-Y.; Frampton, G.; Lippman, S.M.; Connelly, C.; Fabrizio, D.; Miller, V.; et al. Prevalence of PDL1 Amplification and Preliminary Response to Immune Checkpoint Blockade in Solid Tumors. JAMA Oncol. 2018, 4, 1237–1244. [Google Scholar] [CrossRef]
- Iyer, P.C.; Dadu, R.; Gule-Monroe, M.; Busaidy, N.L.; Ferrarotto, R.; Habra, M.A.; Zafereo, M.; Williams, M.D.; Gunn, G.B.; Grosu, H.; et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J. Immunother. Cancer 2018, 6, 68. [Google Scholar] [CrossRef]
- Dierks, C.; Seufert, J.; Aumann, K.; Ruf, J.; Klein, C.; Kiefer, S.; Rassner, M.; Boerries, M.; Zielke, A.; la Rosee, P.; et al. Combination of Lenvatinib and Pembrolizumab Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid 2021, 31, 1076–1085. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Victor, C.T.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA Damage Sensing by the ATM and ATR Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Hosseinimehr, S.J.; Khalaj, A. The paradox role of caspase cascade in ionizing radiation therapy. J. Biomed. Sci. 2016, 23, 88. [Google Scholar] [CrossRef]
- Wächter, S.; Wunderlich, A.; Roth, S.; Mintziras, I.; Maurer, E.; Hoffmann, S.; Verburg, F.A.; Fellinger, S.A.; Holzer, K.; Bartsch, D.K.; et al. Individualised Multimodal Treatment Strategies for Anaplastic and Poorly Differentiated Thyroid Cancer. J. Clin. Med. 2018, 7, 115. [Google Scholar] [CrossRef]
- Wächter, S.; Knauff, F.; Roth, S.; Keber, C.; Holzer, K.; Manoharan, J.; Maurer, E.; Bartsch, D.K.; Di Fazio, P. Synergic induction of autophagic cell death in anaplastic thyroid carcinoma. Cancer Investig. 2023, 41, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Oweida, A.; Phan, A.; VanCourt, B.; Robin, T.; Hararah, M.K.; Bhatia, S.; Milner, D.; Lennon, S.; Pike, L.; Raben, D.; et al. Hypofractionated Radiotherapy Is Superior to Conventional Fractionation in an Orthotopic Model of Anaplastic Thyroid Cancer. Thyroid 2018, 28, 739–747. [Google Scholar] [CrossRef]
- Wagener, N.; Buchholz, M.; Bertolino, P.; Zhang, C.X.; Di Fazio, P. Exploring the MEN1 dependent modulation of caspase 8 and caspase 3 in human pancreatic and murine embryo fibroblast cells. Apoptosis 2021, 27, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Jannin, A.; Escande, A.; Al Ghuzlan, A.; Blanchard, P.; Hartl, D.; Chevalier, B.; Deschamps, F.; Lamartina, L.; Lacroix, L.; Dupuy, C.; et al. Anaplastic Thyroid Carcinoma: An Update. Cancers 2022, 14, 1061. [Google Scholar] [CrossRef]
- Roukoz, C.; Gregoire, V. Indications of external beams radiation for thyroid cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 137–144. [Google Scholar] [CrossRef]
- Augustin, T.; Oliinyk, D.; Rauch, J.; Koehler, V.F.; Spitzweg, C.; Belka, C.; Käsmann, L. Radiation to the Primary Tumor in Metastatic Anaplastic Thyroid Cancer. In Vivo 2021, 35, 461–465. [Google Scholar] [CrossRef]
- Zhou, W.; Yue, Y.; Zhang, X. Radiotherapy Plus Chemotherapy Leads to Prolonged Survival in Patients with Anaplastic Thyroid Cancer Compared With Radiotherapy Alone Regardless of Surgical Resection and Distant Metastasis: A Retrospective Population Study. Front. Endocrinol. 2021, 12, 748023. [Google Scholar] [CrossRef]
- De Leo, S.; Trevisan, M.; Fugazzola, L. Recent advances in the management of anaplastic thyroid cancer. Thyroid. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Qiao, P.-P.; Tian, K.-S.; Han, L.-T.; Ma, B.; Shen, C.-K.; Zhao, R.-Y.; Zhang, Y.; Wei, W.-J.; Chen, X.-P. Correlation of mismatch repair deficiency with clinicopathological features and programmed death-ligand 1 expression in thyroid carcinoma. Endocrine 2022, 76, 660–670. [Google Scholar] [CrossRef]
- de Pedro, I.; Galan-Vidal, J.; Freije, A.; de Diego, E.; Gandarillas, A. p21CIP1 controls the squamous differentiation response to replication stress. Oncogene 2021, 40, 152–162. [Google Scholar] [CrossRef]
- Aylon, Y.; Oren, M. p53: Guardian of ploidy. Mol. Oncol. 2011, 5, 315–323. [Google Scholar] [CrossRef]
- Chu, I.M.; Hengst, L.; Slingerland, J.M. The Cdk inhibitor p27 in human cancer: Prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer 2008, 8, 253–267. [Google Scholar] [CrossRef]
- Kasten-Pisula, U.; Saker, J.; Eicheler, W.; Krause, M.; Yaromina, A.; Meyer-Staeckling, S.; Scherkl, B.; Kriegs, M.; Brandt, B.; Grénman, R.; et al. Cellular and Tumor Radiosensitivity is Correlated to Epidermal Growth Factor Receptor Protein Expression Level in Tumors Without EGFR Amplification. Int. J. Radiat. Oncol. 2011, 80, 1181–1188. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Ruffilli, I.; La Motta, C.; Paparo, S.R.; Patrizio, A.; Vita, R.; Benvenga, S.; Materazzi, G.; et al. Novel treatments for anaplastic thyroid carcinoma. Gland. Surg. 2020, 9 (Suppl. 1), S28–S42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wächter, S.; Roth, S.; Gercke, N.; Schötz, U.; Dikomey, E.; Engenhart-Cabillic, R.; Maurer, E.; Bartsch, D.K.; Di Fazio, P. Anti-Proliferative Effect of Radiotherapy and Implication of Immunotherapy in Anaplastic Thyroid Cancer Cells. Life 2023, 13, 1397. https://doi.org/10.3390/life13061397
Wächter S, Roth S, Gercke N, Schötz U, Dikomey E, Engenhart-Cabillic R, Maurer E, Bartsch DK, Di Fazio P. Anti-Proliferative Effect of Radiotherapy and Implication of Immunotherapy in Anaplastic Thyroid Cancer Cells. Life. 2023; 13(6):1397. https://doi.org/10.3390/life13061397
Chicago/Turabian StyleWächter, Sabine, Silvia Roth, Norman Gercke, Ulrike Schötz, Ekkehard Dikomey, Rita Engenhart-Cabillic, Elisabeth Maurer, Detlef K. Bartsch, and Pietro Di Fazio. 2023. "Anti-Proliferative Effect of Radiotherapy and Implication of Immunotherapy in Anaplastic Thyroid Cancer Cells" Life 13, no. 6: 1397. https://doi.org/10.3390/life13061397
APA StyleWächter, S., Roth, S., Gercke, N., Schötz, U., Dikomey, E., Engenhart-Cabillic, R., Maurer, E., Bartsch, D. K., & Di Fazio, P. (2023). Anti-Proliferative Effect of Radiotherapy and Implication of Immunotherapy in Anaplastic Thyroid Cancer Cells. Life, 13(6), 1397. https://doi.org/10.3390/life13061397





