Effectiveness of One-Year Pemafibrate Therapy on Non-Alcoholic Fatty Liver Disease Refractory to Long-Term Sodium Glucose Cotransporter-2 Inhibitor Therapy: A Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Markers of Hepatic Inflammation, Function, and Fibrosis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Changes in Parameters during One Year of Pemafibrate Therapy
3.3. Liver Enzyme Profiles One Year before and One Year after Starting Pemafibrate Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wai-Sun Wong, V.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Arase, Y.; Ikeda, K.; Seko, Y.; Imai, N.; Hosaka, T.; Kobayashi, M.; Saitoh, S.; Sezaki, H.; Akuta, N.; et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am. J. Gastroenterol. 2012, 107, 253–261. [Google Scholar] [CrossRef]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol. Res. 2021, 51, 1013–1025. [Google Scholar] [CrossRef]
- Dwinata, M.; Putera, D.D.; Hasan, I.; Raharjo, M. SGLT2 inhibitors for improving hepatic fibrosis and steatosis in non-alcoholic fatty liver disease complicated with type 2 diabetes mellitus: A systematic review. Clin. Exp. Hepatol. 2020, 6, 339–346. [Google Scholar] [CrossRef]
- Shinozaki, S.; Tahara, T.; Lefor, A.K.; Ogura, M. Long-term empagliflozin therapy improves levels of hepatic fibrosis marker in patients with non-alcoholic fatty liver disease complicated by type 2 diabetes mellitus. J. Med. Investig. 2020, 67, 280–284. [Google Scholar] [CrossRef]
- Das Pradhan, A.; Glynn, R.J.; Fruchart, J.C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N. Engl. J. Med. 2022, 387, 1923–1934. [Google Scholar] [CrossRef]
- Shinozaki, S.; Tahara, T.; Lefor, A.K.; Ogura, M. Pemafibrate decreases markers of hepatic inflammation in patients with non-alcoholic fatty liver disease. Clin. Exp. Hepatol. 2020, 6, 270–274. [Google Scholar] [CrossRef]
- Shinozaki, S.; Tahara, T.; Lefor, A.K.; Ogura, M. Pemafibrate improves hepatic inflammation, function and fibrosis in patients with non-alcoholic fatty liver disease: A one-year observational study. Clin. Exp. Hepatol. 2021, 7, 172–177. [Google Scholar] [CrossRef]
- Shinozaki, S.; Tahara, T.; Miura, K.; Lefor, A.K.; Yamamoto, H. Pemafibrate therapy for non-alcoholic fatty liver disease is more effective in lean patients than obese patients. Clin. Exp. Hepatol. 2022, 8, 278–283. [Google Scholar] [PubMed]
- Murakami, K.; Sasaki, Y.; Asahiyama, M.; Yano, W.; Takizawa, T.; Kamiya, W.; Matsumura, Y.; Anai, M.; Osawa, T.; Fruchart, J.C.; et al. Selective PPARα Modulator Pemafibrate and Sodium-Glucose Cotransporter 2 Inhibitor Tofogliflozin Combination Treatment Improved Histopathology in Experimental Mice Model of Non-Alcoholic Steatohepatitis. Cells 2022, 11, 720. [Google Scholar] [PubMed]
- Ekstedt, M.; Franzen, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Yamaguchi, K.; Moriguchi, M.; et al. Serum alanine aminotransferase predicts the histological course of non-alcoholic steatohepatitis in Japanese patients. Hepatol. Res. 2015, 45, E53–E61. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Kudo, M.; Hirooka, M.; Tsuji, K.; Itobayashi, E.; Kariyama, K.; Ishikawa, T.; Tajiri, K.; Ochi, H.; et al. Albumin-Bilirubin (ALBI) Grade as Part of the Evidence-Based Clinical Practice Guideline for HCC of the Japan Society of Hepatology: A Comparison with the Liver Damage and Child-Pugh Classifications. Liver Cancer 2017, 6, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Miyake, T.; Kuno, A.; Imai, Y.; Sawai, Y.; Hino, K.; Hara, Y.; Hige, S.; Sakamoto, M.; Yamada, G.; et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 2015, 50, 776–784. [Google Scholar]
- Komiya, C.; Tsuchiya, K.; Shiba, K.; Miyachi, Y.; Furuke, S.; Shimazu, N.; Yamaguchi, S.; Kanno, K.; Ogawa, Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS ONE 2016, 11, e0151511. [Google Scholar]
- Ohki, T.; Isogawa, A.; Toda, N.; Tagawa, K. Effectiveness of Ipragliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor, as a Second-line Treatment for Non-Alcoholic Fatty Liver Disease Patients with Type 2 Diabetes Mellitus Who Do Not Respond to Incretin-Based Therapies Including Glucagon-like Peptide-1 Analogs and Dipeptidyl Peptidase-4 Inhibitors. Clin. Drug Investig. 2016, 36, 313–319. [Google Scholar]
- Sumida, Y.; Murotani, K.; Saito, M.; Tamasawa, A.; Osonoi, Y.; Yoneda, M.; Osonoi, T. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective, single-arm trial (LEAD trial). Hepatol. Res. 2019, 49, 64–71. [Google Scholar]
- Kahl, S.; Gancheva, S.; Strassburger, K.; Herder, C.; Machann, J.; Katsuyama, H.; Kabisch, S.; Henkel, E.; Kopf, S.; Lagerpusch, M.; et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care 2020, 43, 298–305. [Google Scholar] [CrossRef]
- Sasaki, Y.; Asahiyama, M.; Tanaka, T.; Yamamoto, S.; Murakami, K.; Kamiya, W.; Matsumura, Y.; Osawa, T.; Anai, M.; Fruchart, J.C.; et al. Pemafibrate, a selective PPARα modulator, prevents non-alcoholic steatohepatitis development without reducing the hepatic triglyceride content. Sci. Rep. 2020, 10, 7818. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Eguchi, Y.; Yoneda, M.; Imajo, K.; Tamaki, N.; Suganami, H.; Nojima, T.; Tanigawa, R.; Iizuka, M.; Iida, Y.; et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2021, 54, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Yamashita, S.; Arai, H.; Yokote, K.; Satoh, J.; Inoguchi, T.; Nakamura, J.; Maegawa, H.; Yoshioka, N.; Tanizawa, Y.; et al. Effects of Pemafibrate, a Novel Selective PPARα Modulator, on Lipid and Glucose Metabolism in Patients With Type 2 Diabetes and Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care 2018, 41, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.S.; Duffy, D.; Gadi, R.; Bloedon, L.T.; Dunbar, R.L.; Wolfe, M.L.; Movva, R.; Shah, A.; Fuki, I.V.; McCoy, M.; et al. Potent and selective PPAR-alpha agonist LY518674 upregulates both ApoA-I production and catabolism in human subjects with the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 140–146. [Google Scholar] [CrossRef]
- Stienstra, R.; Mandard, S.p.; Patsouris, D.; Maass, C.; Kersten, S.; Müller, M. Peroxisome Proliferator-Activated Receptor α Protects against Obesity-Induced Hepatic Inflammation. Endocrinology 2007, 148, 2753–2763. [Google Scholar] [CrossRef]
- Fujita, Y.; Inagaki, N. Update on the efficacy and safety of sodium-glucose cotransporter 2 inhibitors in Asians and non-Asians. J. Diabetes Investig. 2019, 10, 1408–1410. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K.; et al. Effect of Empagliflozin on Liver Fat in Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care 2018, 41, 1801–1808. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Csermely, A.; Beatrice, G.; Targher, G. Sodium-Glucose Cotransporter-2 Inhibitors for Treatment of Nonalcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Metabolites 2020, 11, 22. [Google Scholar] [CrossRef]
- Imamura, T.; Kinugawa, K. Combination Therapy Using Pemafibrate and Dapagliflozin for Metabolic Dysfunction-associated Fatty Liver Disease. Intern. Med. 2022, 62, 1371–1373. [Google Scholar] [CrossRef]
- Pinyopornpanish, K.; Leerapun, A.; Pinyopornpanish, K.; Chattipakorn, N. Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels. Gut Liver 2021, 15, 827–840. [Google Scholar] [CrossRef]
- Khoshbaten, M.; Beheshtirouy, S.; Shayanrad, S.; Gharekhani, A.; Rezaee, H. Comparison of the efficacy of pioglitazone and metformin on ultrasound grade and liver enzymes level in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Drug Res. 2023, 73, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Galiero, R.; Loffredo, G.; Vetrano, E.; Medicamento, G.; Acierno, C.; Rinaldi, L.; Marrone, A.; Salvatore, T.; Monda, M.; et al. Effects of a Combination of Empagliflozin Plus Metformin vs. Metformin Monotherapy on NAFLD Progression in Type 2 Diabetes: The IMAGIN Pilot Study. Biomedicines 2023, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Xu, B.; Li, P.; Weng, J. Statins for the Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Am. J. Ther. 2023, 30, e17–e25. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Petta, S.; Mannisto, V.; Mancina, R.M.; Pipitone, R.; Karja, V.; Maggioni, M.; Kakela, P.; Wiklund, O.; Mozzi, E.; et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J. Hepatol. 2015, 63, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Kargiotis, K.; Athyros, V.G.; Giouleme, O.; Katsiki, N.; Katsiki, E.; Anagnostis, P.; Boutari, C.; Doumas, M.; Karagiannis, A.; Mikhailidis, D.P. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J. Gastroenterol. 2015, 21, 7860–7868. [Google Scholar] [CrossRef]
- Neto-Ferreira, R.; Rocha, V.N.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; de Carvalho, J.J. Pleiotropic effects of rosuvastatin on the glucose metabolism and the subcutaneous and visceral adipose tissue behavior in C57Bl/6 mice. Diabetol. Metab. Syndr. 2013, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: A histopathological follow-up study. J. Hepatol. 2007, 47, 135–141. [Google Scholar] [CrossRef]
- Hyogo, H.; Tazuma, S.; Arihiro, K.; Iwamoto, K.; Nabeshima, Y.; Inoue, M.; Ishitobi, T.; Nonaka, M.; Chayama, K. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism 2008, 57, 1711–1718. [Google Scholar] [CrossRef]
- Ohki, T.; Isogawa, A.; Iwamoto, M.; Ohsugi, M.; Yoshida, H.; Toda, N.; Tagawa, K.; Omata, M.; Koike, K. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. Sci. World J. 2012, 2012, 496453. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Shao, N.; Kuang, H.Y.; Hao, M.; Gao, X.Y.; Lin, W.J.; Zou, W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2014, 30, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Kitajima, Y.; Hyogo, H.; Takahashi, H.; Kojima, M.; Ono, M.; Araki, N.; Tanaka, K.; Yamaguchi, M.; Matsuda, Y.; et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol. Res. 2015, 45, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Irwin, A.; Gardner, C.J.; Daousi, C.; Purewal, T.; Furlong, N.; Goenka, N.; Thomas, E.L.; Adams, V.L.; Pushpakom, S.P.; et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS ONE 2012, 7, e50117. [Google Scholar] [CrossRef] [PubMed]
- Firneisz, G.; Varga, T.; Lengyel, G.; Fehér, J.; Ghyczy, D.; Wichmann, B.; Selmeci, L.; Tulassay, Z.; Rácz, K.; Somogyi, A. Serum dipeptidyl peptidase-4 activity in insulin resistant patients with non-alcoholic fatty liver disease: A novel liver disease biomarker. PLoS ONE 2010, 5, e12226. [Google Scholar] [CrossRef] [PubMed]
- Joy, T.R.; McKenzie, C.A.; Tirona, R.G.; Summers, K.; Seney, S.; Chakrabarti, S.; Malhotra, N.; Beaton, M.D. Sitagliptin in patients with non-alcoholic steatohepatitis: A randomized, placebo-controlled trial. World J. Gastroenterol. 2017, 23, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.A.; Guirguis, E.; Thornby, K.A. A Systematic Review of Newer Antidiabetic Agents in the Treatment of Nonalcoholic Fatty Liver Disease. Ann. Pharmacother. 2021, 55, 65–79. [Google Scholar] [CrossRef] [PubMed]
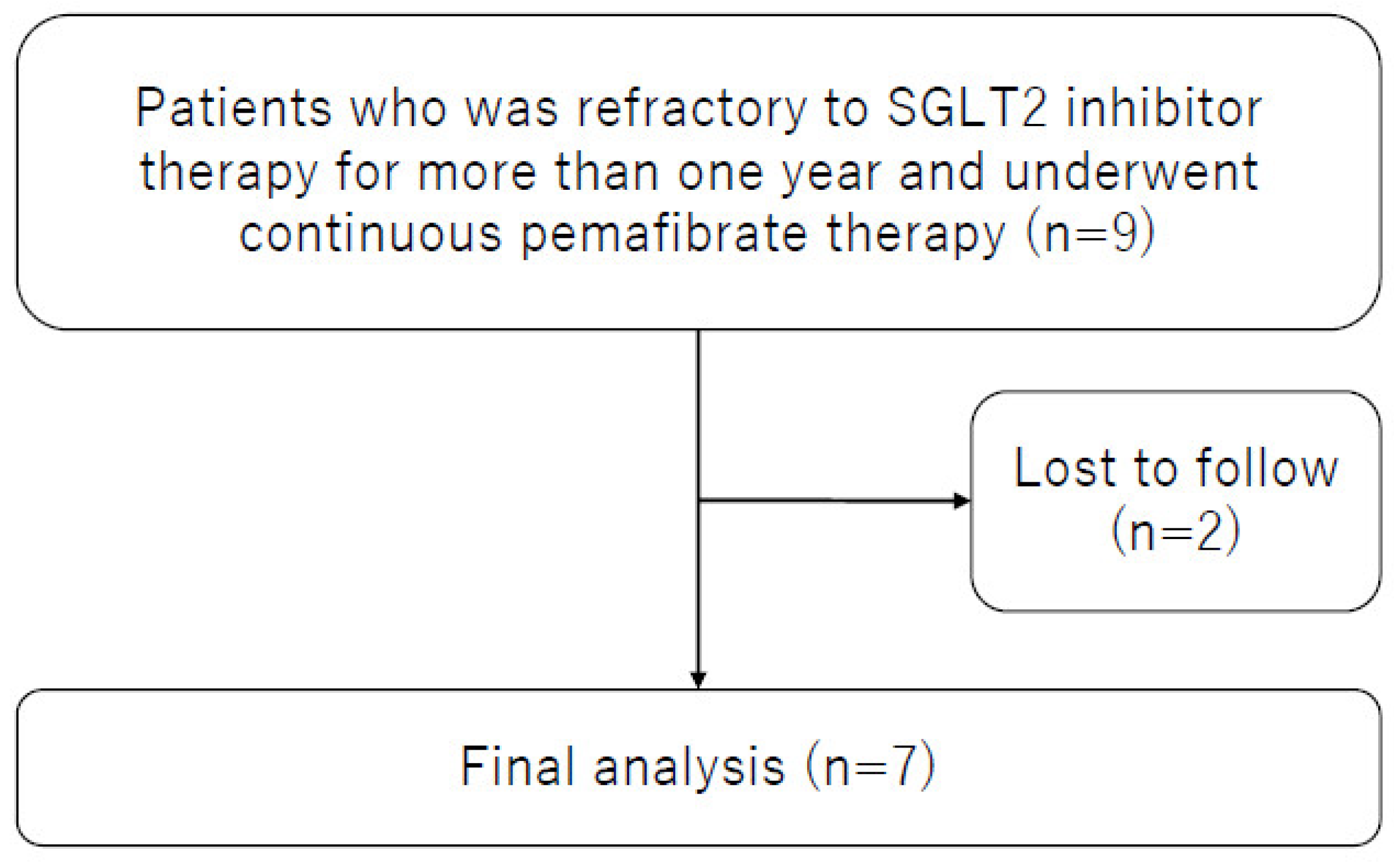
| n = 7 | |
|---|---|
| Age, years, median (range) | 49 (42–68) |
| Gender, male, n | 7 (100%) |
| Current smoker, n | 1 (14%) |
| Complications treated with medications, n | |
| Hypertension | 4 (57%) |
| Gastroesophageal reflux disease | 5 (71%) |
| Diabetes mellitus | 7 (100%) |
| Concurrent medications, n | |
| SGLT2 inhibitor | 7 (100%) |
| Metformin | 3 (43%) |
| Dipeptidyl peptidase 4 inhibitor | 3 (43%) |
| Statins | 2 (29%) |
| Glucagon-like peptide-1 receptor agonist | 1 (14%) |
| Angiotensin II receptor blocker | 0 (0%) |
| Interval from starting SGLT2 inhibitor to starting pemafibrate, day, median (range) | 845 (406–1288) |
| Baseline | One Year Later | p-Value | Reference Value | |
|---|---|---|---|---|
| Weight, kg, median (range) | 73.3 (66.2–112.1) | 74.6 (64.6–114.8) | 0.937 | - |
| Body mass index | 28.3 (22.2–37.4) | 27.8 (21.7–38.3) | 1.000 | - |
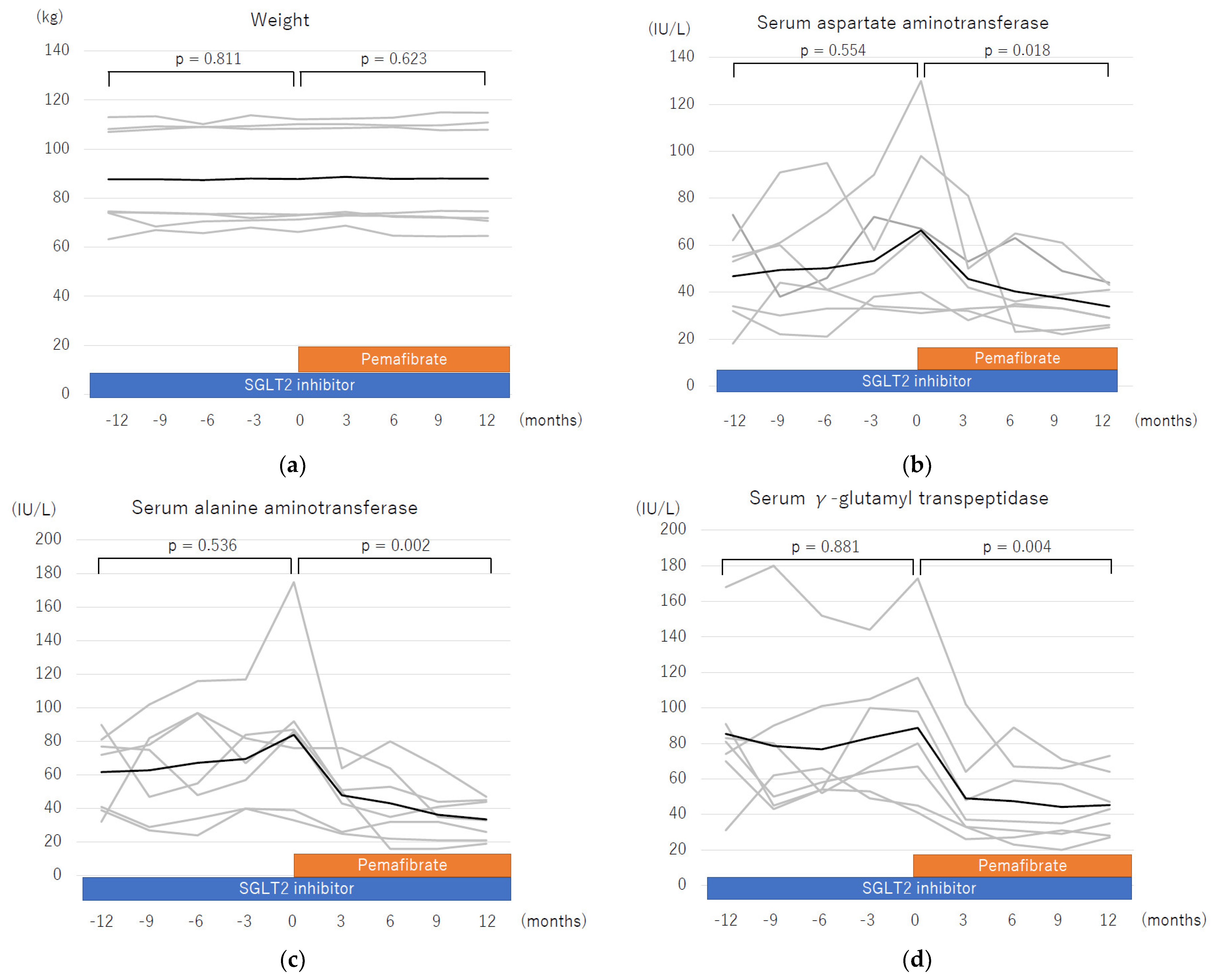
| AST, U/L | 65 (31–130) | 29 (25–44) | 0.015 | 10–40 |
| ALT, U/L | 86 (33–175) | 33 (19–47) | 0.015 | 5–45 |
| γ-GTP, U/L | 80 (41–173) | 43 (27–73) | 0.015 | M ≦ 80 F ≦30 |
| Platelet count, ×104/μL | 22.9 (11.1–31.9) | 22.5 (13.2–26.5) | 0.937 | 14.0–34.0 |
| Estimated GFR, mL/min/1.73 m2 | 73.9 (63.8–89.7) | 64.1 (54.3–92.9) | 0.296 | - |
| LDL cholesterol, mg/dL | 122 (103–190) | 114 (64–169) | 0.132 | 65–139 |
| HDL cholesterol, mg/dL | 45 (30–62) | 51 (34–65) | 0.156 | M 40–85 F 40–95 |
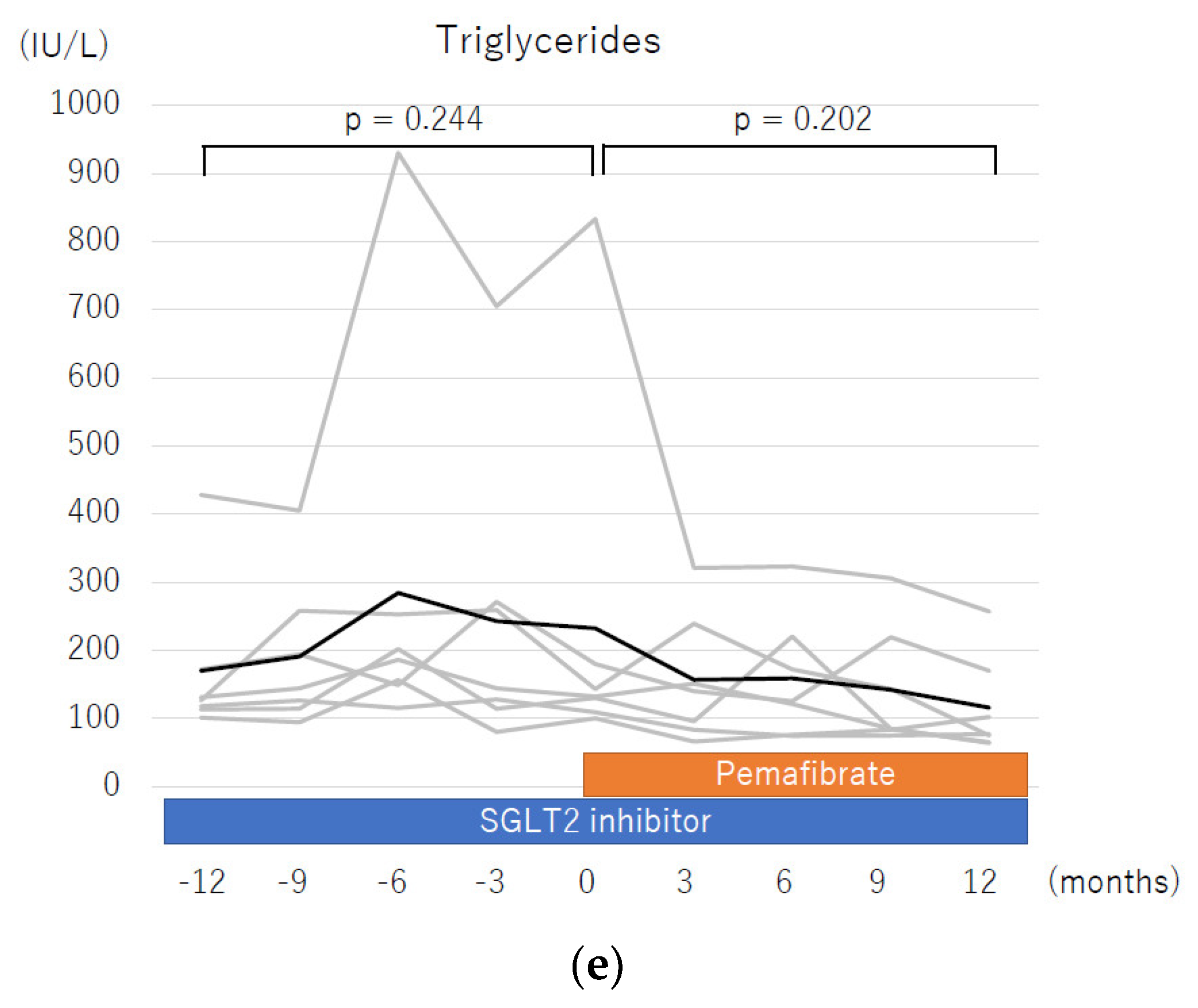
| Triglyceride, mg/dL | 132 (100–833) | 77 (64–257) | 0.031 | 30–149 |
| Uric acid, mg/dL | 5.9 (3.8–7) | 5.9 (4.2–6.8) | 0.687 | M 3.8–7.0 F 2.5–7.0 |
| Fasting plasma glucose, mg/dL | 130 (100–198) | 112 (94–215) | 0.578 | <110 |
| Serum insulin, µU/mL | 9.8 (1.2–14) | 5.5 (2.6–47.6) | 1.000 | 1.7–10.4 |
| Hemoglobin A1c, % | 6.8 (6.2–7.6) | 6.5 (6.2–7.8) | 0.765 | 4.6–6.2 |
| Total bilirubin, mg/dL | 1.0 (0.4–1.5) | 1.0 (0.3–1.3) | 0.218 | 0.2–1.2 |
| Serum albumin, g/dL | 4.4 (4.2–4.6) | 4.5 (4.2–4.9) | 0.250 | 3.8–5.2 |
| ALBI score | −2.9 (−3.1–2.7) | −3.1 (−3.4–2.8) | 0.031 | ≦−2.6 |
| M2BPGi | 0.73 (0.4–2.32) | 0.62 (0.34–1.66) | 0.046 | <1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinozaki, S.; Tahara, T.; Miura, K.; Lefor, A.K.; Yamamoto, H. Effectiveness of One-Year Pemafibrate Therapy on Non-Alcoholic Fatty Liver Disease Refractory to Long-Term Sodium Glucose Cotransporter-2 Inhibitor Therapy: A Pilot Study. Life 2023, 13, 1327. https://doi.org/10.3390/life13061327
Shinozaki S, Tahara T, Miura K, Lefor AK, Yamamoto H. Effectiveness of One-Year Pemafibrate Therapy on Non-Alcoholic Fatty Liver Disease Refractory to Long-Term Sodium Glucose Cotransporter-2 Inhibitor Therapy: A Pilot Study. Life. 2023; 13(6):1327. https://doi.org/10.3390/life13061327
Chicago/Turabian StyleShinozaki, Satoshi, Toshiyuki Tahara, Kouichi Miura, Alan Kawarai Lefor, and Hironori Yamamoto. 2023. "Effectiveness of One-Year Pemafibrate Therapy on Non-Alcoholic Fatty Liver Disease Refractory to Long-Term Sodium Glucose Cotransporter-2 Inhibitor Therapy: A Pilot Study" Life 13, no. 6: 1327. https://doi.org/10.3390/life13061327
APA StyleShinozaki, S., Tahara, T., Miura, K., Lefor, A. K., & Yamamoto, H. (2023). Effectiveness of One-Year Pemafibrate Therapy on Non-Alcoholic Fatty Liver Disease Refractory to Long-Term Sodium Glucose Cotransporter-2 Inhibitor Therapy: A Pilot Study. Life, 13(6), 1327. https://doi.org/10.3390/life13061327








