Transcranial Focal Electric Stimulation Avoids P-Glycoprotein Over-Expression during Electrical Amygdala Kindling and Delays Epileptogenesis in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experiment 1: Evaluation of P-gp Expression during EAK-Induced Epileptogenesis
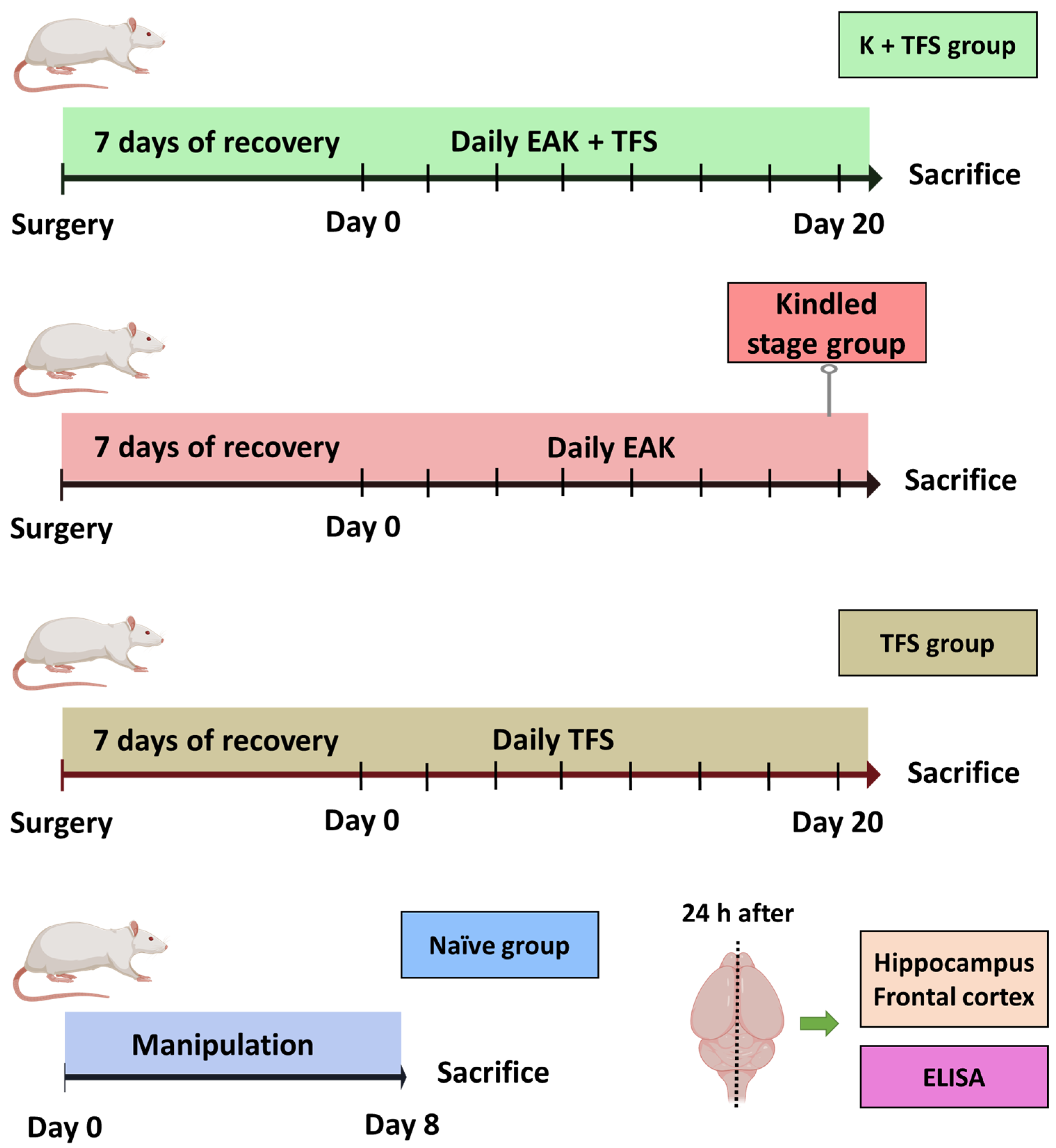
2.2.1. Experimental Groups
2.2.2. ADT, EAK, and EEG
2.2.3. Western Blotting
2.3. Experiment 2: Evaluation of TFS on the EAK-Induced P-gp Overexpression
Experimental Groups
2.4. Statistical Analysis
3. Results
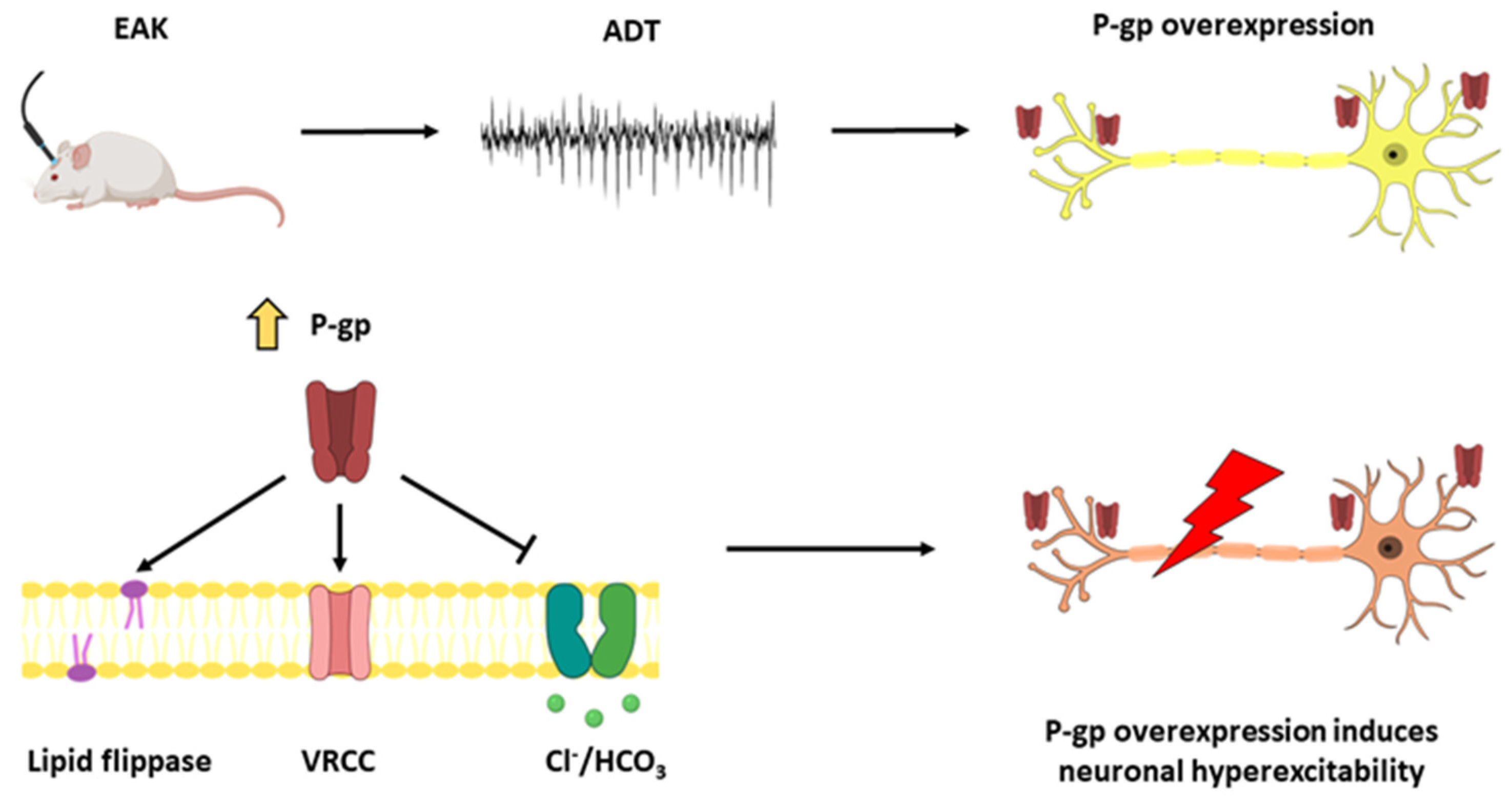
3.1. EAK-Induced Epileptogenesis Is Associated with P-gp Overexpression
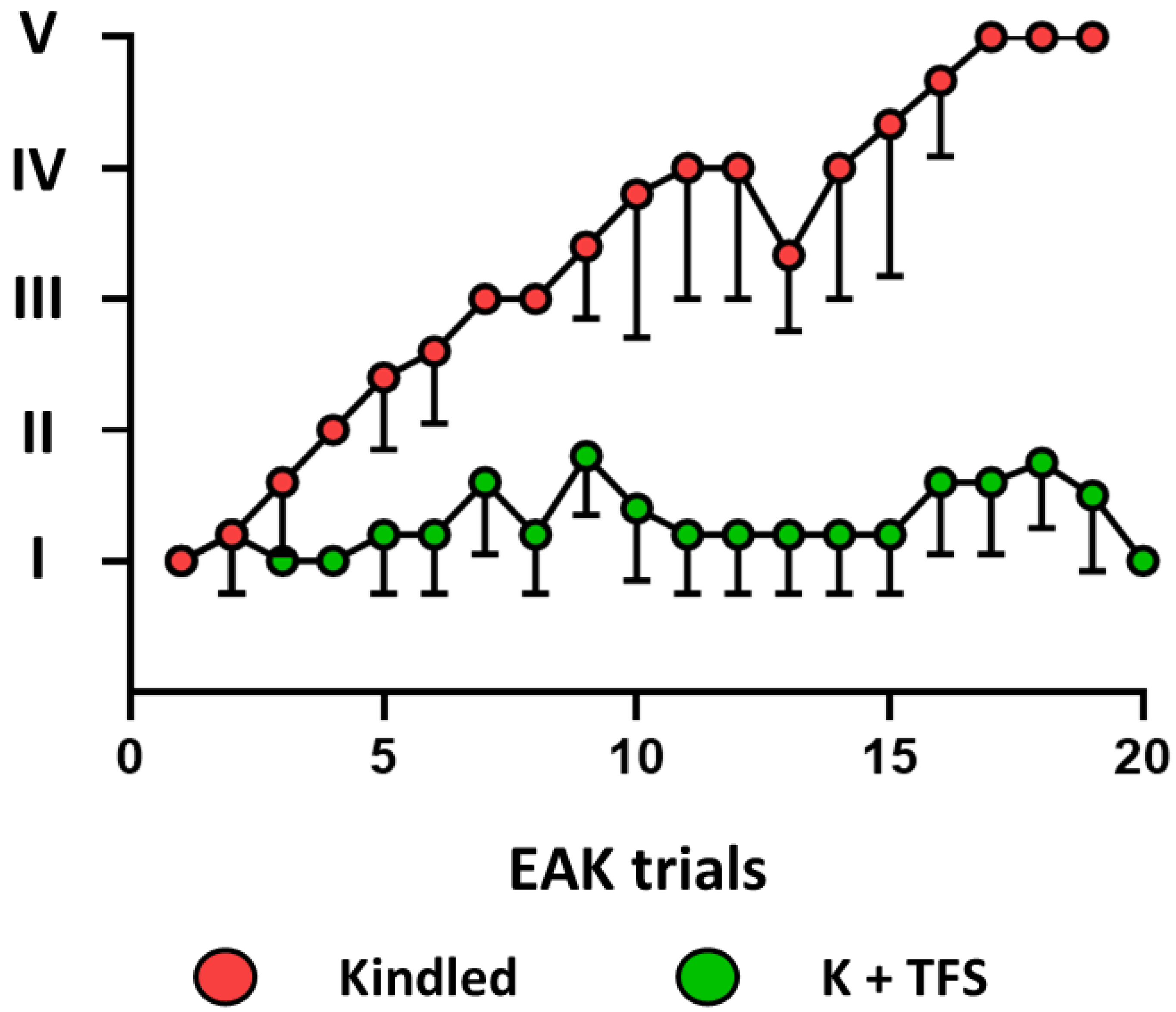
3.2. TFS Avoids P-gp Overexpression during the EAK-Induced Epileptogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC Efflux Transporters at Blood-Brain Barrier in Health and Neurological Disorders. Brain Res. 2015, 1628, 298–316. [Google Scholar] [CrossRef] [PubMed]
- Fardel, O.; Lecureur, V.; Guillouzo, A. The P-Glycoprotein Multidrug Transporter. Gen. Pharmac. 1996, 27, 1283–1291. [Google Scholar] [CrossRef]
- Lazarowski, A.; Czornyj, L.; Lubienieki, F.; Girardi, E.; Vazquez, S.; D’Giano, C. ABC Transporters during Epilepsy and Mechanisms Underlying Multidrug Resistance in Refractory Epilepsy. Epilepsia 2007, 48, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, É.; Demeule, M.; Ghitescu, L.; Béliveau, R. P-Glycoprotein Is Strongly Expressed in the Luminal Membranes of the Endothelium of Blood Vessels in the Brain. Biochem. J. 1997, 326, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Cordon-Cardo, C.; O’Brien, J.P.; Casals, D.; Rittman-Grauer, L.; Biedler, J.L.; Melamed, M.R.; Bertino, J.R. Multidrug-Resistance Gene (P-Glycoprotein) Is Expressed by Endothelial Cells at Blood-Brain Barrier Sites. Proc. Natl. Acad. Sci. USA 1989, 86, 695–698. [Google Scholar] [CrossRef]
- Golden, P.L.; Pardridge, W.M. P-Glycoprotein on Astrocyte Foot Processes of Unfixed Isolated Human Brain Capillaries. Brain Res. 1999, 819, 143–146. [Google Scholar] [CrossRef]
- Rao, V.V.; Dahlheimer, J.L.; Bardgett, M.E.; Snyder, A.Z.; Finch, R.A.; Sartorelli, A.C.; Piwnica-Worms, D. Choroid Plexus Epithelial Expression of MDR1 P Glycoprotein and Multidrug Resistance-Associated Protein Contribute to the Blood-Cerebrospinal-Fluid Drug-Permeability Barrier. Proc. Natl. Acad. Sci. USA 1999, 96, 3900–3905. [Google Scholar] [CrossRef]
- Schlachetzki, F.; Pardridge, W.M. P-Glycoprotein and Caveolin-1α in Endothelium and Astrocytes of Primate Brain. Neuroreport 2003, 14, 695–698. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Wagenaar, E.; Mol, C.A.A.M.; Van Deemter, L. P-Glycoprotein in the Blood-Brain Barrier of Mice Influences the Brain Penetration and Pharmacological Activity of Many Drugs. J. Clin. Investig. 1996, 97, 2517–2524. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Wagenaar, E.; Van Deemter, L.; Mol, C.A.A.M.; Borst, P. Absence of the Mdr1a P-Glycoprotein in Mice Affects Tissue Distribution and Pharmacokinetics of Dexamethasone, Digoxin, and Cyclosporin A. J. Clin. Investig. 1995, 96, 1698–1705. [Google Scholar] [CrossRef]
- Aronica, E.; Sisodiya, S.M.; Gorter, J.A. Cerebral Expression of Drug Transporters in Epilepsy. Adv. Drug Deliv. Rev. 2012, 64, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, A.; Sevlever, G.; Taratuto, A.; Massaro, M.; Rabinowicz, A. Tuberous Sclerosis Associated with MDR1 Gene Expression and Drug- Resistant Epilepsy. Pediatr. Neurol. 1999, 21, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Friedman, A. Structural, Molecular, and Functional Alterations of the Blood-brain Barrier during Epileptogenesis and Epilepsy: A Cause, Consequence, or Both? Int. J. Mol. Sci. 2020, 21, 591. [Google Scholar] [CrossRef] [PubMed]
- Sisodiya, S.M.; Lin, W.R.; Harding, B.N.; Squier, M.V.; Thom, M. Drug Resistance in Epilepsy: Expression of Drug Resistance Proteins in Common Causes of Refractory Epilepsy. Brain 2002, 125, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tishler, D.M.; Weinberg, K.I.; Hinton, D.R.; Barbaro, N.; Annett, G.M.; Raffel, C. MDR1 Gene Expression in Brain of Patients with Medically Intractable Epilepsy. Epilepsia 1995, 36, 1–6. [Google Scholar] [CrossRef]
- Fonseca-Barriendos, D.; Pérez-Pérez, D.; Fuentes-Mejía, M.; Orozco-Suárez, S.; Alonso-Vanegas, M.; Martínez-Juárez, I.E.; Guevara-Guzmán, R.; Castañeda-Cabral, J.L.; Rocha, L. Protein Expression of P-Glycoprotein in Neocortex from Patients with Frontal Lobe Epilepsy. Epilepsy Res. 2022, 181, 106892. [Google Scholar] [CrossRef]
- Lazarowski, A.; Ramos, A.J.; García-Rivello, H.; Brasco, A.; Girardi, E. Neuronal and Glial Expression of the Multidrug Resistance Gene Product in an Experimental Epilepsy Model. Cell Mol. Neurobiol. 2004, 24, 77–85. [Google Scholar] [CrossRef]
- Marchi, N.; Hallene, K.L.; Kight, K.M.; Cucullo, L.; Moddel, G.; Bingaman, W.; Dini, G.; Vezzani, A.; Janigro, D. Significance of MDR1 and Multiple Drug Resistance in Refractory Human Epileptic Brain. BMC Med. 2004, 2, 37. [Google Scholar] [CrossRef]
- Merelli, A.; Ramos, A.J.; Lazarowski, A.; Auzmendi, J. Convulsive Stress Mimics Brain Hypoxia and Promotes the P-Glycoprotein (P-Gp) and Erythropoietin Receptor Overexpression. Recombinant Human Erythropoietin Effect on P-Gp Activity. Front Neurosci. 2019, 13, 750. [Google Scholar] [CrossRef]
- Wang, G.; Xie, G.; Han, L.; Wang, D.; Du, F.; Kong, X.; Su, G. Involvement of Hypoxia-Inducible Factor-1 Alpha in the Upregulation of P-Glycoprotein in Refractory Epilepsy. Neuroreport 2019, 30, 1191–1196. [Google Scholar] [CrossRef]
- Bauer, B.; Hartz, A.M.S.; Miller, D.S. Tumor Necrosis Factor α and Endothelin-1 Increase P-Glycoprotein Expression and Transport Activity at the Blood-Brain Barrier. Mol. Pharm. 2007, 71, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Hartz, A.M.S.; Pekcec, A.; Toellner, K.; Miller, D.S.; Potschka, H. Seizure-Induced up-Regulation of P-Glycoprotein at the Blood-Brain Barrier through Glutamate and Cyclooxygenase-2 Signaling. Mol. Pharm. 2008, 73, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Bankstahl, J.P.; Hoffmann, K.; Bethmann, K.; Löscher, W. Glutamate Is Critically Involved in Seizure-Induced Overexpression of P-Glycoprotein in the Brain. Neuropharmacology 2008, 54, 1006–1016. [Google Scholar] [CrossRef]
- Bateman, L.M.; Li, C.S.; Seyal, M. Ictal Hypoxemia in Localization-Related Epilepsy: Analysis of Incidence, Severity and Risk Factors. Brain 2008, 131, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Wallace, G.C.; Holmes, C.; McDowell, M.L.; Smith, J.A.; Marshall, J.D.; Bonilha, L.; Edwards, J.C.; Glazier, S.S.; Ray, S.K.; et al. Hippocampal Tissue of Patients with Refractory Temporal Lobe Epilepsy Is Associated with Astrocyte Activation, Inflammation, and Altered Expression of Channels and Receptors. Neuroscience 2012, 220, 237–246. [Google Scholar] [CrossRef]
- During, M.J.; Spencer, D.D. Extracellular Hippocampal Glutamate and Spontaneous Seizure in the Conscious Human Brain. Lancet 1993, 341, 1607–1610. [Google Scholar] [CrossRef]
- Moseley, B.D.; Nickels, K.; Britton, J.; Wirrell, E. How Common Is Ictal Hypoxemia and Bradycardia in Children with Partial Complex and Generalized Convulsive Seizures? Epilepsia 2010, 51, 1219–1224. [Google Scholar] [CrossRef]
- Vezzani, A.; Granata, T. Brain Inflammation in Epilepsy: Experimental and Clinical Evidence. Epilepsia 2005, 46, 1724–1743. [Google Scholar] [CrossRef] [PubMed]
- Besio, W.; Hadidi, R.; Makeyev, O.; Luna-Munguía, H.; Rocha, L. Electric Fields in Hippocampus Due to Transcranial Focal Electrical Stimulation via Concentric Ring Electrodes. In Proceedings of the 33rd Annual International Conference of the IEEE EMBS, Boston, MA, USA, 30 August–3 September 2011; pp. 5488–5491. [Google Scholar]
- Besio, W.; Sharma, V.; Spaulding, J. The Effects of Concentric Ring Electrode Electrical Stimulation on Rat Skin. Ann. Biomed. Eng. 2010, 38, 1111–1118. [Google Scholar] [CrossRef]
- Luby, M.D.; Makeyev, O.; Besio, W. Chronic Transcranial Focal Stimulation from Tripolar Concentric Ring Electrodes Does Not Disrupt Memory Formation in Rats. In Proceedings of the Annual Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 6139–6142. [Google Scholar]
- Mucio-Ramírez, S.; Makeyev, O. Safety of the Transcranial Focal Electrical Stimulation via Tripolar Concentric Ring Electrodes for Hippocampal CA3 Subregion Neurons in Rats. J. Heal. Eng 2017, 2017, 4302810. [Google Scholar] [CrossRef]
- van Oosterom, A.; Strackee, J. Computing the Lead Field of Electrodes with Axial Symmetry. Med. Biol. Eng. Comput. 1983, 21, 473–481. [Google Scholar] [CrossRef]
- Besio, W.G.; Koka, K.; Cole, A.J. Effects of Noninvasive Transcutaneous Electrical Stimulation via Concentric Ring Electrodes on Pilocarpine-Induced Status Epilepticus in Rats. Epilepsia 2007, 48, 2273–2279. [Google Scholar] [CrossRef]
- Besio, W.; Gale, K.N.; Medvedev, A.V. Possible Therapeutic Effects of Transcutaneous Electrical Stimulation via Concentric Ring Electrodes. Epilepsia 2010, 51, 85–87. [Google Scholar] [CrossRef]
- Besio, W.; Liu, X. Transcutaneous Focal Electrical Stimulation via Concentric Ring Electrodes Reduces Synchrony Induced by Pentilenetetrazole in Beta and Gamma Bands in Rats. Int. J. Neural Syst. 2011, 21, 139–149. [Google Scholar] [CrossRef]
- Makeyev, O.; Luna-Munguía, H.; Rogel-Salazar, G.; Liu, X.; Besio, W. Noninvasive Transcranial Focal Stimulation via Tripolar Concentric Ring Electrodes Lessens Behavioral Seizure Activity of Recurrent Pentylenetetrazole Administrations in Rats. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 383–390. [Google Scholar] [CrossRef]
- Pérez-Pérez, D.; Castañeda-Cabral, J.L.; Orozco-Suárez, S.; Sotelo, J.; Besio, W.; Rocha, L. Noninvasive Transcranial Focal Stimulation Affects the Convulsive Seizure-Induced P-Glycoprotein Expression and Function in Rats. Epilepsy Behav. 2021, 115, 107659. [Google Scholar] [CrossRef]
- Santana-Gómez, C.E.; Alcántara-González, D.; Luna-Munguía, H.; Bañuelos-Cabrera, I.; Magdaleno-Madrigal, V.M.; Fernández-Mas, R.; Besio, W.; Rocha, L. Transcranial Focal Electrical Stimulation Reduces the Convulsive Expression and Amino Acid Release in the Hippocampus during Pilocarpine-Induced Status Epilepticus in Rats. Epilepsy Behav. 2015, 49, 33–39. [Google Scholar] [CrossRef]
- Valdés-Cruz, A.; Villasana-Salazar, B.; Williams, B.; Martínez-Vargas, D.; Magdaleno-Madrigal, V.M.; Almazán-Alvarado, S.; Besio, W. Transcranial Focal Electrical Stimulation via Concentric Ring Electrodes in Freely Moving Cats: Antiepileptogenic and Postictal Effects. Exp. Neurol. 2019, 320, 113012. [Google Scholar] [CrossRef]
- Auzmendi, J.; Orozco-Suárez, S.; Bañuelos-Cabrera, I.; Eva González-Trujano, M.; Calixto González, E.; Rocha, L.; Lazarowski, A. P-Glycoprotein Contributes to Cell Membrane Depolarization of Hippocampus and Neocortex in a Model of Repetitive Seizures Induced by Pentylenetetrazole in Rats. Curr. Pharm. Des. 2013, 19, 6732–6738. [Google Scholar] [CrossRef]
- Hoffman, M.M.; Wei, L.-Y.; Roepe, P.D. Are Altered PHi and Membrane Potential in Hu MDR 1 Transfectants Sufficient to Cause MDR Protein-Mediated Multidrug Resistance? J. Gen. Physiol. 1996, 108, 295–313. [Google Scholar] [CrossRef]
- Luz, J.G.; Wei, L.-Y.; Basu, S.; Roepe, P.D. Transfection of Mu MDR 1 Inhibits Na+ -Independent Cl-/-HCO3 Exchange in Chinese Hamster Ovary Cells. Am. Chem. Soc. 1994, 33, 7239–7248. [Google Scholar] [CrossRef]
- Romsicki, Y.; Sharom, F.J. Phospholipid Flippase Activity of the Reconstituted P-Glycoprotein Multidrug Transporter. Biochemistry 2001, 40, 6937–6947. [Google Scholar] [CrossRef]
- Valverde, M.A.; Díaz, M.; Sepúlveda, F.V.; Gill, D.R.; Hyde, S.C.; Higgins, C.F. Volume-Regulated Chloride Channels Associated with the Human Multidrug-Resistance P-Glycoprotein. Nature 1992, 355, 830–833. [Google Scholar] [CrossRef]
- Blumenfeld, H.; Rivera, M.; Vasquez, J.G.; Shah, A.; Ismail, D.; Enev, M.; Zaveri, H.P. Neocortical and Thalamic Spread of Amygdala Kindled Seizures. Epilepsia 2007, 48, 254–262. [Google Scholar] [CrossRef]
- Cavazos, J.E.; Golarai, G.; Sutula, T.P. Mossy Fiber Synaptic Reorganization Induced by Kindling: Time Course of Development, Progression, and Permanence. J. Neurosci. 1991, 11, 2795–2803. [Google Scholar] [CrossRef]
- Hewapathirane, D.S.; Burnham, W.M.I. Propagation of Amygdala-Kindled Seizures to the Hippocampus in the Rat: Electroencephalographic Features and Behavioural Correlates. Neurosci. Res. 2005, 53, 369–375. [Google Scholar] [CrossRef]
- Löscher, W.; Ebert, U. Basic Mechanisms of Seizure Propagation: Targets for Rational Drug Design and Rational Polypharmacy. Epilepsy Res. Suppl. 1996, 11, 17–43. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006; ISBN 9780080475158. [Google Scholar]
- Loscher, W.; Rundfeldt, C. Kindling as a Model of Drug-Resistant Partial Epilepsy: Selection of Phenytoin-Resistant and Nonresistant Rats. J. Pharmacol. Exp. Ther. 1991, 258, 483–489. [Google Scholar]
- Racine, R.J. Modification of Seizures Activity by Electrical Stimulation: Motor Seizure. Electroencephalogr. Clin. Neurophisiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Valdés-Cruz, A.; Negrete-Díaz, J.V.; Magdaleno-Madrigal, V.M.; Martínez-Vargas, D.; Fernández-Mas, R.; Almazán-Alvarado, S.; Torres-García, M.E.; Flores, G. Electroencephalographic Activity in Neonatal Ventral Hippocampus Lesion in Adult Rats. Synapse 2012, 66, 738–746. [Google Scholar] [CrossRef]
- Ghosh, R.; Gilda, J.E.; Gomes, A.V. The Necessity of and Strategies for Improving Confidence in the Accuracy of Western Blots. Expert Rev. Proteom. 2014, 11, 549–560. [Google Scholar] [CrossRef]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-Linked Immunosorbent Assay for the Quantitative/Qualitative Analysis of Plant Secondary Metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef]
- Potschka, H.; Löscher, W. In Vivo Evidence for P-Glycoprotein-Mediated Transport of Phenytoin at the Blood-Brain Barrier of Rats. Epilepsia 2001, 42, 1231–1240. [Google Scholar] [CrossRef]
- Potschka, H.; Fedrowitz, M.; Löscher, W. P-Glycoprotein and Multidrug Resistance-Associated Protein Are Involved in the Regulation of Extracellular Levels of the Major Antiepileptic Drug Carbamazepine in the Brain. Neuroreport 2001, 12, 3557–3560. [Google Scholar] [CrossRef]
- Potschka, H.; Fedrowitz, M.; Löscher, W. P-Glycoprotein-Mediated Efflux of Phenobarbital, Lamotrigine, and Felbamate at the Blood-Brain Barrier: Evidence from Microdialysis Experiments in Rats. Neurosci. Lett. 2002, 327, 173–176. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Cheng, H.; Guo, Y.; Xu, C.; Wang, S.; Zhang, J.; Ding, M.; Chen, Z. Low-Frequency Stimulation Inhibits Epileptogenesis by Modulating the Early Network of the Limbic System as Evaluated in Amygdala Kindling Model. Brain Struct. Funct. 2014, 219, 1685–1696. [Google Scholar] [CrossRef]
- Dalsgaard, M.K.; Madsen, F.F.; Secher, N.H.; Laursen, H.; Quistorff, B. High Glycogen Levels in the Hippocampus of Patients with Epilepsy. J. Cereb. Blood Flow Metab. 2007, 27, 1137–1141. [Google Scholar] [CrossRef]
- Dienel, G.A.; Gillinder, L.; McGonigal, A.; Borges, K. Potential New Roles for Glycogen in Epilepsy. Epilepsia 2023, 64, 29–53. [Google Scholar] [CrossRef]
- Rothman, D.L.; Dienel, G.A.; Behar, K.L.; Hyder, F.; DiNuzzo, M.; Giove, F.; Mangia, S. Glucose Sparing by Glycogenolysis (GSG) Determines the Relationship between Brain Metabolism and Neurotransmission. J. Cereb. Blood Flow Metab. 2022, 42, 844–860. [Google Scholar] [CrossRef]
- DiNuzzo, M.; Giove, F.; Maraviglia, B.; Mangia, S. Computational Flux Balance Analysis Predicts That Stimulation of Energy Metabolism in Astrocytes and Their Metabolic Interactions with Neurons Depend on Uptake of K+ Rather than Glutamate. Neurochem. Res. 2017, 42, 202–216. [Google Scholar] [CrossRef]
- Rahman, B.; Kussmaul, L.; Hamprecht, B.; Dringen, R. Glycogen Is Mobilized during the Disposal of Peroxides by Cultured Astroglial Cells from Rat Brain. Neurosci. Lett. 2000, 290, 169–172. [Google Scholar] [CrossRef]
- Swanson, R.A.; Choi, D.W. Glial Glycogen Stores Affect Neuronal Survival during Glucose Deprivation in Vitro. J. Cereb. Blood Flow Metab. 1993, 13, 162–169. [Google Scholar] [CrossRef]
- Rocha, L.; Briones, M.; Ackermann, R.F.; Anton, B.; Maidment, N.T.; Evans, C.J.; Engel, J. Pentylenetetrazol-Induced Kindling: Early Involvement of Excitatory and Inhibitory Systems. Epilepsy Res. 1996, 26, 105–113. [Google Scholar] [CrossRef]
- Minamoto, Y.; Itano, T.; Tokuda, M.; Matsui, H.; Janjua, N.A.; Hosokawa, K.; Okada, Y.; Murakami, T.H.; Negi, T.; Hatase, O. In Vivo Microdialysis of Amino Acid Neurotransmitters in the Hippocampus in Amygdaloid Kindled Rat. Brain Res. 1992, 573, 345–348. [Google Scholar] [CrossRef]
- Corcoran, M.E.; Urstad, H.; Mccaughran, J.A.; Wada, J.A. Frontal Lobe and Kindling in the Rat. Can. J. Neurol. Sci./J. Can. Des Sci. Neurol. 1975, 2, 501–508. [Google Scholar] [CrossRef]
- Batten, S.R.; Matveeva, E.A.; Whiteheart, S.W.; Vanaman, T.C.; Gerhardt, G.A.; Slevin, J.T. Linking Kindling to Increased Glutamate Release in the Dentate Gyrus of the Hippocampus through the STXBP5/Tomosyn-1 Gene. Brain Behav. 2017, 7, e00795. [Google Scholar] [CrossRef]
- Kaura, S.; Bradford, H.F.; Young, A.M.J.; Croucher, M.J.; Hughes, P.D. Effect of Amygdaloid Kindling on the Content and Release of Amino Acids from the Amygdaloid Complex: In Vivo and In Vitro Studies. J. Neurochem. 1995, 65, 1240–1249. [Google Scholar] [CrossRef]
- Luna-Munguia, H.; Orozco-Suarez, S.; Rocha, L. Effects of High Frequency Electrical Stimulation and R-Verapamil on Seizure Susceptibility and Glutamate and GABA Release in a Model of Phenytoin-Resistant Seizures. Neuropharmacology 2011, 61, 807–814. [Google Scholar] [CrossRef]
- Czornyj, L.; Auzmendi, J.; Lazarowski, A. Transporter Hypothesis in Pharmacoresistant Epilepsies Is It at the Central or Peripheral Level? Epilepsia Open 2021, 7, S34–S46. [Google Scholar] [CrossRef]
- Seegers, U.; Potschka, H.; Löscher, W. Expression of the Multidrug Transporter P-Glycoprotein in Brain Capillary Endothelial Cells and Brain Parenchyma of Amygdala-Kindled Rats. Epilepsia 2002, 43, 675–684. [Google Scholar] [CrossRef]
- Volk, H.; Potschka, H.; Löscher, W. Increased Expression of the Multidrug Transporter P-Glycoprotein in Limbic Brain Regions after Amygdala-Kindled Seizures in Rats. Epilepsy Res. 2004, 58, 67–79. [Google Scholar] [CrossRef]
- Volk, H.; Potschka, H.; Löscher, W. Immunohistochemical Localization of P-Glycoprotein in Rat Brain and Detection of Its Increased Expression by Seizures Are Sensitive to Fixation and Staining Variables. J. Histochem. Cytochem. 2005, 53, 517–531. [Google Scholar] [CrossRef]
- Peterson, D.W.; Collins, J.F.; Bradford, H.F. The kindled amygdala model of epilepsy: Anticonvulsant action of amino acid antagonists. Brain Res. 1983, 275, 169–172. [Google Scholar] [CrossRef]
- Sato, K.; Morimoto, K.; Okamoto, M. Anticonvulsant action of a non-competitive antagonist of nmda receptors (MK-801) in the kindling model of epilepsy. Brain Res. 1988, 463, 12–20. [Google Scholar] [CrossRef]
- Bikson, M.; Lian, J.; Hahn, P.J.; Stacey, W.C.; Sciortino, C.; Durand, D.M. Suppression of Epileptiform Activity by High Frequency Sinusoidal Fields in Rat Hippocampal Slices. J. Physiol. 2001, 531, 181–191. [Google Scholar] [CrossRef]
- Jensen, A.L.; Durand, D.M. High Frequency Stimulation Can Block Axonal Conduction. Exp. Neurol. 2009, 220, 57–70. [Google Scholar] [CrossRef]
- Pérez-Pérez, D.; Feria-Romero, I.; Besio, W.; Rocha, L.; Bautista-Orozco, L.; Orozco-Suárez, S. Transcranial Focal Stimulation Modies the Genetic Expression in the Cerebral Cortex and the Hippocampus of Healthy Rats. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xu, Z.; Xu, C.; Ying, X.; Wang, S.; Zhang, S.; Xiao, B.; Chen, Z. Consecutive 15 Min Is Necessary for Focal Low Frequency Stimulation to Inhibit Amygdaloid-Kindling Seizures in Rats. Epilepsy Res. 2013, 106, 47–53. [Google Scholar] [CrossRef]
- Wu, D.C.; Xu, Z.H.; Wang, S.; Fang, Q.; Hu, D.Q.; Li, Q.; Sun, H.L.; Zhang, S.H.; Chen, Z. Time-Dependent Effect of Low-Frequency Stimulation on Amygdaloid-Kindling Seizures in Rats. Neurobiol. Dis. 2008, 31, 74–79. [Google Scholar] [CrossRef]
- Feldmann, M.; Asselin, M.C.; Liu, J.; Wang, S.; McMahon, A.; Anton-Rodriguez, J.; Walker, M.; Symms, M.; Brown, G.; Hinz, R.; et al. P-Glycoprotein Expression and Function in Patients with Temporal Lobe Epilepsy: A Case-Control Study. Lancet Neurol. 2013, 12, 777–785. [Google Scholar] [CrossRef]
- Elkhayat, H.A.; Aly, R.H.; Elagouza, I.A.; El-Kabarity, R.H.; Galal, Y.I. Role of P-Glycoprotein Inhibitors in Children with Drug-Resistant Epilepsy. Acta Neurol. Scand. 2017, 136, 639–644. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Stewart, G.R.; Abrams, D.J.; Sharan, A. Prevalence and Incidence of Drug-Resistant Mesial Temporal Lobe Epilepsy in the United States. World Neurosurg. 2017, 99, 662–666. [Google Scholar] [CrossRef]



| Site | P-gp Expression | Kindling Trials | Stage I | Stage II | Stage III | Stage IV | Stage V | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq | Dur | Freq | Dur | Freq | Dur | Freq | Dur | Freq | Dur | |||
| Ipsilateral | Hippocampus | −0.791 | −0.1 | 0.8 | −0.4 | 0.6 | 0.3 | −0.3 | −0.5 | −0.8 | −0.4 | −0.5 |
| Cortex | −0.316 | −0.7 | 0.1 | −0.4 | 0.6 | −0.6 | −0.4 | −0.6 | −0.6 | 0.5 | −0.1 | |
| Contralateral | Hippocampus | 0.316 | −0.8 | −0.6 | −0.8 | −0.2 | −0.9 | −0.1 | 0.1 | 0.1 | 0.96 | 0.6 |
| Cortex | 0 | −0.6 | −0.2 | −0.4 | 0.6 | −0.7 | −0.3 | −0.5 | −0.3 | 0.6 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca-Barriendos, D.; Castañeda-Cabral, J.L.; Martínez-Cuevas, F.; Besio, W.; Valdés-Cruz, A.; Rocha, L. Transcranial Focal Electric Stimulation Avoids P-Glycoprotein Over-Expression during Electrical Amygdala Kindling and Delays Epileptogenesis in Rats. Life 2023, 13, 1294. https://doi.org/10.3390/life13061294
Fonseca-Barriendos D, Castañeda-Cabral JL, Martínez-Cuevas F, Besio W, Valdés-Cruz A, Rocha L. Transcranial Focal Electric Stimulation Avoids P-Glycoprotein Over-Expression during Electrical Amygdala Kindling and Delays Epileptogenesis in Rats. Life. 2023; 13(6):1294. https://doi.org/10.3390/life13061294
Chicago/Turabian StyleFonseca-Barriendos, Daniel, José Luis Castañeda-Cabral, Frida Martínez-Cuevas, Walter Besio, Alejandro Valdés-Cruz, and Luisa Rocha. 2023. "Transcranial Focal Electric Stimulation Avoids P-Glycoprotein Over-Expression during Electrical Amygdala Kindling and Delays Epileptogenesis in Rats" Life 13, no. 6: 1294. https://doi.org/10.3390/life13061294
APA StyleFonseca-Barriendos, D., Castañeda-Cabral, J. L., Martínez-Cuevas, F., Besio, W., Valdés-Cruz, A., & Rocha, L. (2023). Transcranial Focal Electric Stimulation Avoids P-Glycoprotein Over-Expression during Electrical Amygdala Kindling and Delays Epileptogenesis in Rats. Life, 13(6), 1294. https://doi.org/10.3390/life13061294








