Stimulation of Angiotensin II Type 2 Receptor Modulates Pro-Inflammatory Response in Microglia and Macrophages: Therapeutic Implications for the Treatment of Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Maintenance, Culture, and Treatment Conditions
2.2. RNA Isolation, cDNA Reverse Transcription, and Quantitative Real-Time rt-PCR
2.3. ELISA
2.4. MTS Viability Assay
2.5. Trypan Blue Staining: Viability Analysis
2.6. Cellular ROS Generation
2.7. Nitrite and Nitrate Measurement
2.8. Statistical Analysis
3. Results
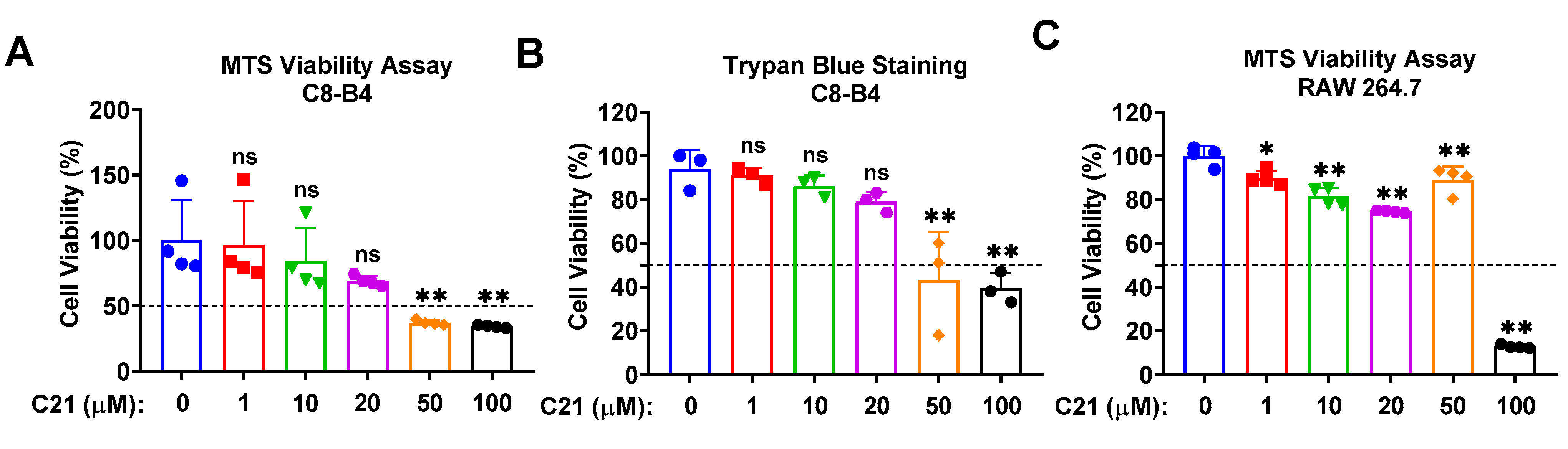
3.1. Compound 21 Demonstrates Acceptable Cytotoxicity at Lower Concentrations
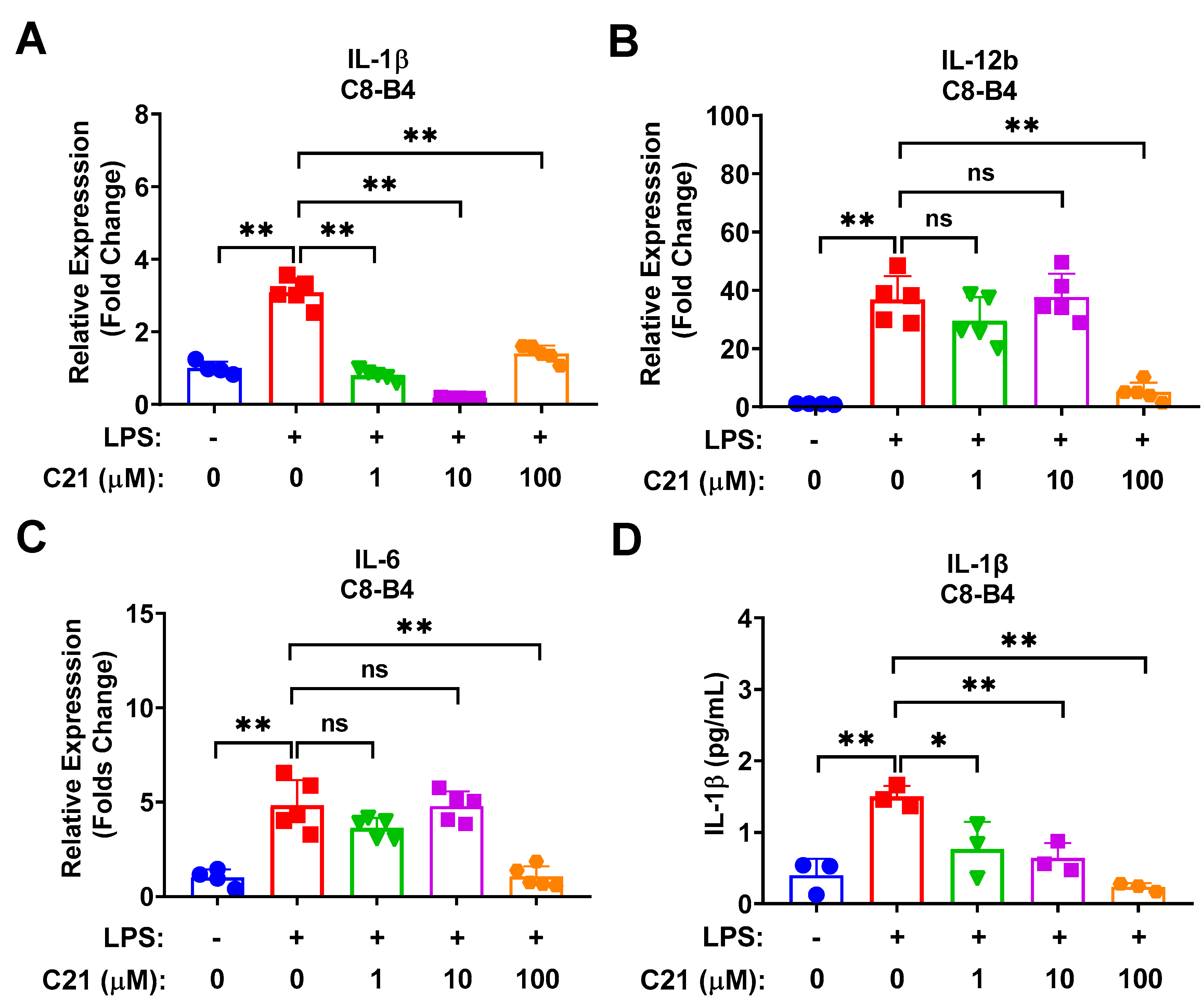
3.2. Compound 21 Exhibits an Anti-Inflammatory Response in Microglia via Decreasing the Expression of LPS-Induced Pro-Inflammatory Cytokines/Chemokines in a Dose-Dependent Manner
3.3. Compound 21 Significantly Reduces the Expression of Pro-Inflammatory Cytokines/Chemokines in Macrophages in a Dose-Dependent Fashion
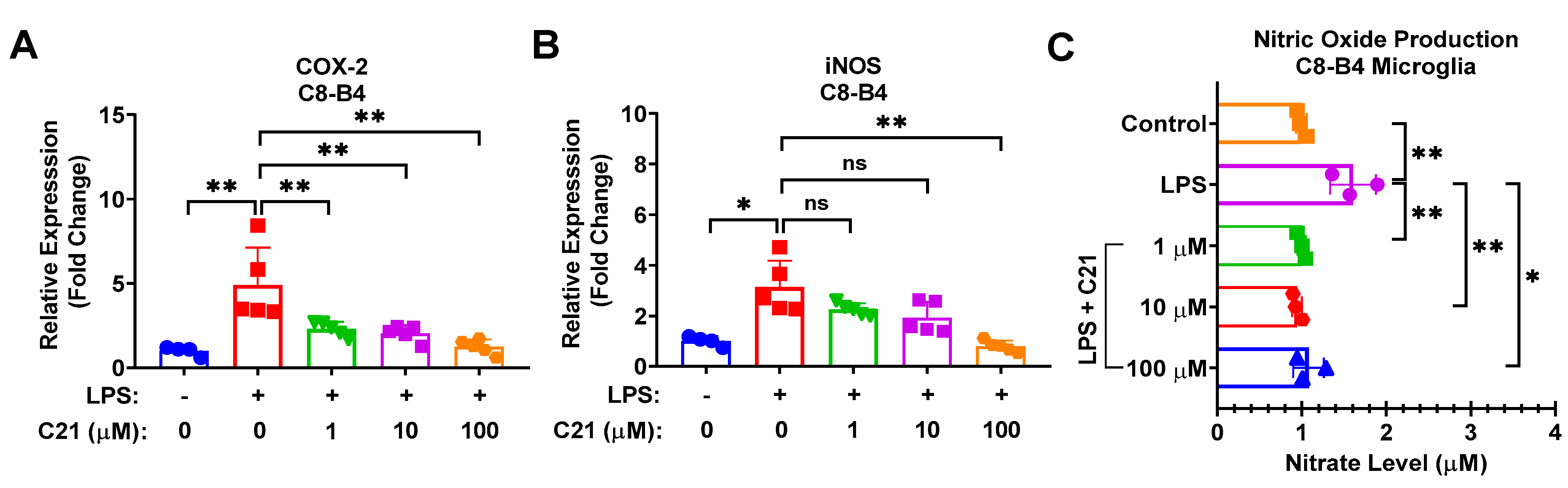
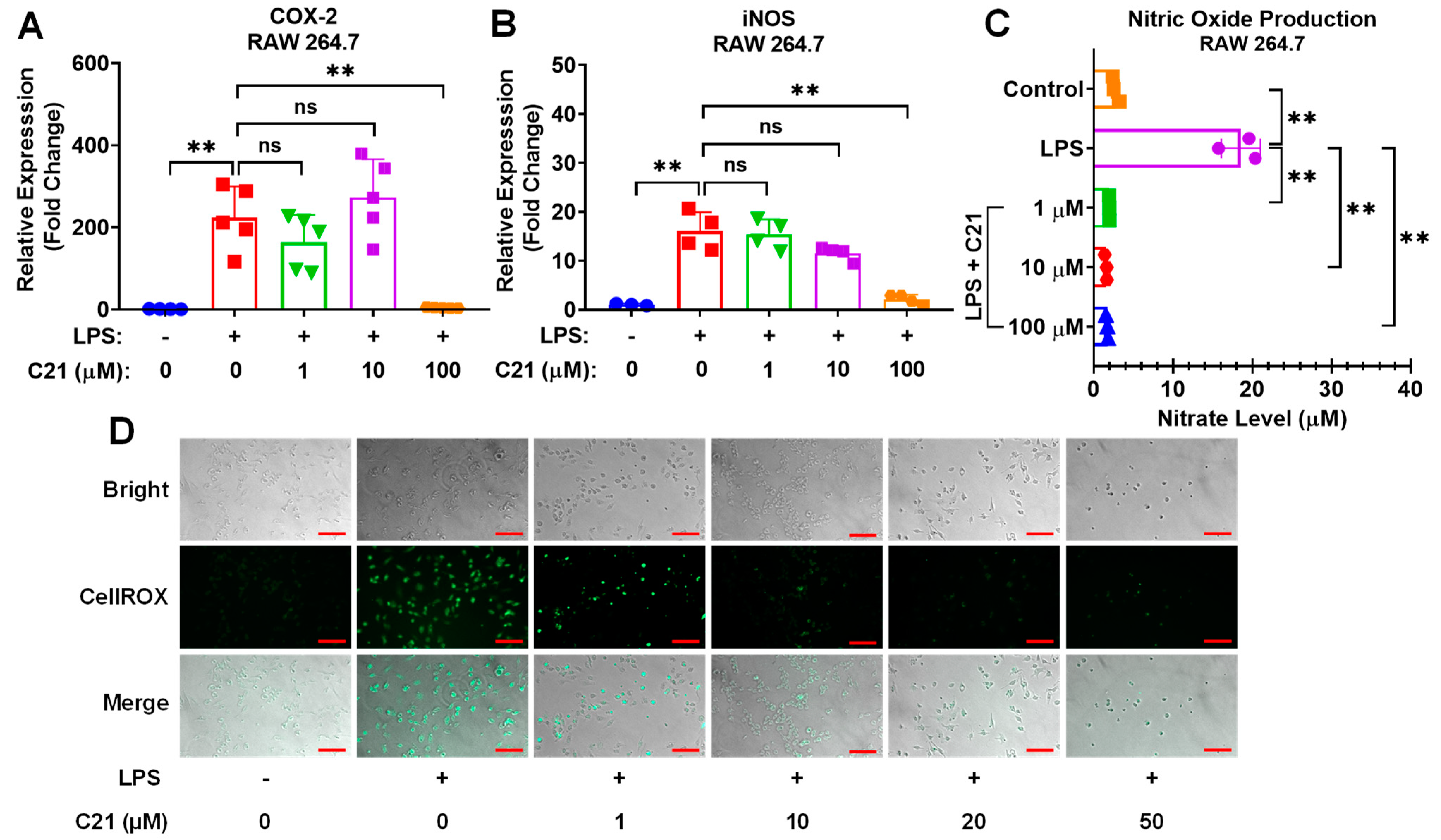
3.4. Compound 21 Effectively Regulates the Production of Reactive Oxygen Species (ROS) and Nitric Oxide (NO), and the Activity of Pro-Inflammatory Enzymes, iNOS, and COX-2, in Microglia/Macrophages
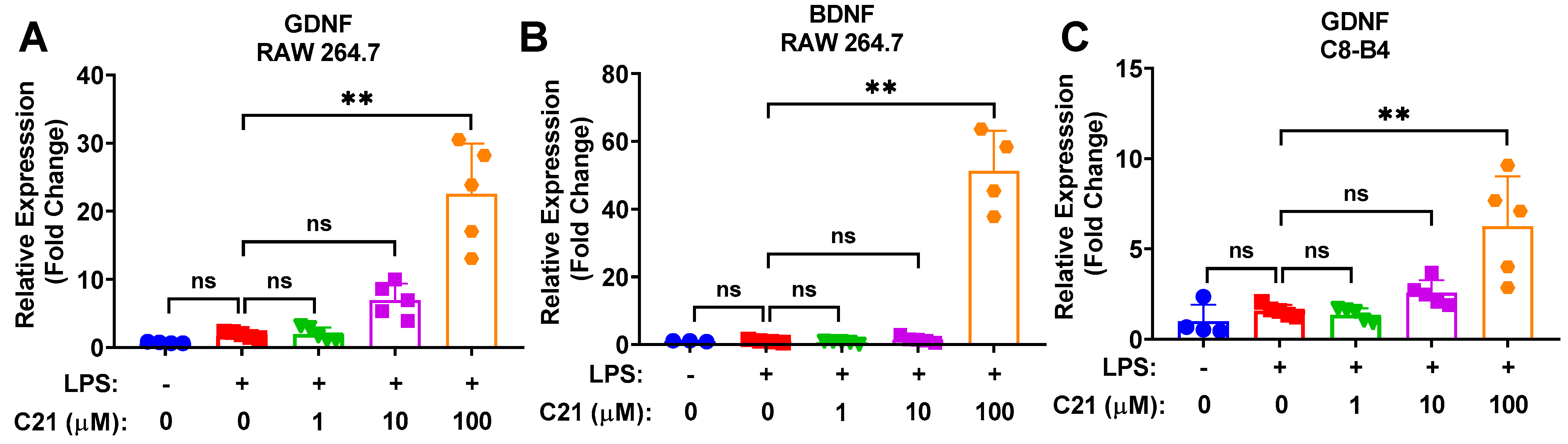
3.5. Compound 21 Is Associated with Increased Neuroprotective Activity in Microglia/Macrophages
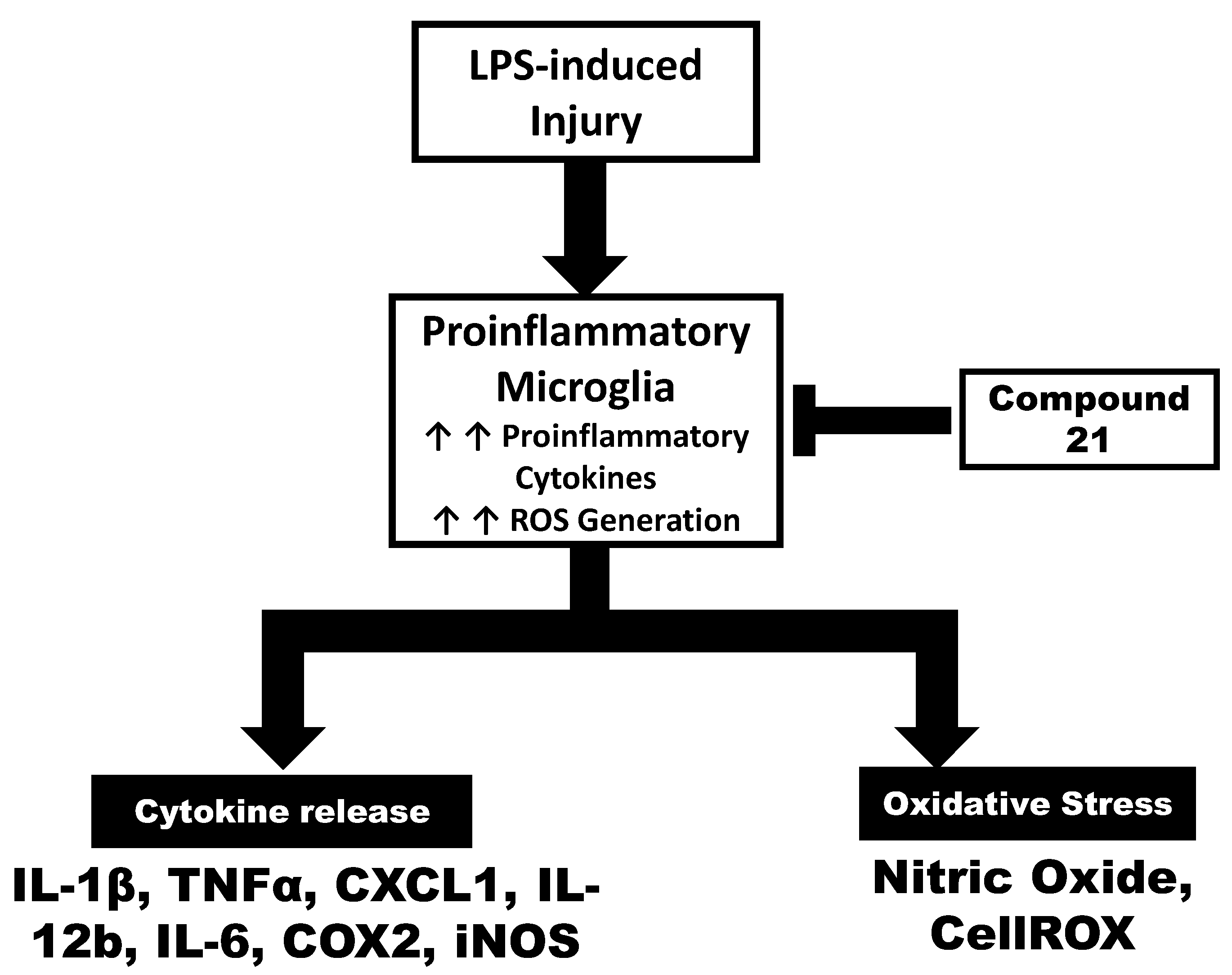
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thurgur, H.; Pinteaux, E. Microglia in the Neurovascular Unit: Blood-Brain Barrier-microglia Interactions After Central Nervous System Disorders. Neuroscience 2019, 405, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S. Innate immunity and neuroinflammation in the CNS: The role of microglia in Toll-like receptor-mediated neuronal injury. Glia 2010, 58, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, O.; Potjewyd, G.; Pinteaux, E. Regenerative Medicine Therapies for Targeting Neuroinflammation After Stroke. Front. Neurol. 2018, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Stieler, K.; Schumacher, U.; Horst, A.K.; Fischer, N. XMRV induces cell migration, cytokine expression and tumor angiogenesis: Are 22Rv1 cells a suitable prostate cancer model? PLoS ONE 2012, 7, e42321. [Google Scholar] [CrossRef]
- Perego, C.; Fumagalli, S.; Zanier, E.R.; Carlino, E.; Panini, N.; Erba, E.; De Simoni, M.G. Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury. Neurobiol. Dis. 2016, 96, 284–293. [Google Scholar] [CrossRef]
- Shechter, R.; Miller, O.; Yovel, G.; Rosenzweig, N.; London, A.; Ruckh, J.; Kim, K.W.; Klein, E.; Kalchenko, V.; Bendel, P.; et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 2013, 38, 555–569. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 2007, 38, 1345–1353. [Google Scholar] [CrossRef]
- Chu, H.X.; Broughton, B.R.; Kim, H.A.; Lee, S.; Drummond, G.R.; Sobey, C.G. Evidence That Ly6C(hi) Monocytes are Protective in Acute Ischemic Stroke by Promoting M2 Macrophage Polarization. Stroke 2015, 46, 1929–1937. [Google Scholar] [CrossRef]
- Wattananit, S.; Tornero, D.; Graubardt, N.; Memanishvili, T.; Monni, E.; Tatarishvili, J.; Miskinyte, G.; Ge, R.; Ahlenius, H.; Lindvall, O.; et al. Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice. J. Neurosci. 2016, 36, 4182–4195. [Google Scholar] [CrossRef]
- Pedragosa, J.; Miro-Mur, F.; Otxoa-de-Amezaga, A.; Justicia, C.; Ruiz-Jaen, F.; Ponsaerts, P.; Pasparakis, M.; Planas, A.M. CCR2 deficiency in monocytes impairs angiogenesis and functional recovery after ischemic stroke in mice. J. Cereb. Blood Flow Metab. 2020, 40, S98–S116. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the Brain: The Renin Angiotensin System. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; Ishrat, T.; Pillai, B.; Fouda, A.Y.; Sayed, M.A.; Eldahshan, W.; Waller, J.L.; Ergul, A.; Fagan, S.C. RAS modulation prevents progressive cognitive impairment after experimental stroke: A randomized, blinded preclinical trial. J. Neuroinflamm. 2018, 15, 229. [Google Scholar] [CrossRef]
- Alhusban, A.; Kozak, A.; Ergul, A.; Fagan, S.C. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J. Pharmacol. Exp. Ther. 2013, 344, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Ishrat, T.; Pillai, B.; Soliman, S.; Fouda, A.Y.; Kozak, A.; Johnson, M.H.; Ergul, A.; Fagan, S.C. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol. Neurobiol. 2015, 51, 1542–1553. [Google Scholar] [CrossRef]
- Alhusban, A.; Fouda, A.Y.; Bindu, P.; Ishrat, T.; Soliman, S.; Fagan, S.C. Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. J. Hypertens. 2015, 33, 170–180. [Google Scholar] [CrossRef]
- Fouda, A.Y.; Alhusban, A.; Ishrat, T.; Pillai, B.; Eldahshan, W.; Waller, J.L.; Ergul, A.; Fagan, S.C. Brain-Derived Neurotrophic Factor Knockdown Blocks the Angiogenic and Protective Effects of Angiotensin Modulation After Experimental Stroke. Mol. Neurobiol. 2017, 54, 661–670. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Ishrat, T.; Pillai, B.; Bunting, K.M.; Patel, A.; Vazdarjanova, A.; Waller, J.L.; Arbab, A.S.; Ergul, A.; Fagan, S.C. Role of angiotensin system modulation on progression of cognitive impairment and brain MRI changes in aged hypertensive animals-A randomized double- blind pre-clinical study. Behav. Brain Res. 2018, 346, 29–40. [Google Scholar] [CrossRef]
- Walther, T.; Olah, L.; Harms, C.; Maul, B.; Bader, M.; Hortnagl, H.; Schultheiss, H.P.; Mies, G. Ischemic injury in experimental stroke depends on angiotensin II. FASEB J. 2002, 16, 169–176. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Ishrat, T.; Pillai, B.; Bunting, K.M.; Vazdarjanova, A.; Waller, J.L.; Ergul, A.; Fagan, S.C. Angiotensin receptor (AT2R) agonist C21 prevents cognitive decline after permanent stroke in aged animals-A randomized double- blind pre-clinical study. Behav. Brain Res. 2019, 359, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Dong, G.; Althomali, W.; Sayed, M.A.; Eldahshan, W.; Baban, B.; Johnson, M.H.; Filosa, J.; Fagan, S.C.; Ergul, A. Delayed Administration of Angiotensin II Type 2 Receptor (AT2R) Agonist Compound 21 Prevents the Development of Post-stroke Cognitive Impairment in Diabetes Through the Modulation of Microglia Polarization. Transl. Stroke Res. 2020, 11, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Sood, A.; Shukla, R.; Hanif, K. AT2R Activation Prevents Microglia Pro-inflammatory Activation in a NOX-Dependent Manner: Inhibition of PKC Activation and p47(phox) Phosphorylation by PP2A. Mol. Neurobiol. 2019, 56, 3005–3023. [Google Scholar] [CrossRef] [PubMed]
- Valero-Esquitino, V.; Lucht, K.; Namsolleck, P.; Monnet-Tschudi, F.; Stubbe, T.; Lucht, F.; Liu, M.; Ebner, F.; Brandt, C.; Danyel, L.A.; et al. Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin. Sci. 2015, 128, 95–109. [Google Scholar] [CrossRef]
- Isaksson, R.; Casselbrant, A.; Elebring, E.; Hallberg, M.; Larhed, M.; Fandriks, L. Direct stimulation of angiotensin II type 2 receptor reduces nitric oxide production in lipopolysaccharide treated mouse macrophages. Eur. J. Pharmacol. 2020, 868, 172855. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef]
- Perego, C.; Fumagalli, S.; De Simoni, M.G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflamm. 2011, 8, 174. [Google Scholar] [CrossRef]
- Nogawa, S.; Forster, C.; Zhang, F.; Nagayama, M.; Ross, M.E.; Iadecola, C. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 10966–10971. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.H.; Lee, C.K.; Ha, N.J.; Yim, D.; et al. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-kappaB pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef]
- Cho, H.; Yun, C.W.; Park, W.K.; Kong, J.Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Zhou, P.; Iadecola, C. iNOS and COX-2 in Ischemic Stroke. In Handbook of Neurochemistry and Molecular Neurobiology: Acute Ischemic Injury and Repair in the Nervous System; Lajtha, A., Chan, P.H., Eds.; Springer: Boston, MA, USA, 2007; pp. 33–45. [Google Scholar]
- Greenhalgh, A.D.; Zarruk, J.G.; Healy, L.M.; Baskar Jesudasan, S.J.; Jhelum, P.; Salmon, C.K.; Formanek, A.; Russo, M.V.; Antel, J.P.; McGavern, D.B.; et al. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 2018, 16, e2005264. [Google Scholar] [CrossRef] [PubMed]
- Menk, M.; Graw, J.A.; von Haefen, C.; Sifringer, M.; Schwaiberger, D.; Unger, T.; Steckelings, U.; Spies, C.D. Stimulation of the Angiotensin II AT2 Receptor is Anti-inflammatory in Human Lipopolysaccharide-Activated Monocytic Cells. Inflammation 2015, 38, 1690–1699. [Google Scholar] [CrossRef] [PubMed]

| Microglia | Macrophages | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genes | LPS | LPS + C21 (1 μm) | LPS + C21 (10 μm) | LPS + C21 (100 μm) | Genes | LPS | LPS + C21 (1 μm) | LPS + C21 (10 μm) | LPS + C21 (100 μm) | |
| mRNA | IL-1β | ⬆⬆⬆ | ⬇⬇ | ⬇⬇⬇ | ⬇ | IL-1β | ⬆⬆⬆ | ⬇ | ⬇⬇ | ⬇⬇⬇ |
| IL-12B | ⬆⬆⬆ | No Change | No Change | ⬇⬇⬇ | CXCL1 | ⬆⬆⬆ | No Change | No Change | ⬇⬇⬇ | |
| IL-6 | ⬆⬆⬆ | No Change | No Change | ⬇⬇⬇ | TNF-α | ⬆⬆⬆ | No Change | No Change | ⬇⬇ | |
| COX-2 | ⬆⬆⬆ | ⬇⬇ | ⬇⬇ | ⬇⬇ | COX-2 | ⬆⬆⬆ | No Change | No Change | ⬇⬇⬇ | |
| iNOS | ⬆⬆⬆ | No Change | No Change | ⬇⬇⬇ | iNOS | ⬆⬆⬆ | No Change | No Change | ⬇⬇⬇ | |
| Gdnf | No Change | No Change | ⬆⬆ | ⬆⬆⬆ | Gdnf | No Change | No Change | No Change | ⬆⬆⬆ | |
| BDNF | No Change | No Change | No Change | ⬆⬆⬆ | ||||||
| Protein | IL-1β | ⬆⬆⬆ | ⬇ | ⬇⬇ | ⬇⬇⬇ | IL-1β | ⬆⬆⬆ | ⬇ | ⬇⬇ | ⬇⬇⬇ |
| CXCL1 | ⬆⬆⬆ | ⬇⬇ | ⬇⬇ | ⬇⬇⬇ | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, A.; Han, Y.; Jones, T.W.; Pillai, B.; Zhang, D.; Ergul, A.; Somanath, P.R.; Fagan, S.C. Stimulation of Angiotensin II Type 2 Receptor Modulates Pro-Inflammatory Response in Microglia and Macrophages: Therapeutic Implications for the Treatment of Stroke. Life 2023, 13, 1274. https://doi.org/10.3390/life13061274
Alshammari A, Han Y, Jones TW, Pillai B, Zhang D, Ergul A, Somanath PR, Fagan SC. Stimulation of Angiotensin II Type 2 Receptor Modulates Pro-Inflammatory Response in Microglia and Macrophages: Therapeutic Implications for the Treatment of Stroke. Life. 2023; 13(6):1274. https://doi.org/10.3390/life13061274
Chicago/Turabian StyleAlshammari, Abdulkarim, Yohan Han, Timothy W. Jones, Bindu Pillai, Duo Zhang, Adviye Ergul, Payaningal R. Somanath, and Susan C. Fagan. 2023. "Stimulation of Angiotensin II Type 2 Receptor Modulates Pro-Inflammatory Response in Microglia and Macrophages: Therapeutic Implications for the Treatment of Stroke" Life 13, no. 6: 1274. https://doi.org/10.3390/life13061274
APA StyleAlshammari, A., Han, Y., Jones, T. W., Pillai, B., Zhang, D., Ergul, A., Somanath, P. R., & Fagan, S. C. (2023). Stimulation of Angiotensin II Type 2 Receptor Modulates Pro-Inflammatory Response in Microglia and Macrophages: Therapeutic Implications for the Treatment of Stroke. Life, 13(6), 1274. https://doi.org/10.3390/life13061274







