Social Isolation: A Narrative Review on the Dangerous Liaison between the Autonomic Nervous System and Inflammation
Abstract
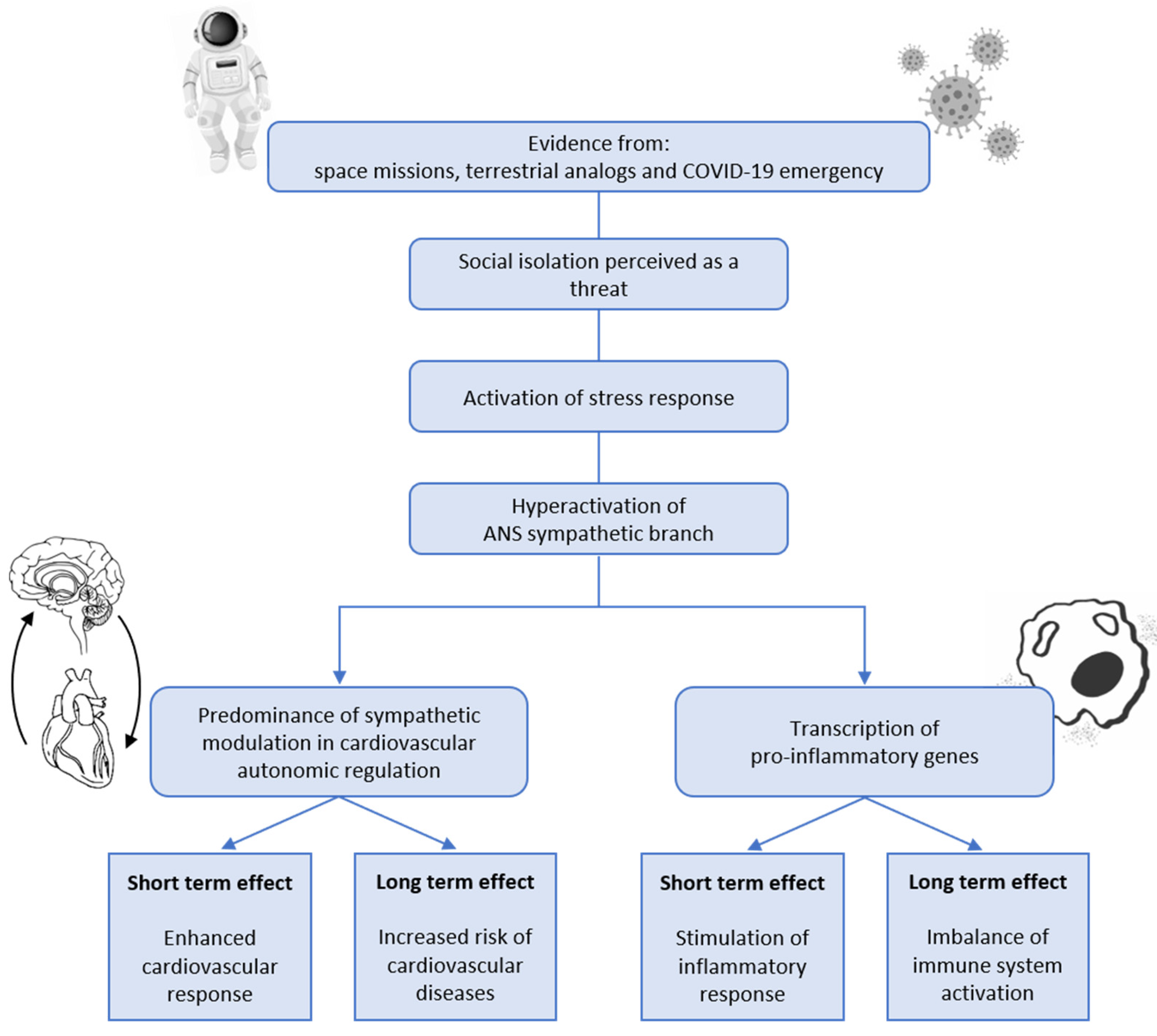
1. Introduction
2. Social Isolation and Stress Response
3. Social Isolation and Cardiovascular Autonomic Control Alterations
4. Social Isolation and Inflammation
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eisenberger, N.I. Social Pain and the Brain: Controversies, Questions, and Where to Go from Here. Annu. Rev. Psychol. 2015, 66, 601–629. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.E.; Panksepp, J. Brain Substrates of Infant–Mother Attachment: Contributions of Opioids, Oxytocin, and Norepinephrine. Neurosci. Biobehav. Rev. 1998, 22, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Bzdok, D.; Dunbar, R.I.M. The Neurobiology of Social Distance. Trends Cogn. Sci. 2020, 24, 717–733. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Cacioppo, S.; Capitanio, J.P.; Cole, S.W. The Neuroendocrinology of Social Isolation. Annu. Rev. Psychol. 2015, 66, 733–767. [Google Scholar] [CrossRef] [PubMed]
- Eng, P.M.; Rimm, E.B.; Fitzmaurice, G.; Kawachi, I. Social Ties and Change in Social Ties in Relation to Subsequent Total and Cause-Specific Mortality and Coronary Heart Disease Incidence in Men. Am. J. Epidemiol. 2002, 155, 700–709. [Google Scholar] [CrossRef]
- Steptoe, A.; Shankar, A.; Demakakos, P.; Wardle, J. Social Isolation, Loneliness, and All-Cause Mortality in Older Men and Women. Proc. Natl. Acad. Sci. USA 2013, 110, 5797–5801. [Google Scholar] [CrossRef]
- Holt-Lunstad, J.; Smith, T.B.; Baker, M.; Harris, T.; Stephenson, D. Loneliness and Social Isolation as Risk Factors for Mortality: A Meta-Analytic Review. Perspect. Psychol. Sci. 2015, 10, 227–237. [Google Scholar] [CrossRef]
- Grippo, A.J.; Lamb, D.G.; Carter, C.S.; Porges, S.W. Social Isolation Disrupts Autonomic Regulation of the Heart and Influences Negative Affective Behaviors. Biol. Psychiatry 2007, 62, 1162–1170. [Google Scholar] [CrossRef]
- Ramsay, S.; Ebrahim, S.; Whincup, P.; Papacosta, O.; Morris, R.; Lennon, L.; Wannamethee, S.G. Social Engagement and the Risk of Cardiovascular Disease Mortality: Results of a Prospective Population-Based Study of Older Men. Ann. Epidemiol. 2008, 18, 476–483. [Google Scholar] [CrossRef]
- Mattos dos Santos, R. Isolation, Social Stress, Low Socioeconomic Status and Its Relationship to Immune Response in COVID-19 Pandemic Context. Brain Behav. Immun.—Health 2020, 7, 100103. [Google Scholar] [CrossRef]
- Choukér, A.; Stahn, A.C. COVID-19—The Largest Isolation Study in History: The Value of Shared Learnings from Spaceflight Analogs. NPJ Microgravity 2020, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Suedfeld, P.; Weiss, K.; Gaudino, M. Affective, Social, and Cognitive Outcomes During a 1-Year Wintering in Concordia. Environ. Behav. 2016, 48, 1073–1091. [Google Scholar] [CrossRef]
- Palinkas, L. Psychosocial Issues in Long-Term Space Flight: Overview. Gravit. Space Biol. Bull. Publ. Am. Soc. Gravit. Space Biol. 2001, 14, 25–33. [Google Scholar]
- Palinkas, L.A.; Suedfeld, P. Psychosocial Issues in Isolated and Confined Extreme Environments. Neurosci. Biobehav. Rev. 2021, 126, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Hawkley, L.C.; Cacioppo, J.T. Loneliness and Pathways to Disease. Brain Behav. Immun. 2003, 17 (Suppl. S1), S98–S105. [Google Scholar] [CrossRef]
- Stickley, A.; Koyanagi, A. Loneliness, Common Mental Disorders and Suicidal Behavior: Findings from a General Population Survey. J. Affect. Disord. 2016, 197, 81–87. [Google Scholar] [CrossRef]
- Morphew, E. Psychological and Human Factors in Long Duration Spaceflight. MJM 2020, 6. [Google Scholar] [CrossRef]
- Pagel, J.I.; Choukèr, A. Effects of Isolation and Confinement on Humans-Implications for Manned Space Explorations. J. Appl. Physiol. 2016, 120, 1449–1457. [Google Scholar] [CrossRef]
- Patel, Z.S.; Brunstetter, T.J.; Tarver, W.J.; Whitmire, A.M.; Zwart, S.R.; Smith, S.M.; Huff, J.L. Red Risks for a Journey to the Red Planet: The Highest Priority Human Health Risks for a Mission to Mars. NPJ Microgravity 2020, 6, 33. [Google Scholar] [CrossRef]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Abeln, V.; MacDonald-Nethercott, E.; Piacentini, M.F.; Meeusen, R.; Kleinert, J.; Strueder, H.K.; Schneider, S. Exercise in Isolation- A Countermeasure for Electrocortical, Mental and Cognitive Impairments. PLoS ONE 2015, 10, e0126356. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.; Simpson, R.J.; Mehta, S.; Stowe, R.; Chouker, A.; Hwang, S.-A.; Actor, J.K.; Salam, A.P.; Pierson, D.; Sams, C. Terrestrial Stress Analogs for Spaceflight Associated Immune System Dysregulation. Brain Behav. Immun. 2014, 39, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Palinkas, L.A.; Suedfeld, P. Psychological Effects of Polar Expeditions. Lancet 2008, 371, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Basner, M.; Dinges, D.F.; Mollicone, D.J.; Savelev, I.; Ecker, A.J.; Di Antonio, A.; Jones, C.W.; Hyder, E.C.; Kan, K.; Morukov, B.V.; et al. Psychological and Behavioral Changes during Confinement in a 520-Day Simulated Interplanetary Mission to Mars. PLoS ONE 2014, 9, e93298. [Google Scholar] [CrossRef]
- Lugg, D.J. Behavioral Health in Antarctica: Implications for Long-Duration Space Missions. Aviat. Space Environ. Med. 2005, 76, B74–B77. [Google Scholar]
- Palinkas, L.A.; Browner, D. Effects of Prolonged Isolation in Extreme Environments on Stress, Coping, and Depression. J. Appl. Soc. Psychol. 1995, 25, 557–576. [Google Scholar] [CrossRef]
- O’Sullivan, R.; Burns, A.; Leavey, G.; Leroi, I.; Burholt, V.; Lubben, J.; Holt-Lunstad, J.; Victor, C.; Lawlor, B.; Vilar-Compte, M.; et al. Impact of the COVID-19 Pandemic on Loneliness and Social Isolation: A Multi-Country Study. Int. J. Environ. Res. Public. Health 2021, 18, 9982. [Google Scholar] [CrossRef]
- Varga, T.V.; Bu, F.; Dissing, A.S.; Elsenburg, L.K.; Bustamante, J.J.H.; Matta, J.; van Zon, S.K.R.; Brouwer, S.; Bültmann, U.; Fancourt, D.; et al. Loneliness, Worries, Anxiety, and Precautionary Behaviours in Response to the COVID-19 Pandemic: A Longitudinal Analysis of 200,000 Western and Northern Europeans. Lancet Reg. Health—Eur. 2021, 2, 100020. [Google Scholar] [CrossRef]
- Passavanti, M.; Argentieri, A.; Barbieri, D.M.; Lou, B.; Wijayaratna, K.; Foroutan Mirhosseini, A.S.; Wang, F.; Naseri, S.; Qamhia, I.; Tangerås, M.; et al. The Psychological Impact of COVID-19 and Restrictive Measures in the World. J. Affect. Disord. 2021, 283, 36–51. [Google Scholar] [CrossRef]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 Pandemic on Mental Health in the General Population: A Systematic Review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef]
- Fiorenzato, E.; Zabberoni, S.; Costa, A.; Cona, G. Cognitive and Mental Health Changes and Their Vulnerability Factors Related to COVID-19 Lockdown in Italy. PLoS ONE 2021, 16, e0246204. [Google Scholar] [CrossRef]
- Nogueira, J.; Gerardo, B.; Silva, A.R.; Pinto, P.; Barbosa, R.; Soares, S.; Baptista, B.; Paquete, C.; Cabral-Pinto, M.; Vilar, M.M.; et al. Effects of Restraining Measures Due to COVID-19: Pre- and Post-Lockdown Cognitive Status and Mental Health. Curr. Psychol. 2022, 41, 7383–7392. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. Stress and the General Adaptation Syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef]
- Henry, J.P. Biological Basis of the Stress Response. Integr. Physiol. Behav. Sci. 1992, 27, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, C.M. Hormones and Resistance. By Hans Selye. Springer-Verlag, 175 Fifth Ave., New York, NY 10010, 1971. Xviii + 1140 Pp. 17 × 25 Cm. Price $79.40 (Two Parts, Not Sold Separately). J. Pharm. Sci. 1972, 61, 2012. [Google Scholar] [CrossRef]
- Szabo, S.; Yoshida, M.; Filakovszky, J.; Juhasz, G. “Stress” is 80 Years Old: From Hans Selye Original Paper in 1936 to Recent Advances in GI Ulceration. Curr. Pharm. Des. 2017, 23, 4029–4041. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Chrousos, G.P. The Neuroendocrinology of Stress: The Stress-Related Continuum of Chronic Disease Development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef]
- Hering, D.; Lachowska, K.; Schlaich, M. Role of the Sympathetic Nervous System in Stress-Mediated Cardiovascular Disease. Curr. Hypertens. Rep. 2015, 17, 80. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.-K. Stress, the Autonomic Nervous System, and the Immune-Kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Hypercortisolism Associated With Social Subordinance or Social Isolation among Wild Baboons. Arch. Gen. Psychiatry 1997, 54, 1137. [Google Scholar] [CrossRef]
- Grippo, A.J.; Scotti, M.-A.L.; Wardwell, J.; McNeal, N.; Bates, S.L.; Chandler, D.L.; Ihm, E.; Jadia, N. Cardiac and Behavioral Effects of Social Isolation and Experimental Manipulation of Autonomic Balance. Auton. Neurosci. 2018, 214, 1–8. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Ernst, J.M.; Burleson, M.H.; Malarkey, W.B.; Hawkley, L.C.; Paulsen, A.; Hobson, J.A.; Hugdahl, K.; Spiegel, D.; Berntson, G.G. Lonely Traits and Concomitant Physiological Processes: The MacArthur Social Neuroscience Studies. Int. J. Psychophysiol. 2000, 35, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Jacubowski, A.; Abeln, V.; Vogt, T.; Yi, B.; Choukèr, A.; Fomina, E.; Strüder, H.K.; Schneider, S. The Impact of Long-Term Confinement and Exercise on Central and Peripheral Stress Markers. Physiol. Behav. 2015, 152, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Grippo, A.J.; Ihm, E.; Wardwell, J.; McNeal, N.; Scotti, M.-A.L.; Moenk, D.A.; Chandler, D.L.; LaRocca, M.A.; Preihs, K. The Effects of Environmental Enrichment on Depressive and Anxiety-Relevant Behaviors in Socially Isolated Prairie Voles. Psychosom. Med. 2014, 76, 277–284. [Google Scholar] [CrossRef]
- McNeal, N.; Scotti, M.-A.L.; Wardwell, J.; Chandler, D.L.; Bates, S.L.; LaRocca, M.; Trahanas, D.M.; Grippo, A.J. Disruption of Social Bonds Induces Behavioral and Physiological Dysregulation in Male and Female Prairie Voles. Auton. Neurosci. 2014, 180, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N. The Role of Oxidative Stress in Cardiovascular Disease Caused by Social Isolation and Loneliness. Redox Biol. 2020, 37, 101585. [Google Scholar] [CrossRef]
- Xia, N.; Li, H. Loneliness, Social Isolation, and Cardiovascular Health. Antioxid. Redox Signal. 2018, 28, 837–851. [Google Scholar] [CrossRef]
- Steptoe, A.; Owen, N.; Kunz-Ebrecht, S.R.; Brydon, L. Loneliness and Neuroendocrine, Cardiovascular, and Inflammatory Stress Responses in Middle-Aged Men and Women. Psychoneuroendocrinology 2004, 29, 593–611. [Google Scholar] [CrossRef]
- Hawkley, L.C.; Thisted, R.A.; Masi, C.M.; Cacioppo, J.T. Loneliness Predicts Increased Blood Pressure: 5-Year Cross-Lagged Analyses in Middle-Aged and Older Adults. Psychol. Aging 2010, 25, 132–141. [Google Scholar] [CrossRef]
- Valtorta, N.K.; Kanaan, M.; Gilbody, S.; Ronzi, S.; Hanratty, B. Loneliness and Social Isolation as Risk Factors for Coronary Heart Disease and Stroke: Systematic Review and Meta-Analysis of Longitudinal Observational Studies. Heart 2016, 102, 1009–1016. [Google Scholar] [CrossRef]
- Umberson, D. Family Status and Health Behaviors: Social Control as a Dimension of Social Integration. J. Health Soc. Behav. 1987, 28, 306. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the Heart-Brain Connection: Further Elaboration of a Model of Neurovisceral Integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, J. The Emotional Brain: The Mysterious Underpinnings of Emotional Life; Simon and Schuster: New York, NY, USA, 1998; ISBN 978-0-684-83659-1. [Google Scholar]
- Nakagawa, S.; Takeuchi, H.; Taki, Y.; Nouchi, R.; Sekiguchi, A.; Kotozaki, Y.; Miyauchi, C.M.; Iizuka, K.; Yokoyama, R.; Shinada, T.; et al. White Matter Structures Associated with Loneliness in Young Adults. Sci. Rep. 2015, 5, 17001. [Google Scholar] [CrossRef]
- Kamarck, T.W.; Manuck, S.B.; Jennings, J.R. Social Support Reduces Cardiovascular Reactivity to Psychological Challenge: A Laboratory Model. Psychosom. Med. 1990, 52, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The Relationship of Autonomic Imbalance, Heart Rate Variability and Cardiovascular Disease Risk Factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Montano, N.; Porta, A.; Cogliati, C.; Costantino, G.; Tobaldini, E.; Casali, K.R.; Iellamo, F. Heart Rate Variability Explored in the Frequency Domain: A Tool to Investigate the Link between Heart and Behavior. Neurosci. Biobehav. Rev. 2009, 33, 71–80. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Esler, M. Mental Stress and Human Cardiovascular Disease. Neurosci. Biobehav. Rev. 2017, 74, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Kivimäki, M. Stress and Cardiovascular Disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Ursin, H. ISEMSI (Isolation Study for European Manned Space Infrastructures): A Space Psychology Experiment. Adv. Space Biol. Med. 1993, 3, 23–34. [Google Scholar]
- Vaernes, R.J. EXEMSI: Description of Facilities, Organization, Crew Selection, and Operational Aspects. Experimental Campaign for the European Manned Space Infrastructure. Adv. Space Biol. Med. 1996, 5, 7–38. [Google Scholar]
- Sasahara, S.; Oi, Y.; Doki, S.; Hori, D.; Ohtaki, Y.; Andrea, C.-S.; Takahashi, T.; Shiraki, N.; Ikeda, Y.; Ikeda, T.; et al. Structured Review: Psychosocial Stress During Long-Term Stays in Space. Aerosp. Technol. Jpn. 2020, 18, 180–185. [Google Scholar] [CrossRef]
- Cromwell, R.L.; Huff, J.L.; Simonsen, L.C.; Patel, Z.S. Earth-Based Research Analogs to Investigate Space-Based Health Risks. New Space 2021, 9, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Maillet, A.; Gunga, H.C.; Gauquelin, G.; Fortrat, J.O.; Hope, A.; Røcker, L.; Kirsch, K.; Gharib, C. Effects of 28-Day Isolation (ESA-ISEMSI’90) on Blood Pressure and Blood Volume Regulating Hormones. Aviat. Space Environ. Med. 1993, 64, 287–294. [Google Scholar]
- Maillet, A.; Normand, S.; Gunga, H.C.; Allevard, A.M.; Cottet-Emard, J.M.; Kihm, E.; Strollo, F.; Pachiaudi, C.; Kirsch, K.A.; Bizollon, C.A.; et al. Hormonal, Water Balance, and Electrolyte Changes during Sixty-Day Confinement. Adv. Space Biol. Med. 1996, 5, 55–78. [Google Scholar] [CrossRef]
- Pagani, M.; Iellamo, F.; Lucini, D.; Pizzinelli, P.; Castrucci, F.; Peruzzi, G.; Malliani, A. Adaptational Changes in the Neural Control of Cardiorespiratory Function in a Confined Environment: The CNEC#3 Experiment. Acta Astronaut. 1995, 36, 449–461. [Google Scholar] [CrossRef]
- Wientjes, C.J.E.; Veltman, J.A.; Gaillard, A.W.K. Chapter 8 Cardiovascular and Respiratory Responses During a Complex Decision-Making Task Under Prolonged Isolation. Adv. Space Biol. Med. 1996, 5, 133–155. [Google Scholar]
- Farrace, S.; Ferrara, M.; De Angelis, C.; Trezza, R.; Cenni, P.; Peri, A.; Casagrande, M.; De Gennaro, L. Reduced Sympathetic Outflow and Adrenal Secretory Activity during a 40-Day Stay in the Antarctic. Int. J. Psychophysiol. 2003, 49, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Vigo, D.E.; Tuerlinckx, F.; Ogrinz, B.; Wan, L.; Simonelli, G.; Bersenev, E.; Van den Bergh, O.; Aubert, A.E. Circadian Rhythm of Autonomic Cardiovascular Control During Mars500 Simulated Mission to Mars. Aviat. Space Environ. Med. 2013, 84, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Germain, A.; Kupfer, D.J. Circadian Rhythm Disturbances in Depression. Hum. Psychopharmacol. Clin. Exp. 2008, 23, 571–585. [Google Scholar] [CrossRef]
- Jarczok, M.; Gündel, H.; McGrath, J.; Balint, E. Circadian Rhythms of the Autonomic Nervous System: Scientific Implication and Practical Implementation. In Chronobiology—The Science of Biological Time Structure; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78984-901-1. [Google Scholar]
- Bourdillon, N.; Yazdani, S.; Schmitt, L.; Millet, G.P. Effects of COVID-19 Lockdown on Heart Rate Variability. PLoS ONE 2020, 15, e0242303. [Google Scholar] [CrossRef]
- Makovac, E.; Carnevali, L.; Medina, S.; Sgoifo, A.; Petrocchi, N.; Ottaviani, C. Safe in My Heart: Resting Heart Rate Variability Longitudinally Predicts Emotion Regulation, Worry, and Sense of Safeness during COVID-19 Lockdown. Stress 2022, 25, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Abelaira, H.M.; Réus, G.Z.; Petronilho, F.; Barichello, T.; Quevedo, J. Neuroimmunomodulation in Depression: A Review of Inflammatory Cytokines Involved in This Process. Neurochem. Res. 2014, 39, 1634–1639. [Google Scholar] [CrossRef]
- Konsman, J.P.; Parnet, P.; Dantzer, R. Cytokine-Induced Sickness Behaviour: Mechanisms and Implications. Trends Neurosci. 2002, 25, 154–159. [Google Scholar] [CrossRef]
- Smith, K.J.; Gavey, S.; RIddell, N.E.; Kontari, P.; Victor, C. The Association between Loneliness, Social Isolation and Inflammation: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 112, 519–541. [Google Scholar] [CrossRef]
- Mehta, S.K.; Crucian, B.E.; Stowe, R.P.; Simpson, R.J.; Ott, C.M.; Sams, C.F.; Pierson, D.L. Reactivation of Latent Viruses Is Associated with Increased Plasma Cytokines in Astronauts. Cytokine 2013, 61, 205–209. [Google Scholar] [CrossRef]
- Mehta, S.K.; Pierson, D.L.; Cooley, H.; Dubow, R.; Lugg, D. Epstein-Barr Virus Reactivation Associated with Diminished Cell-Mediated Immunity in Antarctic Expeditioners. J. Med. Virol. 2000, 61, 235–240. [Google Scholar] [CrossRef]
- Mhatre, S.D.; Iyer, J.; Puukila, S.; Paul, A.M.; Tahimic, C.G.T.; Rubinstein, L.; Lowe, M.; Alwood, J.S.; Sowa, M.B.; Bhattacharya, S.; et al. Neuro-Consequences of the Spaceflight Environment. Neurosci. Biobehav. Rev. 2022, 132, 908–935. [Google Scholar] [CrossRef] [PubMed]
- Krieger, S.S.; Zwart, S.R.; Mehta, S.; Wu, H.; Simpson, R.J.; Smith, S.M.; Crucian, B. Alterations in Saliva and Plasma Cytokine Concentrations During Long-Duration Spaceflight. Front. Immunol. 2021, 12, 725748. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, S.; Kalinin, S.; Sadova, A.; Rykova, M.; Orlova, K.; Crucian, B. Immunological Aspects of Isolation and Confinement. Front. Immunol. 2021, 12, 697435. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.E.; Zwart, S.R.; Mehta, S.; Uchakin, P.; Quiriarte, H.D.; Pierson, D.; Sams, C.F.; Smith, S.M. Plasma Cytokine Concentrations Indicate That in Vivo Hormonal Regulation of Immunity Is Altered during Long-Duration Spaceflight. J. Interferon Cytokine Res. 2014, 34, 778–786. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic Inflammatory Response to Exhaustive Exercise. Cytokine Kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar]
- Feuerecker, M.; Crucian, B.; Salam, A.; Rybka, A.; Kaufmann, I.; Moreels, M.; Quintens, R.; Schelling, G.; Thiel, M.; Baatout, S.; et al. Early Adaption to the Antarctic Environment at Dome C: Consequences on Stress-Sensitive Innate Immune Functions. High Alt. Med. Biol. 2014, 15, 341–348. [Google Scholar] [CrossRef]
- Feuerecker, M.; Crucian, B.E.; Quintens, R.; Buchheim, J.-I.; Salam, A.P.; Rybka, A.; Moreels, M.; Strewe, C.; Stowe, R.; Mehta, S.; et al. Immune Sensitization during 1 Year in the Antarctic High-Altitude Concordia Environment. Allergy 2019, 74, 64–77. [Google Scholar] [CrossRef]
- Yi, B.; Rykova, M.; Feuerecker, M.; Jäger, B.; Ladinig, C.; Basner, M.; Hörl, M.; Matzel, S.; Kaufmann, I.; Strewe, C.; et al. 520-d Isolation and Confinement Simulating a Flight to Mars Reveals Heightened Immune Responses and Alterations of Leukocyte Phenotype. Brain Behav. Immun. 2014, 40, 203–210. [Google Scholar] [CrossRef]
- Cole, S.W. Human Social Genomics. PLOS Genet. 2014, 10, e1004601. [Google Scholar] [CrossRef]
- Cole, S.W. Social Regulation of Human Gene Expression: Mechanisms and Implications for Public Health. Am. J. Public Health 2013, 103 (Suppl. S1), S84–S92. [Google Scholar] [CrossRef]
- Cole, S.W.; Hawkley, L.C.; Arevalo, J.M.; Sung, C.Y.; Rose, R.M.; Cacioppo, J.T. Social Regulation of Gene Expression in Human Leukocytes. Genome Biol. 2007, 8, R189. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W. The Conserved Transcriptional Response to Adversity. Curr. Opin. Behav. Sci. 2019, 28, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, Neuroinflammation and Biological Remodeling as Key Factors in Pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Brusaferri, L.; Alshelh, Z.; Martins, D.; Kim, M.; Weerasekera, A.; Housman, H.; Morrissey, E.J.; Knight, P.C.; Castro-Blanco, K.A.; Albrecht, D.S.; et al. The Pandemic Brain: Neuroinflammation in Non-Infected Individuals during the COVID-19 Pandemic. Brain Behav. Immun. 2022, 102, 89–97. [Google Scholar] [CrossRef]
- Tona, F.; Plebani, M.; Gregori, D.; Carretta, G.; Lorenzoni, G.; Donato, D.; Iliceto, S. “Stay Home Stay Safe?” Systemic Inflammation in Subjects Undergoing Routine Hematology Tests during the Lockdown Period of COVID-19. Clin. Chem. Lab. Med. 2020, 58, e315–e316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scatà, C.; Carandina, A.; Della Torre, A.; Arosio, B.; Bellocchi, C.; Dias Rodrigues, G.; Furlan, L.; Tobaldini, E.; Montano, N. Social Isolation: A Narrative Review on the Dangerous Liaison between the Autonomic Nervous System and Inflammation. Life 2023, 13, 1229. https://doi.org/10.3390/life13061229
Scatà C, Carandina A, Della Torre A, Arosio B, Bellocchi C, Dias Rodrigues G, Furlan L, Tobaldini E, Montano N. Social Isolation: A Narrative Review on the Dangerous Liaison between the Autonomic Nervous System and Inflammation. Life. 2023; 13(6):1229. https://doi.org/10.3390/life13061229
Chicago/Turabian StyleScatà, Costanza, Angelica Carandina, Alice Della Torre, Beatrice Arosio, Chiara Bellocchi, Gabriel Dias Rodrigues, Ludovico Furlan, Eleonora Tobaldini, and Nicola Montano. 2023. "Social Isolation: A Narrative Review on the Dangerous Liaison between the Autonomic Nervous System and Inflammation" Life 13, no. 6: 1229. https://doi.org/10.3390/life13061229
APA StyleScatà, C., Carandina, A., Della Torre, A., Arosio, B., Bellocchi, C., Dias Rodrigues, G., Furlan, L., Tobaldini, E., & Montano, N. (2023). Social Isolation: A Narrative Review on the Dangerous Liaison between the Autonomic Nervous System and Inflammation. Life, 13(6), 1229. https://doi.org/10.3390/life13061229










