The Mitochondrial tRNASer(UCN) Gene: A Novel m.7484A>G Mutation Associated with Mitochondrial Encephalomyopathy and Literature Review
Abstract
1. Introduction
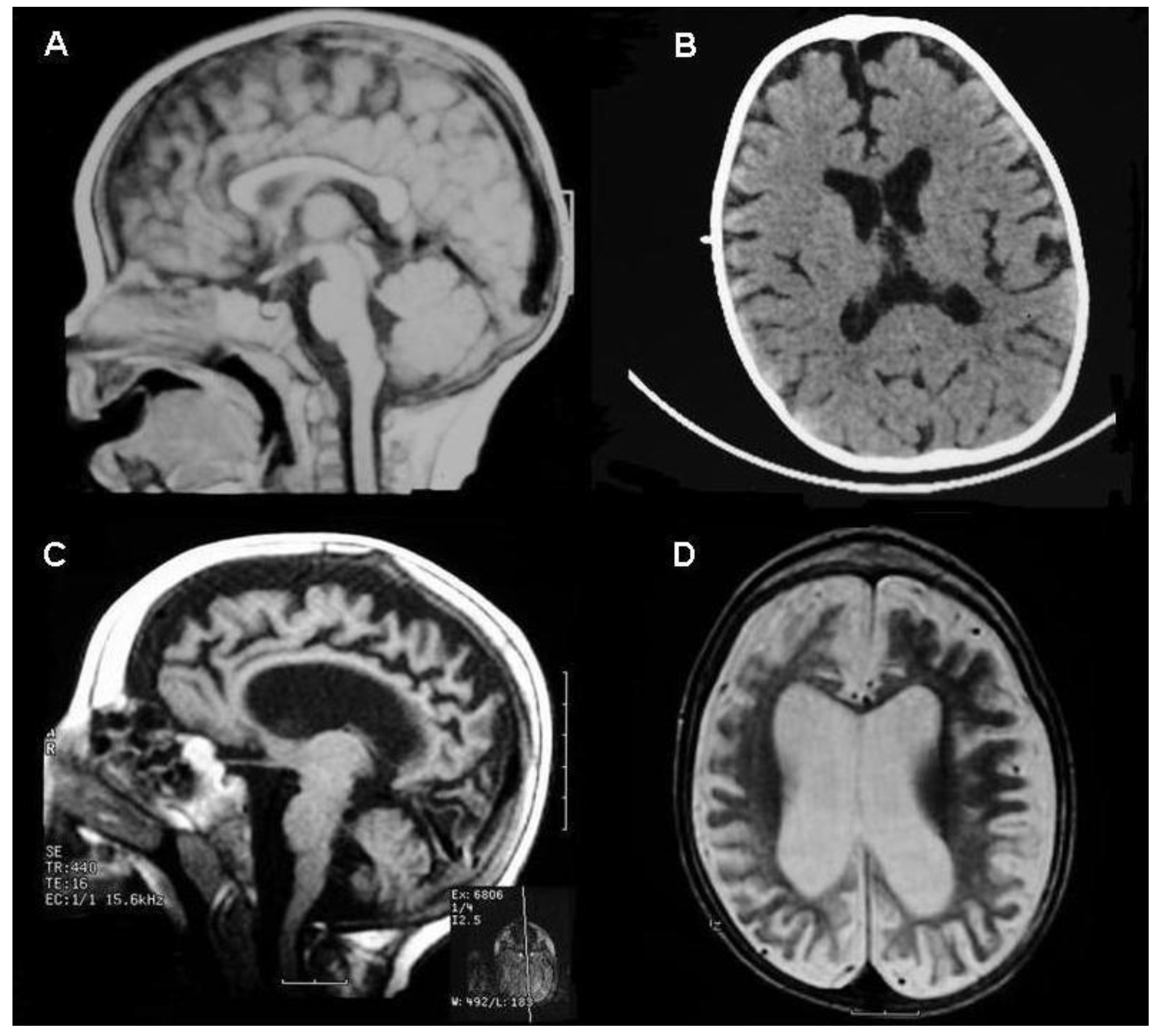
2. Case Report
3. Materials and Methods
3.1. Morphologic and Biochemical Analysis
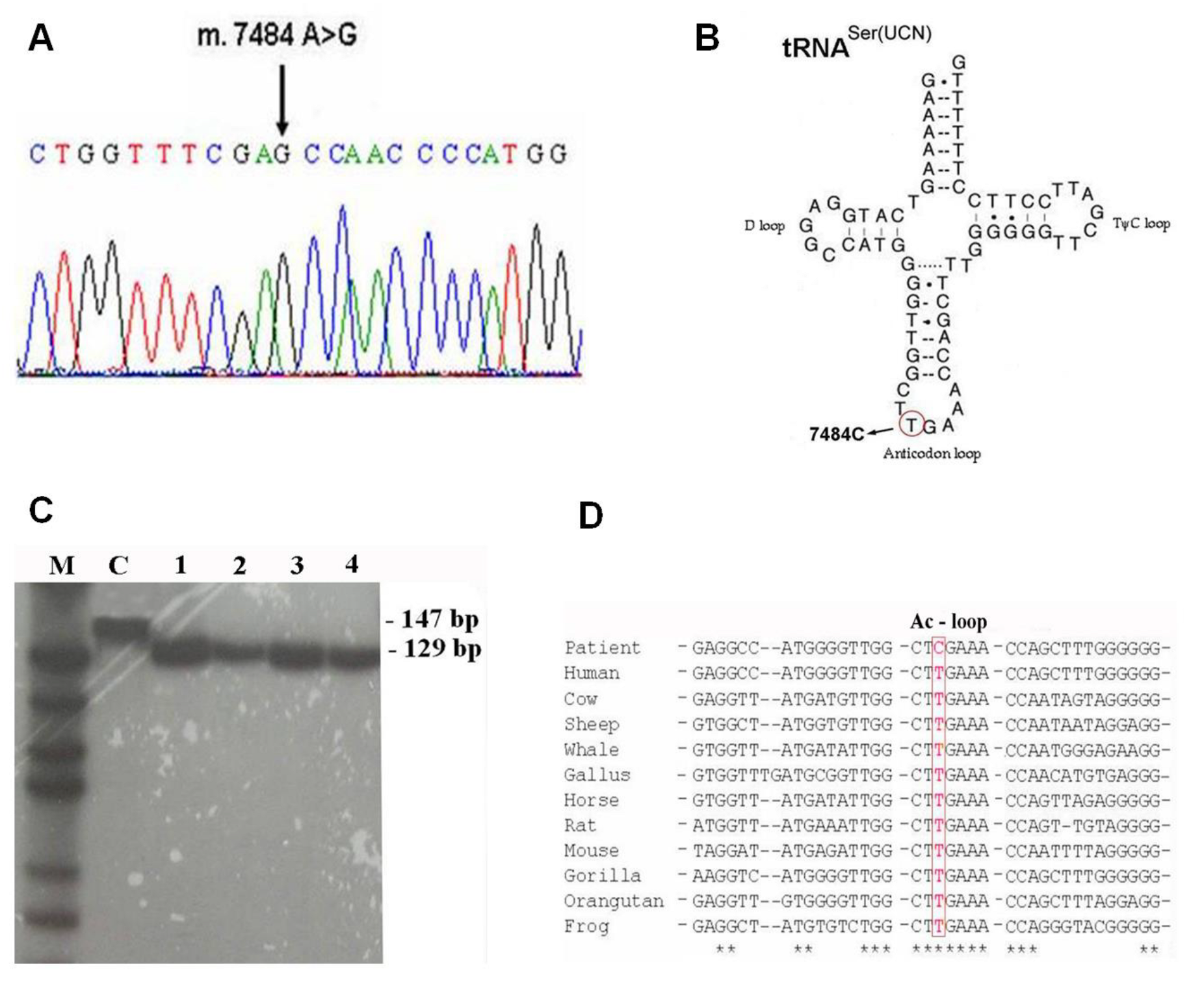
3.2. Molecular Genetic Analysis
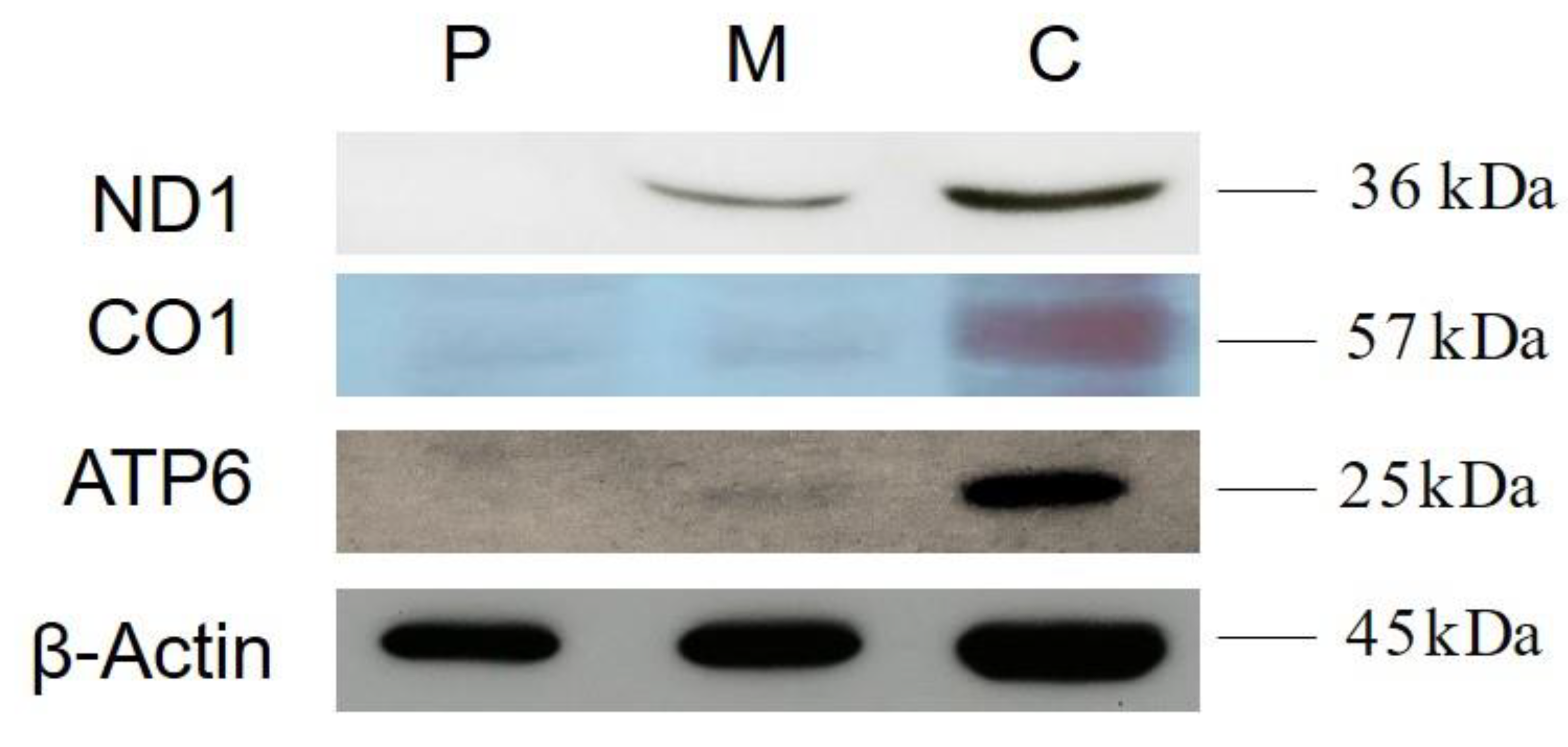
3.3. Western Blot Analysis
4. Results
5. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, F.; Xu, H. Human mitochondrial DNA diseases and Drosophila models. J. Genet. Genom. 2019, 46, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.-J.C.; Liang, M.-H.; Kwon, H.; Park, J.; Bai, R.-K.; Tan, D.-J. Comprehensive Scanning of the Entire Mitochondrial Genome for Mutations. Clin. Chem. 2002, 48, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Nonaka, I.; Horai, S. A Mutation in the tRNALeu(UUR) Gene Associated with the MELAS Subgroup of Mitochondrial Encephalomyopathies. Nature 1990, 348, 651–653. [Google Scholar] [CrossRef]
- Shoffner, J.M.; Lott, M.T.; Lezza, A.M.S.; Seibel, P.; Ballinger, S.W.; Wallace, D.C. Myoclonic Epilepsy and Ragged-Red Fiber Disease (MERRF) Is Associated with a Mitochondrial DNA tRNALys Mutation. Cell 1990, 61, 931–937. [Google Scholar] [CrossRef]
- Zeviani, M.; Gellera, C.; Antozzi, C.; Rimoldi, M.; Morandi, L.; Tiranti, V.; DiDonato, S.; Villani, F. Maternally Inherited Myopathy and Cardiomyopathy: Association with Mutation in Mitochondrial DNA tRNALeu(UUR). Lancet 1991, 338, 143–147. [Google Scholar] [CrossRef]
- Jaksch, M.; Klopstock, T.; Kurlemann, G.; Dörner, M.; Hofmann, S.; Kleinle, S.; Hegemann, S.; Weissert, M.; MüLler-Höcker, J.; Pongratz, D.; et al. Progressive Myoclonus Epilepsy and Mitochondrial Myopathy Associated with Mutations in the tRNAser(UCN) Gene. Ann. Neurol. 1998, 44, 635–640. [Google Scholar] [CrossRef]
- Sciacco, M.; Bonilla, E. [43]Cytochemistry and Immunocytochemistry of Mitochondria in Tissue Sections. In Methods in Enzymology; Mitochondrial Biogenesis and Genetics Part B; Academic Press: Cambridge, MA, USA, 1996; Volume 264, pp. 509–521. [Google Scholar] [CrossRef]
- DiMauro, S.; Servidei, S.; Zeviani, M.; DiRocco, M.; DeVivo, D.C.; DiDonato, S.; Uziel, G.; Berry, K.; Hoganson, G.; Johnsen, S.D.; et al. Cytochrome c Oxidase Deficiency in Leigh Syndrome. Ann. Neurol. 1987, 22, 498–506. [Google Scholar] [CrossRef]
- Rieder, M. Automating the Identification of DNA Variations Using Quality-Based Fluorescence Re-Sequencing: Analysis of the Human Mitochondrial Genome. Nucleic Acids Res. 1998, 26, 967–973. [Google Scholar] [CrossRef]
- Pavlakis, S.G.; Phillips, P.C.; DiMauro, S.; De Vivo, D.C.; Rowland, L.P. Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis, and Strokelike Episodes: A Distinctive Clinical Syndrome. Ann. Neurol. 1984, 16, 481–488. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, Y.; Tang, X.; Wang, J.; Yang, L.; Liao, Z.; Li, R.; Ji, J.; Li, Z.; Chen, J.; et al. Aminoglycoside-Induced and Non-Syndromic Hearing Loss Is Associated with the G7444A Mutation in the Mitochondrial COI/tRNASer(UCN) Genes in Two Chinese Families. Biochem. Biophys. Res. Commun. 2006, 342, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.; Xia, X.-J.; Erdenetungalag, R.; Amendola, M.; Landa, B.; Radnaabazar, J.; Dangaasuren, B.; Van Tuyle, G.; Nance, W.E. Heterogenous Point Mutations in the Mitochondrial tRNA Ser(UCN) Precursor Coexisting with the A1555G Mutation in Deaf Students from Mongolia. Am. J. Hum. Genet. 1999, 65, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Reid, F.M.; Vernham, G.A.; Jacobs, H.T. A Novel Mitochondrial Point Mutation in a Maternal Pedigree with Sensorineural Deafness. Hum. Mutat. 1994, 3, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Hutchin, T.; Parker, M.; Young, I.; Davis, A.; Pulleyn, L.; Deeble, J.; Lench, N.; Markham, A.; Mueller, R. A Novel Mutation in the Mitochondrial tRNASer(UCN) Gene in a Family with Non-Syndromic Sensorineural Hearing Impairment. J. Med. Genet. 2000, 37, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Sue, C.M.; Tanji, K.; Hadjigeorgiou, G.; Andreu, A.L.; Nishino, I.; Krishna, S.; Bruno, C.; Hirano, M.; Shanske, S.; Bonilla, E.; et al. Maternally Inherited Hearing Loss in a Large Kindred with a Novel T7511C Mutation in the Mitochondrial DNA tRNASer(UCN) Gene. Neurology 1999, 52, 1905. [Google Scholar] [CrossRef]
- Cardaioli, E.; Da Pozzo, P.; Gallus, G.N.; Malandrini, A.; Gambelli, S.; Gaudiano, C.; Malfatti, E.; Viscomi, C.; Zicari, E.; Berti, G.; et al. A Novel Heteroplasmic tRNASer(UCN) mtDNA Point Mutation Associated with Progressive External Ophthalmoplegia and Hearing Loss. Neuromuscul. Disord. 2007, 17, 681–683. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Tang, X.-W.; Lan, J.-S.; Lv, J.-X.; Yang, L.; Li, Z.-Y.; Zhu, Y.; Sun, D.-M.; Yang, A.-F.; Wang, J.-D.; et al. Hearing loss and epilepsy may be associated with the novel mitochondrial tRNASer(UCN) 7472delC mutation in a Chinese family. Yi Chuan 2008, 30, 1557–1562. [Google Scholar] [CrossRef]
- Jaksch, M.; Hofmann, S.; Kleinle, S.; Liechti-Gallati, S.; Pongratz, D.E.; Müller-Höcker, J.; Jedele, K.B.; Meitinger, T.; Gerbitz, K.D. A Systematic Mutation Screen of 10 Nuclear and 25 Mitochondrial Candidate Genes in 21 Patients with Cytochrome c Oxidase (COX) Deficiency Shows tRNA(Ser)(UCN) Mutations in a Subgroup with Syndromal Encephalopathy. J. Med. Genet. 1998, 35, 895–900. [Google Scholar] [CrossRef]
- Schuelke, M.; Bakker, M.; Stoltenburg, G.; Sperner, J.; von Moers, A. Epilepsia Partialis Continua Associated with a Homoplasmic Mitochondrial tRNASer(UCN) Mutation. Ann. Neurol. 1998, 44, 700–704. [Google Scholar] [CrossRef]
- Zaragoza, M.V.; Fass, J.; Diegoli, M.; Lin, D.; Arbustini, E. Mitochondrial DNA Variant Discovery and Evaluation in Human Cardiomyopathies through Next-Generation Sequencing. PLoS ONE 2010, 5, e12295. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Q.; Zhu, C.; Wang, X.; Yang, J.; Yin, T.; Gao, J.; Li, Z.; Ma, Q.; Guan, M.; et al. Systematic Analysis of the Clinical and Biochemical Characteristics of Maternally Inherited Hypertension in Chinese Han Families Associated with Mitochondrial. BMC Med. Genom. 2014, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Imasawa, T.; Tanaka, M.; Yamaguchi, Y.; Nakazato, T.; Kitamura, H.; Nishimura, M. 7501 T > A Mitochondrial DNA Variant in a Patient with Glomerulosclerosis. Ren. Fail. 2014, 36, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xia, B.-H.; Zhang, C.-J.; Zhuo, G.-C. Mutations in Mitochondrial tRNA Genes May Be Related to Insulin Resistance in Women with Polycystic Ovary Syndrome. Am. J. Transl. Res. 2017, 9, 2984–2996. [Google Scholar] [PubMed]
- Brown, M.D.; Torroni, A.; Reckord, C.L.; Wallace, D.C. Phylogenetic Analysis of Leber’s Hereditary Optic Neuropathy Mitochondrial DNA’s Indicates Multiple Independent Occurrences of the Common Mutations. Hum. Mutat. 1995, 6, 311–325. [Google Scholar] [CrossRef]
- Yuan, H.; Qian, Y.; Xu, Y.; Cao, J.; Bai, L.; Shen, W.; Ji, F.; Zhang, X.; Kang, D.; Mo, J.Q.; et al. Cosegregation of the G7444A Mutation in the Mitochondrial COI/tRNASer(UCN) Genes with the 12S RRNA A1555G Mutation in a Chinese Family with Aminoglycoside-Induced and Nonsyndromic Hearing Loss. Am. J. Med. Genet. A 2005, 138A, 133–140. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, J.; Liu, X.; Cheng, J.; Wang, X.; Yang, L.; Yang, S.; Cao, J.; Kang, D.; Dai, P.; et al. Coexistence of Mitochondrial 12S rRNA C1494T and CO1/tRNASer(UCN) G7444A Mutations in Two Han Chinese Pedigrees with Aminoglycoside-Induced and Non-Syndromic Hearing Loss. Biochem. Biophys. Res. Commun. 2007, 362, 94–100. [Google Scholar] [CrossRef]
- Mkaouar-Rebai, E.; Chamkha, I.; Kammoun, T.; Alila-Fersi, O.; Aloulou, H.; Hachicha, M.; Fakhfakh, F. A Novel MT-CO1 m.6498C>A Variation Associated with the m.7444G>A Mutation in the Mitochondrial COI/tRNASer(UCN) Genes in a Patient with Hearing Impairment, Diabetes and Congenital Visual Loss. Biochem. Biophys. Res. Commun. 2013, 430, 585–591. [Google Scholar] [CrossRef]
- Sevior, K.B.; Hatamochi, A.; Stewart, I.A.; Bykhovskaya, Y.; Allen-Powell, D.R.; Fischel-Ghodsian, N.; Maw, M.A. Mitochondrial A7445G Mutation in Two Pedigrees with Palmoplantar Keratoderma and Deafness. Am. J. Med. Genet. 1998, 75, 179–185. [Google Scholar] [CrossRef]
- Martin, L.; Toutain, A.; Guillen, C.; Haftek, M.; Machet, M.C.; Toledano, C.; Arbeille, B.; Lorette, G.; Rötig, A.; Vaillant, L. Inherited Palmoplantar Keratoderma and Sensorineural Deafness Associated with A7445G Point Mutation in the Mitochondrial Genome. Br. J. Dermatol. 2000, 143, 876–883. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, H.; Lu, J.; Liu, X.; Wang, G.; Zhu, Y.; Wang, X.; Han, B.; Yang, L.; Yang, S.; et al. Mutations at Position 7445 in the Precursor of Mitochondrial TRNA(Ser(UCN)) Gene in Three Maternal Chinese Pedigrees with Sensorineural Hearing Loss. Mitochondrion 2008, 8, 285–292. [Google Scholar] [CrossRef]
- Blakely, E.L.; Yarham, J.W.; Alston, C.L.; Craig, K.; Poulton, J.; Brierley, C.; Park, S.-M.; Dean, A.; Xuereb, J.H.; Anderson, K.N.; et al. Pathogenic Mitochondrial tRNA Point Mutations: Nine Novel Mutations Affirm Their Importance as a Cause of Mitochondrial Disease. Hum. Mutat. 2013, 34, 1260–1268. [Google Scholar] [CrossRef]
- Götz, A.; Isohanni, P.; Liljeström, B.; Rummukainen, J.; Nikolajev, K.; Herrgård, E.; Marjavaara, S.; Suomalainen, A. Fatal Neonatal Lactic Acidosis Caused by a Novel de Novo Mitochondrial G7453A tRNA-Serine ((UCN)) Mutation. Pediatr. Res. 2012, 72, 90–94. [Google Scholar] [CrossRef]
- Riley, L.G.; Cowley, M.J.; Gayevskiy, V.; Minoche, A.E.; Puttick, C.; Thorburn, D.R.; Rius, R.; Compton, A.G.; Menezes, M.J.; Bhattacharya, K.; et al. The Diagnostic Utility of Genome Sequencing in a Pediatric Cohort with Suspected Mitochondrial Disease. Genet. Med. 2020, 22, 1254–1261. [Google Scholar] [CrossRef]
- Jacobs, H.T.; Hutchin, T.P.; Käppi, T.; Gillies, G.; Minkkinen, K.; Walker, J.; Thompson, K.; Rovio, A.T.; Carella, M.; Melchionda, S.; et al. Mitochondrial DNA Mutations in Patients with Postlingual, Nonsyndromic Hearing Impairment. Eur. J. Hum. Genet. 2005, 13, 26–33. [Google Scholar] [CrossRef]
- Sihem, S.; Mounir, K.; Mancuso, M.; Nesti, C.; Faycal, H.; Rim, A. A Novel Heteroplasmic tRNA Ser(UCN) MtDNA Point Mutation Associated with Progressive Ophthalmoplegia and Dysphagia. Diagn. Mol. Pathol. Am. J. Surg. Pathol. Part B 2010, 19, 28–32. [Google Scholar] [CrossRef]
- Uehara, D.T.; Rincon, D.; Abreu-Silva, R.S.; de Mello Auricchio, M.T.B.; Tabith, A.; Kok, F.; Mingroni-Netto, R.C. Role of the Mitochondrial Mutations, m.827A>G and the Novel m.7462C>T, in the Origin of Hearing Loss. Genet. Test. Mol. Biomarkers 2010, 14, 611–616. [Google Scholar] [CrossRef]
- Yang, P.; Wu, P.; Liu, X.; Feng, J.; Zheng, S.; Wang, Y.; Fan, Z. Mitochondrial tRNASer(UCN) 7471delC May Be a Novel Mutation Associated with Maternally Transmitted Hypertension. Ir. J. Med. Sci. 2020, 189, 489–496. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, J.; Ying, Z.; Cai, Z.; Gao, Y.; He, Z.; Yu, H.; Yao, J.; Yang, Y.; Wang, H.; et al. Mitochondrial tRNA(Ser(UCN)) Variants in 2651 Han Chinese Subjects with Hearing Loss. Mitochondrion 2015, 23, 17–24. [Google Scholar] [CrossRef]
- Tiranti, V.; Chariot, P.; Carella, F.; Toscano, A.; Soliveri, P.; Girlanda, P.; Carrara, F.; Fratta, G.M.; Reid, F.M.; Mariotti, C.; et al. Maternally Inherited Hearing Loss, Ataxia and Myoclonus Associated with a Novel Point Mutation in Mitochondrial tRNA Ser(UCN) Gene. Hum. Mol. Genet. 1995, 4, 1421–1427. [Google Scholar] [CrossRef]
- Fetoni, V.; Briem, E.; Carrara, F.; Mora, M.; Zeviani, M. Monomelic Amyotrophy Associated with the 7472insC Mutation in the MtDNA TRNASer(UCN) Gene. Neuromuscul. Disord. 2004, 14, 723–726. [Google Scholar] [CrossRef]
- Hutchin, T.; Thompson, K.; Parker, M.; Newton, V.; Bitner-Glindzicz, M.; Mueller, R. Prevalence of Mitochondrial DNA Mutations in Childhood/Congenital Onset Non-Syndromal Sensorineural Hearing Impairment. J. Med. Genet. 2001, 38, 229–231. [Google Scholar] [CrossRef]
- Pulkes, T.; Liolitsa, D.; Eunson, L.H.; Rose, M.; Nelson, I.P.; Rahman, S.; Poulton, J.; Marchington, D.R.; Landon, D.N.; Debono, A.G.; et al. New Phenotypic Diversity Associated with the Mitochondrial TRNASer(UCN) Gene Mutation. Neuromuscul. Disord. 2005, 15, 364–371. [Google Scholar] [CrossRef]
- Cardaioli, E.; Pozzo, P.D.; Cerase, A.; Sicurelli, F.; Malandrini, A.; Stefano, N.D.; Stromillo, M.L.; Battisti, C.; Dotti, M.T.; Federico, A. Rapidly Progressive Neurodegeneration in a Case with the 7472insC Mutation and the A7472C Polymorphism in the mtDNA tRNAser(UCN) Gene. Neuromuscul. Disord. 2006, 16, 26–31. [Google Scholar] [CrossRef]
- Zheng, J.; Bai, X.; Xiao, Y.; Ji, Y.; Meng, F.; Aishanjiang, M.; Gao, Y.; Wang, H.; Fu, Y.; Guan, M.-X. Mitochondrial tRNA Mutations in 887 Chinese Subjects with Hearing Loss. Mitochondrion 2020, 52, 163–172. [Google Scholar] [CrossRef]
- Bidooki, S.; Jackson, M.J.; Johnson, M.A.; Chrzanowska-Lightowlers, Z.M.A.; Taylor, R.W.; Venables, G.; Lightowlers, R.N.; Turnbull, D.M.; Bindoff, L.A. Sporadic Mitochondrial Myopathy Due to a New Mutation in the Mitochondrial tRNASer(UCN) Gene. Neuromuscul. Disord. 2004, 14, 417–420. [Google Scholar] [CrossRef]
- Bacalhau, M.; Simões, M.; Rocha, M.C.; Hardy, S.A.; Vincent, A.E.; Durães, J.; Macário, M.C.; Santos, M.J.; Rebelo, O.; Lopes, C.; et al. Disclosing the Functional Changes of Two Genetic Alterations in a Patient with Chronic Progressive External Ophthalmoplegia: Report of the Novel mtDNA m.7486G>A Variant. Neuromuscul. Disord. 2018, 28, 350–360. [Google Scholar] [CrossRef]
- Peng, W.; Zhong, Y.; Zhao, X.; Yuan, J. Low Penetrance of Hearing Loss in Two Chinese Families Carrying the Mitochondrial tRNASer(UCN) Mutations. Mol. Med. Rep. 2020, 22, 77–86. [Google Scholar] [CrossRef]
- Grafakou, O.; Hol, F.A.; Otfried Schwab, K.; Siers, M.H.; ter Laak, H.; Trijbels, F.; Ensenauer, R.; Boelen, C.; Smeitink, J. Exercise Intolerance, Muscle Pain and Lactic Acidaemia Associated with a 7497G>A Mutation in the tRNASer(UCN) Gene. J. Inherit. Metab. Dis. 2003, 26, 593–600. [Google Scholar] [CrossRef]
- Müller, T.; Deschauer, M.; Neudecker, S.; Zierz, S. Late-Onset Mitochondrial Myopathy with Dystrophic Changes Due to a G7497A Mutation in the Mitochondrial tRNASer (UCN) Gene. Acta Neuropathol. 2005, 110, 426–430. [Google Scholar] [CrossRef]
- Jiang, P.; Ling, Y.; Zhu, T.; Luo, X.; Tao, Y.; Meng, F.; Cheng, W.; Ji, Y. Mitochondrial tRNA Mutations in Chinese Children with Tic Disorders. Biosci. Rep. 2020, 40, BSR20201856. [Google Scholar] [CrossRef]
- Tang, X.; Li, R.; Zheng, J.; Cai, Q.; Zhang, T.; Gong, S.; Zheng, W.; He, X.; Zhu, Y.; Xue, L.; et al. Maternally Inherited Hearing Loss Is Associated with the Novel Mitochondrial tRNASer(UCN) 7505T>C Mutation in a Han Chinese Family. Mol. Genet. Metab. 2010, 100, 57–64. [Google Scholar] [CrossRef] [PubMed]
- McCann, B.J.; Tuppen, H.A.L.; Küsters, B.; Lammens, M.; Smeitink, J.A.M.; Taylor, R.W.; Rodenburg, R.J.; Wortmann, S.B. A Novel Mitochondrial DNA m.7507A>G Mutation Is Only Pathogenic at High Levels of Heteroplasmy. Neuromuscul. Disord. 2015, 25, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nakano, S.; Goto, Y.; Ozawa, M.; Nagahama, Y.; Fukuyama, H.; Akiguchi, I.; Kaji, R.; Kimura, J. A Novel Point Mutation in the Mitochondrial tRNASer(UCN) Gene Detected in a Family with MERRF/MELAS Overlap Syndrome. Biochem. Biophys. Res. Commun. 1995, 214, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ramelli, G.P.; Gallati, S.; Weis, J.; Krähenbühl, S.; Burgunder, J.-M. Point Mutation tRNASer(UCN) in a Child With Hearing Loss and Myoclonus Epilepsy. J. Child Neurol. 2006, 21, 253–255. [Google Scholar] [CrossRef]
- Lindberg, C.; Moslemi, A.-R.; Oldfors, A. MELAS Syndrome in a Patient with a Point Mutation in MTTS1. Acta Neurol. Scand. 2008, 117, 128–132. [Google Scholar] [CrossRef]
- Toompuu, M.; Yasukawa, T.; Suzuki, T.; Hakkinen, T.; Spelbrink, J.N.; Watanabe, K.; Jacobs, H.T. The 7472insC Mitochondrial DNA Mutation Impairs the Synthesis and Extent of Aminoacylation of tRNASer(UCN) but Not Its Structure or Rate of Turnover. J. Biol. Chem. 2002, 277, 22240–22250. [Google Scholar] [CrossRef]
- Normanly, J.; Abelson, J. tRNA IDENTITY. Ann. Rev. Biochem. 1989, 58, 1029–1049. [Google Scholar] [CrossRef]
- Schulman, L.H. Recognition of tRNAs by Aminoacyl-TRNA Synthetases. Prog. Nucleic Acid Res. Mol. Biol. 1991, 41, 23–87. [Google Scholar]
- Schimmel, P.R.; Söll, D. Aminoacyl-tRNA Synthetases: General Features and Recognition of Transfer RNAs. Ann. Rev. Biochem. 1979, 48, 601–648. [Google Scholar] [CrossRef]
- Scuderi, C.; Borgione, E.; Musumeci, S.; Elia, M.; Castello, F.; Fichera, M.; Davidzon, G.; DiMauro, S. Severe Encephalomyopathy in a Patient with Homoplasmic A5814G Point Mutation in Mitochondrial tRNACys Gene. Neuromuscul. Disord. 2007, 17, 258–261. [Google Scholar] [CrossRef]
- Wong, L.-J.C.; Chen, T.; Wang, J.; Tang, S.; Schmitt, E.S.; Landsverk, M.; Li, F.; Wang, Y.; Zhang, S.; Zhang, V.W.; et al. Interpretation of Mitochondrial tRNA Variants. Genet. Med. 2020, 22, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Darin, N.; Kollberg, G.; Andersson Grönlund, M.; Tulinius, M.; Holme, E.; Moslemi, A.-R.; Oldfors, A. A Novel Mitochondrial tRNA Arg Mutation Resulting in an Anticodon Swap in a Patient with Mitochondrial Encephalomyopathy. Eur. J. Hum. Genet. 2013, 21, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Anitori, R.; Manning, K.; Quan, F.; Weleber, R.G.; Buist, N.R.M.; Shoubridge, E.A.; Kennaway, N.G. Contrasting Phenotypes in Three Patients with Novel Mutations in Mitochondrial tRNA Genes. Mol. Genet. Metab. 2005, 84, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Filosto, M.; Mootha, V.K.; Rocchi, A.; Pistolesi, S.; Murri, L.; DiMauro, S.; Siciliano, G. A Novel Mitochondrial tRNAPhe Mutation Causes MERRF Syndrome. Neurology 2004, 62, 2119–2121. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.T.; Ciacci, F.; Bonilla, E.; Ionasescu, V.; Schon, E.A.; DiMauro, S. A Mitochondrial tRNA Anticodon Swap Associated with a Muscle Disease. Nat. Genet. 1993, 4, 284–288. [Google Scholar] [CrossRef]
- Sacconi, S.; Salviati, L.; Nishigaki, Y.; Walker, W.F.; Hernandez-Rosa, E.; Trevisson, E.; Delplace, S.; Desnuelle, C.; Shanske, S.; Hirano, M.; et al. A Functionally Dominant Mitochondrial DNA Mutation. Hum. Mol. Genet. 2008, 17, 1814–1820. [Google Scholar] [CrossRef]
- Zanssen, S.; Molnar, M.; Schröder, J.M.; Buse, G. Multiple Mitochondrial tRNA(Leu[UUR]) Mutations Associated with Infantile Myopathy. Mol. Cell. Biochem. 1997, 174, 231–236. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Ozand, P.T.; Al-Dhalaan, H. Novel Mitochondrial DNA Transversion Mutation in Transfer Ribonucleic Acid for Leucine 2 (CUN) in a Patient With the Clinical Features of MELAS. J. Child Neurol. 2006, 21, 971–972. [Google Scholar] [CrossRef]


| Locus | Mutation | Homoplasmy | Heteroplasmy | Status | MitoTIP | Disease | First Report |
|---|---|---|---|---|---|---|---|
| MT-COI/ MT-TS1 precursor | m.7443A>G | + | - | Reported | - | Hearing loss | Pandya et al., 1999 [13] |
| MT-COI/ MT-TS1 precursor | m.7444G>A | + | - | Reported | - | Aminoglycoside-induced deafness and non syndromic hearing loss | Zhu et al., 2006 [12] |
| m.7444G>A (with m.3460G>A or m.14484T>C) | LHON | Brown et al., 1995 [25] | |||||
| m.7444G>A (with m.1555A>G) | Hearing loss | Pandya et al., 1999 [13] | |||||
| Aminoglycoside-induced deafness | Yuan et al., 2005 [26] | ||||||
| m.7444G>A (with m.1494C>T) | Aminoglycoside-induced and non syndromic hearing loss | Yuan et al., 2007 [27] | |||||
| m.7444G>A (with m.6498C>A) | Non syndromic hearing loss, diabetes and congenital visual loss | Mkaouar-Rebai et al., 2013 [28] | |||||
| MT-TS1 precursor | m.7445A>C | + | - | Reported | - | Hearing loss | Pandya et al., 1999 [13] |
| MT-TS1 precursor | m.7445A>G | + | + | Confirmed | - | Sensorineural hearing loss | Reid et al., 1994 [14] |
| Progressive hearing loss and palmoplantar keratoderma | Sevior et al., 1998 [29] | ||||||
| Sensorineural deafness and NEPPK | Martin et al., 2000 [30] | ||||||
| MT-TS1 precursor | m.7445A>T | + | - | Reported | - | Sensorineural hearing loss | Chen et al., 2008 [31] |
| MT-TS1 | m.7451A>T | - | + | Reported | 80.70% | C-PEO, ptosis | Blakely et al., 2013 [32] |
| MT-TS1 | m.7453G>A | + | - | Reported | 68.00% | Fatal neonatal lactic acidosis | Gotz et al., 2012 [33] |
| Neonatal lactic acidosis, exercise intolerance, mild ID | Riley et al., 2020 [34] | ||||||
| MT-TS1 | m.7456A>G | + | - | Unclear | 16.00% | Deafness | Jacobs et al., 2005 [35] |
| MT-TS1 | m.7458G>A | - | + | Reported | 86.00% | PEO | Souilem et al., 2010 [36] |
| MT-TS1 | m.7462C>T | + | - | Reported | 11.20% | Hearing loss | Uehara et al., 2010 [37] |
| MT-TS1 | m.7471del | nd | nd | Reported | 4.30% | Maternally inherited hypertension | Yang et al., 2020 [38] |
| Deafness | Tang et al., 2015 [39] | ||||||
| MT-TS1 | m.7471_7472insC (reported as m.7472insC) | + | + | Confirmed | - | Hearing loss, ataxia, dysarthria and, occasionally, peripheral sensory neuropathy and focal myoclonus | Tiranti et al., 1995 [40] |
| Sensorineural hearing loss, myoclonic epilepsy, ataxia, MR | Jaksch et al., 1998 [19] | ||||||
| Epilepsia partialis continua, ataxia, lactic acidosis, myopathy, sensorineural hearing loss, severe headaches, and MR | Schuelke et al., 1998 [20] | ||||||
| Non syndromic sensorineural hearing loss and monomelic amyotrophic | Fetoni et al., 2004 [41] | ||||||
| Non syndromic sensorineural hearing loss | Hutchin et al., 2001 [42] | ||||||
| MT-TS1 | m.7472A>C (with m.7471_7472insC) | + | + | Reported | 3.2% | Early onset myopathy and execise intollerance | Pulkes et al., 2005 [43] |
| Bilateral hearing loss, MR, fatal neurodegeneration with cognitive decline, epilepsia partialis continua, myopathy, lactic acidosis and ataxia | Cardaioli et al., 2006 [44] | ||||||
| MT-TS1 | m.7474A>G | nd | nd | Reported | 0.00% | Hearing loss | Zheng et al., 2020 [45] |
| MT-TS1 | m.7474del | nd | nd | Reported | 34.80% | Hearing loss and epilepsy | Zhao et al., 2008 [18] |
| MT-TS1 | m.7480T>C | - | + | Reported | 46.60% | Progressive mitochondrial myopathy, deafness, dementia and ataxia | Bidooki et al., 2004 [46] |
| MT-TS1 | m.7486G>A | - | + | Reported | 50.50% | C-PEO | Bacalhau et al., 2018 [47] |
| MT-TS1 | m.7492C>T | + | - | Reported | 0.10% | Hypertension | Liu et al., 2014 [22] |
| Hearing loss | Peng et al., 2020 [48] | ||||||
| Polycystic ovary syndrome-insulin resistance | Dyng et al., 2017 [24] | ||||||
| MT-TS1 | m.7496T>C | nd | nd | Reported | 58.30% | Hearing Loss | Tang et al., 2015 [39] |
| MT-TS1 | m.7497G>A | + | + | Confirmed | Pathogenic | Severe progressive myopathy, muscle weakness and increase exercise intolerance | Jaksch et al., 1998 [7] |
| Exercise intolerance, muscle pain and lactic acidemia | Grafakou et al., 2003 [49] | ||||||
| Muscular weakness, atrophy and severe dystrophic myopathy | Muller et al., 2005 [50] | ||||||
| MT-TS1 | m.7501T>A | nd | nd | Reported | 1.90% | Cardiovascular disease | Zaragoza et al., 2010 [21] |
| Renal disease patient | Imasawa et al., 2014 [23] | ||||||
| MT-TS1 | m.7502C>T | nd | nd | Reported | 8.20% | Tic disorder | Jiang et al., 2020 [51] |
| MT-TS1 | m.7505T>C | + | - | Reported | 58.60% | Maternally inherited hearing loss | Tang et al., 2010 [52] |
| MT-TS1 | m.7506G>A | - | + | Reported | 81.40% | PEO and hearing loss | Cardaioli et al., 2007 [17] |
| MT-TS1 | m.7507A>G | + | - | Reported | - | Cardio-respiratory failure and fatal lactic acidosis, severe hearing loss and progressive exercise intolerance | McCann et al., 2015 [53] |
| MT-TS1 | m.7510T>C | - | + | Confirmed | Pathogenic | Non syndromic sensorineural hearing loss | Hutchin et al., 2000 [15] |
| MT-TS1 | m.7511T>C | + | + | Confirmed | Pathogenic | Non syndromic hearing loss | Sue et al., 1999 [16] |
| MT-TS1 | m.7512T>C | + | + | Reported | 64.20% | MERRF/MELAS overlap syndrome | Nakamura et al., 1995 [54] |
| Sensorineural hearing loss, myoclonic epilepsy, ataxia, MR | Jaksch et al., 1998 [19] | ||||||
| Sensorineural hearing loss, myoclonus epilepsy, ataxia, severe psychomotor retardation, short stature, and diabetes mellitus | Ramelli et al., 2006 [55] | ||||||
| MELAS syndrome | Lindberg et al., 2008 [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgione, E.; Lo Giudice, M.; Santa Paola, S.; Giuliano, M.; Di Blasi, F.D.; Di Stefano, V.; Lupica, A.; Brighina, F.; Pettinato, R.; Romano, C.; et al. The Mitochondrial tRNASer(UCN) Gene: A Novel m.7484A>G Mutation Associated with Mitochondrial Encephalomyopathy and Literature Review. Life 2023, 13, 554. https://doi.org/10.3390/life13020554
Borgione E, Lo Giudice M, Santa Paola S, Giuliano M, Di Blasi FD, Di Stefano V, Lupica A, Brighina F, Pettinato R, Romano C, et al. The Mitochondrial tRNASer(UCN) Gene: A Novel m.7484A>G Mutation Associated with Mitochondrial Encephalomyopathy and Literature Review. Life. 2023; 13(2):554. https://doi.org/10.3390/life13020554
Chicago/Turabian StyleBorgione, Eugenia, Mariangela Lo Giudice, Sandro Santa Paola, Marika Giuliano, Francesco Domenico Di Blasi, Vincenzo Di Stefano, Antonino Lupica, Filippo Brighina, Rosa Pettinato, Corrado Romano, and et al. 2023. "The Mitochondrial tRNASer(UCN) Gene: A Novel m.7484A>G Mutation Associated with Mitochondrial Encephalomyopathy and Literature Review" Life 13, no. 2: 554. https://doi.org/10.3390/life13020554
APA StyleBorgione, E., Lo Giudice, M., Santa Paola, S., Giuliano, M., Di Blasi, F. D., Di Stefano, V., Lupica, A., Brighina, F., Pettinato, R., Romano, C., & Scuderi, C. (2023). The Mitochondrial tRNASer(UCN) Gene: A Novel m.7484A>G Mutation Associated with Mitochondrial Encephalomyopathy and Literature Review. Life, 13(2), 554. https://doi.org/10.3390/life13020554








