Abstract
Lithium is a therapeutic cation used to treat bipolar disorders but also has some important features as an anti-cancer agent. In this review, we provide a general overview of lithium, from its transport into cells, to its innovative administration forms, and based on genomic, transcriptomic, and proteomic data. Lithium formulations such as lithium acetoacetate (LiAcAc), lithium chloride (LiCl), lithium citrate (Li3C6H5O7), and lithium carbonate (Li2CO3) induce apoptosis, autophagy, and inhibition of tumor growth and also participate in the regulation of tumor proliferation, tumor invasion, and metastasis and cell cycle arrest. Moreover, lithium is synergistic with standard cancer therapies, enhancing their anti-tumor effects. In addition, lithium has a neuroprotective role in cancer patients, by improving their quality of life. Interestingly, nano-sized lithium enhances its anti-tumor activities and protects vital organs from the damage caused by lipid peroxidation during tumor development. However, these potential therapeutic activities of lithium depend on various factors, such as the nature and aggressiveness of the tumor, the type of lithium salt, and its form of administration and dosage. Since lithium has been used to treat bipolar disorder, the current study provides an overview of its role in medicine and how this has changed. This review also highlights the importance of this repurposed drug, which appears to have therapeutic cancer potential, and underlines its molecular mechanisms.
1. Introduction
Cancer is a disease characterized by the uncontrolled growth of certain cells. The phenotypic and genotypic complexity of cancer cells translates into the acquisition of functional capabilities, to continue to grow and spread. Capabilities for sustaining proliferative signaling, evading growth suppressors, non-mutational epigenetic reprogramming, avoiding immune destruction, enabling replicative immortality, tumor-promoting inflammation, polymorphic microbiomes, activating invasion and metastasis, inducing or accessing vasculature, senescent cells, genome instability and mutation, resisting cell death, deregulating cellular metabolism, and unlocking phenotypic plasticity are all hallmarks of cancer [1]. Metabolic alterations in cancer, known as the Warburg effect [2], have emerged as an alternative therapeutic pathway to deprive the body and tumor of glucose [3]. Therefore, there has been interest in ketogenic bodies (KBs), to evaluate the mechanism of glucose deprivation as a therapy in cancer. Among the KBs, acetoacetate (AcAc) is the most abundant and relevant [3] and, for in vitro and in vivo assays, AcAc is often used in the form of lithium acetoacetate (LiAcAc). Lithium has various therapeutic effects on neurotrophic factors, neurotransmitters, oxidative metabolism, apoptosis, neuronal structures, and glia [4,5]. In this context, several publications reported that LiAcAc affects cancer cell growth. Other reports, however, indicated that lithium chloride (LiCl) inhibited cell growth in a dose-dependent manner, similarly to LiAcAc. Several questions arise in this regard, is lithium involved in certain hallmarks of cancer? Does lithium have a therapeutic potential in cancer? In this review, genomic, transcriptomic, and proteomic approaches are used to try to answer these questions.
2. The Effect of Lithium on Cancer Progression: Preclinical Approach
Lithium is a highly reactive alkali metal that exists as Li+ in seawater and in minerals [6]. The Li+ cation is therapeutically relevant, given that it has been used to treat bipolar disorders [7,8,9], Alzheimer’s disease [10,11], and as adjunctive therapy in thyroid cancer [12,13]. For bipolar mood disorders, achievable Li levels in plasma are around 0.5–1.0 mM (mEq/L) [14], whereas concentrations higher than 2 mM cause cytotoxicity in patients, resulting in nausea, fine tremors, diarrhea, confusion, slurred speech, and ataxia [14]. Lithium forms a complex with ATP and Mg2+, resulting in the bioactive form of lithium that modulates purine receptor activity in neuronal cells [15]. Lithium had been used over decades for bipolar disorder treatment, preventing relapses, and for the prophylaxis of manic episodes [16]. Some morphological abnormalities have been observed in patients with bipolar disorder. The neuroprotective activity of Li is related to the inhibition of its molecular targets, such as the enzymes inositol-monophosphate (IMPase), glycogen-synthase-kinase 3β (GSK3), and protein kinase C (PKC) [17]. Interestingly, these enzymes are deregulated in cancer, suggesting a potential role for Li, beyond bipolar disorders, in cancer treatment. Recent in vitro and in vivo studies reported the effects of lithium (lithium acetoacetate (LiAcAc), lithium chloride (LiCl), lithium citrate (Li3C6H5O7), and lithium carbonate (Li2CO3) on cell proliferation, tumor growth, and cancer metabolism. According to this evidence, LiAcAc inhibited cell proliferation in colon, breast, pancreatic, and neuroblastoma cancers [18]. Table 1 shows some recent preclinical studies elucidating the value of lithium in cancer treatment.

Table 1.
Recent preclinical studies of lithium in cancer treatment.
3. Lithium Transport
The Li+ ion shares transport and permeation pathways with other ions, such as sodium [38]. Therefore, extracellular and intracellular concentrations of the Li+ ion depend on its ingestion [39]. However, LiAcAc requires specific transporters. In cells, the presence of monocarboxylate transporters (MCTs) is involved in the passive transport of lactate, pyruvate, D-β-hydroxybutyrate, and acetoacetate across the cell membrane [40]. MCT2 has the highest affinity constant (Km) for KBs such as D-β-hydroxybutyrate and acetoacetate [41], but these substrates also are transported by MCT1 [42] and MCT4, which have a lower affinity for them [43]. LiAcAc is imported into cells through MCT1 and MCT2, with Km values of 5.5 mM and 0.8 mM, respectively [41,43,44]. These values indicate that MCT2 has a higher affinity for LiAcAc compared to MCT1, therefore the cellular expression of MCT2 could determine the cellular response to Li+ ion exposure. Differential expression of MCTs in healthy and cancer tissues has been reported. In this way, MCT1 is expressed by healthy or cancer tissues [45]. Furthermore, MCT1 has a specific expression in cardiomyocytes, hepatocytes, and cells of the gastrointestinal tract (stomach, duodenum, jejunum, ileum, cecum, colon, and rectum) [44,46,47,48,49,50,51,52,53,54,55]. In addition, this transporter is found in erythrocytes, lymphocytes, and monocytes [46,56], in adipocytes [57,58] and in mammary glands [59]. Moreover, MCT1 is found in endothelial brain cells, astrocytes, oligodendrocytes, microglial cells, and the hypothalamus [60,61,62,63,64]. Furthermore, this transporter was found in the peripheral nervous system [65,66]. While MCT1 is a highly distributed transporter, MCT2 has a restricted distribution, being found in the liver and kidney, testis, and central nervous system [44,67,68,69,70]. The ratio of intracellular/extracellular lithium concentration is about five, suggesting a passive distribution of Li+ ion. The transport of lithium is in both directions across the cellular membrane through a Na+-dependent counter-transport system. Furthermore, Li+ ion enters the voltage-dependent Na+ channel, but the Na+-K+ pump mediates the lithium uptake but not its release in cells [71]. Therefore, neuronal cells maintained a ratio of internal-external Li+ ion below 1 mM in patients exposed to 1–2 mM of lithium [71].
Lithium Transporters in Cancer
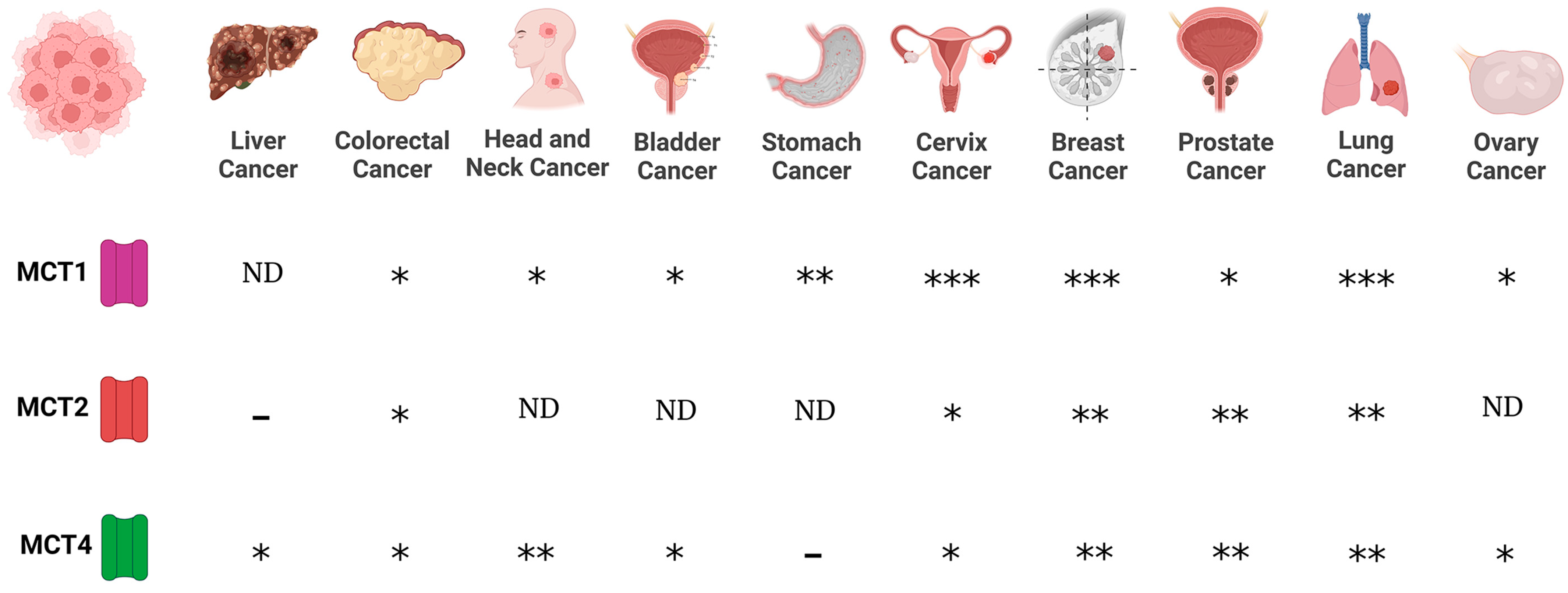
Regarding MCTs, evidence indicates that MCT1 and MCT4 are expressed in cancer cells, whereas scant information is available regarding MCT2 and MCT3 expression in cancer. Expression of MCT1 was reported for lung (non-small cell lung carcinomas, NSCLC), stomach, colon, bladder, prostate, breast, ovarian cervical, head and neck cancers, and gliomas [72,73,74,75,76,77,78]. On the other hand, MCT4 is expressed in colon, bladder, and breast cancers, and gliomas [74,76,77,78]. In addition, while MCT2 is expressed in NSCLS, colon, breast, and ovarian cancers [74,78], MCT3 is found in NSCLC [78] (Figure 1).
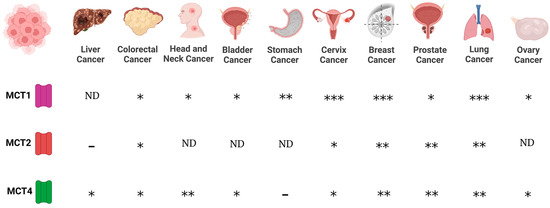
Figure 1.
Expression of monocarboxylate transporters (MCTs) in various cancers. MCTs are involved in the passive transport of lithium acetoacetate across cell membranes, and their abundance is associated with cancer. (-), no expression; (*), medium expression; (**), high expression; (***), very high expression level; ND, not data.
Furthermore, chaperone proteins of MCTs are important mediators in cancer. CD147 and CD44 function as chaperones of MCT1. In this way, the expression of MCT1 and CD147 correlated in bladder and ovarian carcinomas [78], whereas the expression of CD44 is associated with MCT1 expression in lung cancer [74]. Interestingly, the expression of MCT1 and CD147 in bladder tumors showed a poor prognosis [78]. However, when the expression of CD147 was downregulated by silencing using small interfering RNAs, an increase in the chemosensitivity to cisplatin was observed in bladder cell culture [78]. In prostate, breast, and lung cancers, the expression of MCT4 is associated with CD147 expression [74,78], suggesting a link between lithium transporters and their chaperone proteins in various cancers. In addition, evidence indicates that MCT’s differential expression depends on cancer progression. In this way, MCT1 is observed in healthy epithelium and malignant glands, while MCT2 was found in both benign to neoplastic lesions, but also in malignant glands. In contrast, the presence of MCT4 was observed in malignant glands with poor prognosis. These results suggest that MCT1 and MCT2 are involved in tumor maintenance and that MCT4 affects tumor aggressiveness [79]. Since MCTs have been proposed as biomarkers for cancer prognosis [79,80], their substrates such as LiAcAc, might be implicated in some cancer hallmarks, as discussed below. In addition, high expression levels of MCT1 and MCT4 are associated with increased invasion in lung cancer cells [81] and the inhibition of MCTs decreases the migration and invasion process in glioma [77], suggesting that MCTs have an important role in invasion and migration processes in cancer. Since lithium transporters have a pivotal role in some cancer hallmarks, the potential role of lithium in cancer biology needs to be addressed.
Cancer cells use several metabolic substrates such as glucose, glutamine, lipids, and lactate to grow and survive. Therefore, MCTs maintain a high-rate lactate transport, allowing glycolysis to preferentially fuel lactic fermentation [82]. On the other hand, acetoacetate is used to obtain energy, by oxidation through the TCA cycle generating acetyl-CoA [83], suggesting a possible function of lithium transporters in the metabolism of cancer cells; e.g., the BRAFV600E mutation found in malignant melanomas, hairy cell leukemia, colorectal cancer, and multiple myeloma [84,85,86,87]. This mutation activates octamer transcription factor Oct-1, which promotes the expression of 3-hydroxy-3-methylglutaryl-CoA lyase (HMGCL) [88], resulting in an increase of acetoacetate levels, which promotes BRAFV600E binding and activates the protein kinase MEK1 (mitogen-activated protein kinase/extracellular signal-regulated kinase) [88]. Furthermore, depletion of MEK1 reduced the colony formation of cellular culture, suggesting the important role of MEK1 in long-term proliferation and survival [89]. Therefore, HMGCL inhibitors or non-metabolizable acetoacetate compounds might be considered potential therapies for BRAFV600E mutation cancers [88]. Interestingly, MCT1 is expressed in oxidative cancer cells and these types of transporters are capable of transporting lactate and LiAcAc [72,90]. In cells, the enzyme lactate dehydrogenase B (LDHB) oxidizes lactate into pyruvate, which inhibits the prolyl hydroxylases (PHDs) that catalyze the hydroxylation of the Hypoxia-Inducible Factor 1α (HIF-1α) on two proline residues, to initiate the proteasome-dependent degradation pathway of HIF-1α [90,91,92,93]. The question then arises, do lithium transports have a role in metabolism deregulation in cancer cells?
4. Lithium as a Specific Inhibitor of GSK3β
The chlorinated form of lithium inhibits Glycogen Synthase Kinase 3β (GSK3β), which is a serine/threonine protein kinase. GSK3β phosphorylates β-catenin in the Wnt metabolic pathway, leading to growth arrest [94,95]. Moreover, GSK3β participates in cellular proliferation and survival by activating nuclear factor ϰB-dependent gene transcription, suggesting an active role of GSK3β in tumorigenesis in pancreatic, colorectal, and prostate cancers, and gliomas [96,97,98,99,100]. Since GSK3β is involved in repressing Wnt/β-catenin signaling, but also in maintaining cell survival and proliferation via the NF-ϰB pathway, the inhibition of this dual kinase activity by lithium might have a positive effect on cancer hallmarks.
The expression of GSK3β is higher in colon cancer cells and in colorectal cancer patients in comparison to normal counterparts. Furthermore, the inhibition of GSK3β activity resulted in apoptosis induction and reduced proliferation in cell culture [99]. Interestingly, lithium is capable of inhibiting GSK3β; consequently, lithium is a potential therapeutic agent. Inhibition of GSK3β by LiCl takes place through two mechanisms. One of them is a competitive mechanism between lithium and magnesium for binding to GSK3β [101,102]. The other mechanism involves the role of LiCl in the phosphorylation of the serine-9, located in the N-terminal, which is the main regulator region of GSK3β [103]. However, GSK3β has contradictory functions in cancer cells. On one hand, GSK3β phosphorylates p53, leading to its inhibition [104]. In this way, GSK3β binds to p53 in camptothecin-treated neuroblastoma cells, leading to increased mitochondrial apoptosis signaling and the expression of p21 and Bax [105,106]. On the other hand, in lithium-treated cells, the nuclear complex GSK3β/p53 is disrupted, conferring chemoresistance to apoptosis in hepatoblastoma cells (HepG2) through GSK3β inhibition and repression of Cd95 expression [107].
Furthermore, when lithium blocks GSK3β activity, ovarian cancer cell proliferation was reduced and tumor suppressor activity was observed in nude mice inoculated with human ovarian cancer cells [108]. Moreover, proliferation was reduced in Li-treated human medullary thyroid TT cancer cells, due to the inactivation of GSK3β activity [109]. In addition, the inhibitory activity of lithium for GSK3β is linked to cell cycle arrest, by increasing cyclin-dependent kinase inhibitors such as p21, p27, and p15 [109]. In esophageal cancer, lithium decreases the proliferation of Eca-109 cells and affects the cell cycle, causing most cells to be in the G2/M phase [110].
The polycomb group gene Bmi1 is increased in various cancers such as medulloblastoma and glioblastoma multiforme [111]. When Bmi1 is downregulated, it triggers differentiation and reduces the expression of the stem cell markers Sox2 and Nestin [111]. Since GSK3β activity is linked to Bmi1, inhibition of GSK3β activity by lithium triggered tumor cell differentiation, increased apoptosis, the disintegration of tumor spheroids, and decreased clonogenicity [111].
5. Effect of Lithium on Certain Cancer Hallmarks
5.1. Effect of Lithium on Apoptosis
The role of lithium as a proapoptotic or antiapoptotic compound is contradictory. In HepG2 cells, lithium confers resistance to the apoptosis induced by etoposide and camptothecin, by preventing the activation of caspase-8 and caspase-3 and by inhibiting the Bax translocation to mitochondria [107]. Therefore, lithium confers resistance to chemotherapy-induced apoptosis through GSK3β inhibition, disruption of GSK3β/p53 cooperation, and repression of CD95 expression [107]. Furthermore, in human malignant glioma cell lines (T98G and U87MG), non-proapoptotic effects regarding LiAcAc have been reported [112]. In hematological tumors (multiple myeloma), LiCl triggers cell cycle arrest and apoptosis by inhibition of GSK3β and the activation of the Wnt/β-catenin signaling pathway [24].
On the other hand, in human SH-SY5Y neuroblastoma cells, lithium inhibits GSK3β, contributing to antiapoptotic signaling mechanisms [113]. Furthermore, LiCl induces the decrease of proapoptotic proteins p53 and Bax, while increasing Bcl-2 (a major antiapoptotic protein that controls mitochondrial membrane permeability) levels [114]. In addition, the effects of mitochondrial toxins might be attenuated by the inhibition of GSK3β, which can be achieved by the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is inhibited by GSK3β through Akt-mediated phosphorylation of Ser-9 of GSK3β [115] or by GSK3β direct inhibitors such as lithium, suggesting that the inhibition of GSK3β provides protection against the activation of the apoptosis-associated cysteine protease caspase-3 [116]. In human breast and colorectal cancer cell lines with oncogenic phosphatidylinositol 3-kinase subunit PIK3CA, lithium treatment reduced proliferation and reduced the GSK3β-target gene cyclin-D1, suggesting that GSK3β is an effector of oncogenic PIK3CA, suggesting lithium as a promising anti-neoplastic therapy against cancers harboring PIK3CA mutations [117].
In glioblastoma cells, low and higher LiCl doses had no effect on the expression of Bcl-2, procaspase-3, and PARP [118]. In contrast, in neuronal cells, Li blocks caspase-3 activation [119]. Therefore, cells of different origins (neuronal or glial) might have different results when treated with lithium. In cancer, several pathways might be dysregulated, and combinatory therapy with LiCl and pharmacological activators of the Notch1 pathway suppressed the hormonal secretion and reduces cell growth through apoptosis in medullary thyroid cancer (MTC) [120]. Lithium chloride enhances cell death induced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in human prostate cancer cells [121]. In addition to the TRAIL-mediated caspase-8 activation, LiCl enhances BID (BH3 interacting-domain death agonist) cleavage [121]. Li increases the activity of transcription factors such as activator protein-1 (AP-1) and cyclic-AMP response element binding protein (CREB) in vivo and in vitro [122] and activates the mitogen-activated protein (MAP) kinase pathway [123], which are involved in regulating apoptosis, cytokine production, and differentiation of promyelocytic leukemia cells [124]. Initiation and promotion of pancreatic ductal adenocarcinoma are linked to the Hedgehog-GLI signaling pathway, in which the GLI proteins are mediators and regulate cell differentiation [125]. Lithium decreases the expression and activity of glioma-associated oncogene-1 (GLI1) blocking cell proliferation and G1/S cell cycle progression, triggering apoptosis and reducing the tumorigenicity of pancreatic ductal adenocarcinoma cells [125]. In colorectal cancer cells, LiCl increased apoptosis and ROS levels, which could be mediated by the reduction of NF-κB expression [126]. Therefore, LiCl targets ROS/GSK3β/NF-κB pathways, resulting in increased apoptosis of colorectal cancer [126].
5.2. Effect of Lithium on Autophagy
Lithium induces autophagy and enhances the clearance of aggregated proteins, such as mutant huntingtin and α-synucleins neuronal precursor cell lines and non-neuronal cells. Interestingly, this effect is not mediated by GSK3β, but it was mediated by inhibition of IMPase, leading to free inositol depletion and reducing myo-inositol-1,4,5-triphosphate (IP3) levels, resulting in a novel form of autophagy induction that is independent of the mammalian target rapamycin (mTOR) [127,128]. In addition, the inhibition of IMPase and GSK3β showed opposite effects on autophagy. On one hand, Li induced the inhibition of IMPase at lower doses (Ki = 0.8 mM), enhancing autophagy [128]. On the other hand, the inhibition of GSK3β by Li+ ion downregulated autophagy (at higher doses, Ki ≅ 2mM) via activation of mTOR [129,130]. Furthermore, lithium chloride and other chemical forms induced autophagy by reducing the amount of pathological prion protein (PrPSc) in prion-infected neuronal and non-neuronal cells [131]. Lithium carbonate-treated cells showed a high expression of LC3 and LAMP-1 (autophagy markers) and an increase in autophagic structure volume density, suggesting that the activation of autophagy by lithium may promote an increase of protein recycling in kidney proximal tubule cells [132].
Additionally, lithium carbonate promotes autophagic vacuole formation in hepatocellular carcinoma (HCC) cells in vivo, suggesting that lithium-mediated autophagy might be a novel approach for treating hepatocellular carcinoma [133]. Interestingly, in HCC, combinatory treatment of lithium carbonate and rapamycin increases autolysosome formation and the expression of autophagy markers such as LC3β and LAMP1 [134]. Further, 5 mM lithium carbonate produced an antitumor effect, by arresting the cell cycle in G2/M-phase and increasing the number of apoptotic HCC cells, as well as inducing autophagy [33]. In corneal endothelial cells, treatment with lithium carbonate upregulated the expression of P62, Tmem74, Tm9sf1, and Tmem166, indicating that lithium increased autophagy that may have contributed to increased endothelial cell survival in a mouse model of Fuchs endothelial corneal dystrophy [135]. Moreover, in human prostate cancer cells, lithium inhibited GSK3β, inducing cell death by modulating Bif-1, and in parallel induced an extensive autophagic response [136]. Blocking the autophagic response switched GSK3β-inhibition-induced necrosis to apoptotic cell death. Therefore, GSK3β promotes cell survival by modulating Bif-1-dependent autophagic response and cell death [136]. Finally, in acute promyelocytic leukemia, lithium chloride increases apoptosis and leads to cell cycle arrest at the G2/M phase. Although lithium increases the level of Ser9 phosphorylated GSK3β, it decreases the level of Akt1, inhibits c-Myc, and enhances cell death, with a concomitant increase in β-catenin. Therefore, LiCl promotes apoptosis through the Akt signaling pathway and cell cycle arrest by increasing phosphorylation of GSK3β [137]. In addition, lithium carbonate increases the number and volume of autophagic vacuoles and increases levels of LC3β, a biomarker of autophagy death [133]. Additionally, Li2CO3 induces apoptosis and arrests the cell cycle in the G2/M phase in hepatocellular carcinoma-29 cells [33].
5.3. Effect of Lithium on Tumor Growth, Tumor Proliferation, Tumor Invasion and Metastasis, and Cell Cycle Arrest
Tumor cell growth is controlled by various signaling pathways, such as PI3K/Akt and the raf-1/mitogen-regulated extracellular kinase (MEK)/extracellular regulated kinase (ERK) pathway (raf-1/MEK/ERK). LiCl decreases hormonal secretion and suppresses the growth of MTC in vitro and in vivo due to GSK3β inhibition [109]. The raf-1 activation induces GSK3β phosphorylation, therefore the inactivation of GSK3β by LiCl might be a mechanism of rat-1-induced growth arrest [109]. It has been reported that LiAcAc reduced cell growth and ATP concentration in seven human cancer cell lines, including colon and breast cancer cells [138]. Since uncoupling protein 2 (UCP2) was overexpressed in cancer cells, the authors suggested that the effect of LiAcAc might have been associated with an inefficient Randle cycle [138]. Furthermore, LiAcAc and LiCl inhibited bovine lymphocyte proliferation in vitro [139]. However, LiAcAc or LiCl did not affect the growth of glioma cell lines [112]. Moreover, LiAcAc had no effect on proliferation in the various breast cancer lines tested (BT20, BT474, HBL100, MCF-7, MDA-MB 231, MDA-MB 468, and T47D) [140].
Interestingly, low concentrations of LiCl (at 10, 20, 30, and 40 mM) promote the proliferation of mk3 and mk4 cells (metanephric mesenchyme cells), which play an essential role in nephron generation, by increasing the expression of the transcription factor Six2 (sine oculis homeobox homolog 2 gene). In contrast, higher concentrations of LiCl (50 mM) inhibit proliferation and downregulate the expression of Six2 [141]. Therefore, the potential anti-tumor effect of LiCl depends on its dose; low concentrations of LiCl induce proliferation via upregulating Six2 expression, while high LiCl concentration decreases both proliferation and Six2 expression, which is connected to the Wnt/β-catenin signaling pathway. Furthermore, in hippocampal neural stem/progenitor cells, low concentrations of LiCl increase cell proliferation [142]. In addition, LiCl induces G2 arrest in mouse embryonal cells and inhibits the activity of cyclin B/cdc2 kinase through interference with the dephosphorylation of Tyr-15 on cdc2 [143]. In addition, LiCl inhibits the cell proliferation of C6 glioma cells harboring an IDH2 (isocitrate dehydrogenase-2) mutation via GSK3β [144].
Lithium causes nuclear localization of the claudin-1 and β-catenin that form the apical junctional complex, which is associated with colorectal cancer progression. In addition, lithium reduces colony formation, inhibiting cell migration and cell proliferation through G2/M cell cycle arrest. In colorectal cancer, the aberrant activation of epidermal growth factor (EGF) disrupts the association between E-cadherin and β-catenin, increasing cell proliferation and migration [1]. EGFR activates downstream kinases that modulate the GSK3β phosphorylation and consequently activate the Wnt/β-catenin pathway [145,146]. Since LiCl inhibited the tumorigenic effects of EGF, this cation might have tumor suppressor activity in colorectal cancer [147]. LiCl inhibits multiple-myeloma proliferation in a dose-dependent manner and triggers cell cycle arrest and apoptosis through inhibition of GSK3β, which is a crucial mediator of the Wnt/β-catenin pathway [24]. GSK3β regulates cadherin-11 at transcriptional and translational levels. Lithium inhibition of GSK3β suppresses cadherin-11, which is involved in cell–cell adhesion, cancer cell invasion, and metastasis of cancer [148]. Moreover, dedifferentiation, invasion, and metastasis are associated with E-cadherin loss [149]. Although the loss of E-cadherin or β-catenin, along with the downregulation of WNT7, has a poor prognosis in lung cancers, lithium led to the induction of E-cadherin and WNT7 in an inositol-independent manner [149].
In colon cancer, lithium reduces the expression of transforming growth factor-β-induced protein (TGFBIp) by inhibiting Smad3 phosphorylation through GSK3β inhibition [150]. In addition, Li inhibits cell migration of lymphatic endothelial cells and prevents metastasis by inhibiting TGFBIp-induced tumor lymphangiogenesis, resulting in the inhibition of colon cancer metastasis [150]. The effects of lithium on migration are greater than its effects on viability and occur at lower doses and earlier time points. Moreover, the migration effects of Li are reversible, suggesting that migration blockade is not due to cytotoxicity [151]. Moreover, GSK3β and GSK3α inhibition by lithium reduces migration and invasion of glioma cells [151]. In primary bovine aortic endothelial cells, LiCl induced cell cycle arrest in the G2/M phase via over-expression of cyclin-dependent kinase inhibitor (p21Cip) in a p53-dependent pathway, suggesting that lithium stabilizes β-catenin and activates p53 [152]. In the presence of LiCl, stabilized β-catenin is translocated into the nucleus, where it associates with transcription factors such as TCF (T-cell factor), leading to the expression of genes involved in cell adhesion and cell proliferation [153,154,155,156].
Genome-wide screening has been used to determine that LiCl inhibits cell proliferation, reduces replication, and arrests the cell cycle in multiple prostate cancer cells [157]. In prostate cancer, LiCl participates in the reduction of DNA replication and S-phase cell cycle arrest, by disrupting the interaction of E2F factor with DNA, decreasing the expression of the cell division cycle 6 (cdc6), cyclin A, cyclin E, and cdc25C, which are regulated by E2F [157]. Furthermore, the effects of LiCl might be associated with GSK3β inhibition [157]. In addition, promyelocytic leukemia cells evade death by increasing the levels of IL-2 and IL-10, at the same time as decreasing the amount of TNF-α and IL-6 [158]. In this context, LiCl induces cell death by promoting the expression of TNF-α and IL-6, while inhibiting IL-2 and IL-10 production [158].
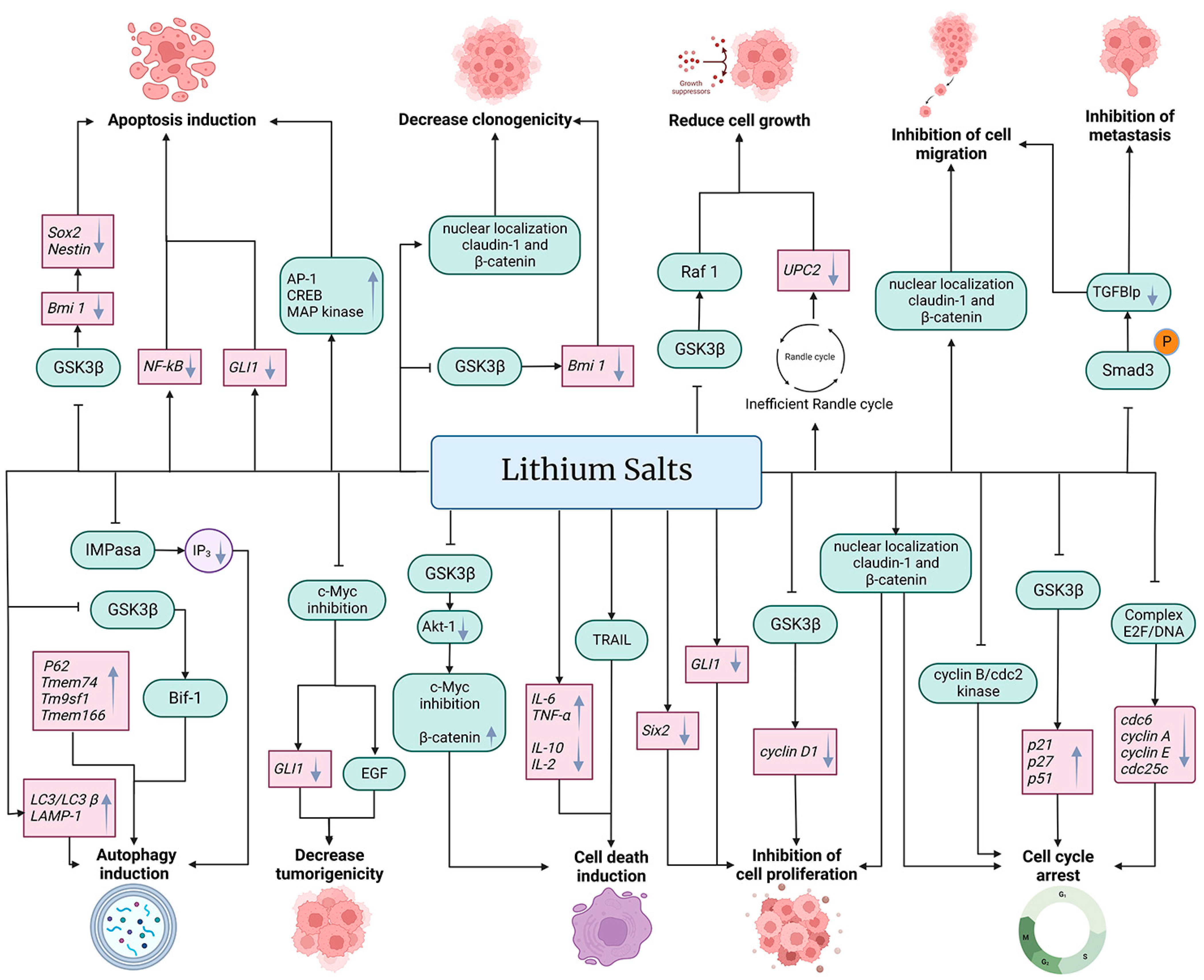
The anti-cancer properties of lithium salts are summarized in Figure 2.
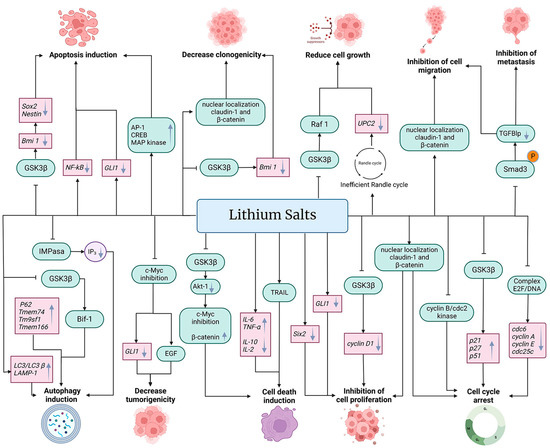
Figure 2.
Effects of lithium on cancer cells. Treatment with salts of lithium such as lithium acetoacetate (LiAcAc), lithium chloride (LiCl), lithium citrate (Li3C6H5O7), and lithium carbonate (Li2CO3) inhibit cell proliferation, cell migration, metastasis, and cell growth, in addition to decreasing tumorigenicity and clonogenicity, arresting the cell cycle and inducing autophagy, cell death, and apoptosis. Lithium affects cancer cells at the transcriptional (gene expression level) (pink boxes) and at post-transcriptional (protein function, expression, and cell localization) (green boxes) levels. In addition, lithium affects metabolic pathways and other metabolic products (purple circle). Black arrows (→) indicate the effect of lithium on the molecule (gray downward arrow (↓) denotes decrease or down-regulation, and gray upward arrow (↑) denotes increase or up-regulation). The truncated black line (⊥) indicates an inhibitory effect of lithium.
6. Anti-Inflammatory Activity of Lithium
In some types of cancer, before the cell becomes malignant, inflammatory conditions occur. Therefore, inflammation is associated with the proliferation and survival of cancer cells. In addition, this promotes angiogenesis and metastasis [159]. Since inflammation is associated with the development and progression of cancer, inflammatory biomarkers such as pro-inflammatory cytokines, tumor necrosis factor (TNF), interleukins 1 and 6, and chemokines can be used to monitor cancer progression [160]. In this way, the anti-inflammatory effect of lithium might be an alternative for prevention and monitoring of cancer. Furthermore, in animal models, LiCl treatment induced therapeutic effects on inflammation-mediated skeletal muscle wasting such as sepsis-induced muscle atrophy and cancer cachexia [161].
The anti-inflammatory activity of lithium results from its strong inhibitory effect on GSK3β, leading to a reduction in TNF-α production via attenuated activation of NF-κB and JNK signaling cascades [124] and induction of cytokine IL-10 [162]. The anti-inflammatory activity of lithium is due to the reduction of cyclooxygenase-2 expression, induction of the nitric oxide synthase expression, inhibition of interleukin 1β and TNFα, and increased production of IL-2 and IL-10 [163,164]. Furthermore, LiCl increases the levels of TNF-induced IL-6 in murine skin [165]. During tumor growth, toxic products are generated that cause damage to organs distal to the tumor site. In this way, lithium carbonate had a protective effect on podocytes during tumor growth induced by inoculation of hepatocellular carcinoma-29 cells in CBA mice, suggesting that lithium may have a protective role in organs during tumor growth [166].
7. Synergism of Lithium with Standard Cancer Therapies
High concentrations of lithium (4–5 mM) inhibit inositol 1-monophosphatase, resulting in a reduction of phosphatidylinositol turnover. However, these high concentrations of Li+ ion have toxic effects in patients [14]. Reported serum Li+ ion levels for therapy were 0.8 to 1.2 mEq/L (0.8–1.2 mM) and the cytotoxicity effect began at 1.5 to 2 mEq/L (1.5–2 mM) [167]. Interestingly, 1 mM of LiCl had no effect on cellular metabolism or cell cycle but its combination with cisplatin or paclitaxel reduced metabolic activity in a serous ovarian cancer cell line (OVCA 433) or primary cultures. However, LiCl by itself or in combination with cisplatin or paclitaxel had no significant effect on cellular proliferation, suggesting that the effects of LiCl are mainly related to cellular metabolism rather than proliferation [167]. These results observed in vitro contradict a phase II study of 15 patients with low-grade neuroendocrine tumors treated with LiCl that showed non-positive results in terms of response evaluation criteria in solid tumors and in overall survival, as well as GSK3β phosphorylation [168], suggesting that therapeutic usage of lithium depends on various factors, such as tumor nature and aggressiveness, lithium salts, and doses that allowed GSK3β phosphorylation, among others.
Conventional cancer therapies such as chemotherapy and radiotherapy inhibit cell division, causing neurocognitive deterioration. Preliminary studies have shown that lithium has a neuroprotective role in cancer patients undergoing radiotherapy or chemotherapy treatment, improving their quality of life [169]. Combination of LiCl with a low dose or IC50 dose of etoposide enhances apoptosis and reduces the percentage of cells in the G2/M phase in prostate cancer LNCap cells [19]. In pancreatic ductal adenocarcinoma cells, lithium increases the anti-tumoral efficacy of gemcitabine [125]. Additionally, histone deacetylase inhibitors with lithium resulted in an effective combinatory therapy, due to inducing the Notch1 pathway and inhibiting GSK3β activity, respectively. [120]. Furthermore, temozolomide combined with a low dose of LiCl (1.2 mM) induced cell death in TP53wt glioma cells by inhibiting GSK3, promoting the nuclear translocation of the nuclear factor of activated T cells (NFAT1), and upregulating the expression of Fas/FasL, resulting in a potential combinatorial therapy for glioblastoma treatment [23]. In addition, breast cancer cells (T47D) treated with LiCl (20 mM) had increased cellular radiosensitivity through decreasing Mre11 mRNA levels, resulting in decreased DNA repair in T47D monolayer and spheroid cells [170]. Neurite growth-promoting factor 2 (NEGF2), also known as Midkine (MK), is a cytokine that promotes cell growth, differentiation, survival, gene expression, and drug resistance. MK, PI3K, and GSK3β inhibitors together with LiCl could be a very effective treatment modality for tumors with a high expression of these molecules [171]. Pre-treatment of breast cancer cells with LiCl induced a synergistic cytotoxicity effect with mitomycin C (MMC), resulting in apoptosis via HMGB1 and Bax signaling, but LiCl also prevented MMC-induced necrosis [25].
Radiotherapy has various consequences in children, such as debilitating cognitive decline, partly linked to impaired neurogenesis. In this context, LiCl has a protective role in the juvenile brain against radiotherapy and improves the quality of life of these children [142]. In addition, LiCl is a promising drug for rescuing neurogenesis in adult and juvenile brains after irradiation, suggesting that Li+ prevents the debilitating cognitive effects of cranial radiotherapy [172,173]. Since lithium is a specific radio-sensitizer for tumor cells, it can be used in combination with radiotherapy [174]. LiCl has an immunomodulatory capability for regulating titanium nanoparticle-stimulated inflammatory responses through the inhibition of the MAPK signaling pathway [175]. In colorectal cancer, the effects of LiCl on the upregulation of pro-apoptotic proteins and the downregulation of expression of a protein associated with survival were enhanced by high-energy photon irradiation [31]. Therefore, lithium might be used as a sensitizer for colon cancer cells, to improve the results of radiation therapy. On the other hand, pre-treatment with lithium in thyroid cancer patients before radioiodine therapy demonstrated no improvement in radioiodine uptake in thyroid tissue [176].
Chemotherapy-induced peripheral neuropathy (CIPN) is an adverse effect of cancer treatment using taxanes, such as paclitaxel. Adding lithium to taxane treatment prevents calcium overloading in mitochondria, resulting in the prevention of calpain activation and preventing the loss of cell functions related to the progression of peripheral neuropathy [177], suggesting that lithium could be used to prevent the adverse effects of cancer treatment with only taxane administration [178]. In addition, transient receptor potential V4 (TRPV4) is involved in the initiation of CIPN. Evidence suggests that prolongated exposure to paclitaxel increases the acute effects of TRPV4 expression, currents, and calcium fluxes, while pre-treatment with lithium decreases the expression of TRPV4, currents, and calcium fluxes, suggesting that lithium prevents the CIPN caused by paclitaxel [179].
In a systems biology analysis, the interactomes of lithium-sensitive enzymes and the pathways associated with several types of cancer suggested that Li affects the incidence and progression of cancer [180]. Despite evidence recommending designing and conducting clinical trials of lithium for cancer, efforts prioritize the use of Li for bipolar disorder. Patients with bipolar disorders who were treated with Li showed a significantly lower cancer risk, suggesting an association between Li and cancer risk in a dose-dependent manner [181]. Furthermore, lithium (300 mg) administrated once daily for 5 days in each course of chemotherapy to 36 breast cancer patients was not effective in preventing the adverse effects of this cancer treatment [182]. Another clinical trial conducted with nine patients with acute myeloid leukemia (AML), who were orally treated with lithium carbonate (300 mg), resulted in the inhibition of GSK3, which induces differentiation of leukemic cells and could target AML stem cells [183].
8. Recent Clinical Studies
Based on the information available (http://www.clinicaltrials.gov (accessed on 20 January 2023)), thirteen clinical trials have been conducted (Table 2). Some of these clinical studies include intervention with lithium and anti-cancer drugs, such as oxaliplatin, capecitabine, temozolomide, bevacizumab, and aripiprazole. Recent clinical studies have focused on the potential use of lithium in colorectal neoplasm, stomach neoplasm, esophageal neoplasm, medullary thyroid cancer, neuroendocrine tumors, prostate cancer, small cell lung cancer, breast cancer, adult acute lymphoblastic leukemia in remission, adult acute myeloid leukemia in remission, osteosarcoma, brain and central nervous system tumors, and familial adenomatous polyposis. Additionally, lithium carbonate was employed in combination with radiation protocols. The results of these clinical trials could provide us with important information for understanding the potential synergism of lithium with current therapies.

Table 2.
Clinical trials elucidating the value of lithium in cancer clinic 1.
9. Trends in the Administration of Lithium
Medicine has adopted nanotechnology strategies for drug administration. Similarly, nanosized lithium being administered as nanoparticles or other nanostructures has been reported. Injection of lithium carbonate nanoparticles in tumors causes cell necrosis, destruction of the vascular bed, and attraction of neutrophils and macrophages to the tumor [189]. During the development of hepatocarcinoma-29 in mice, injection of nanosized lithium carbonate was essential for the functioning of macrophages [20]. Interestingly, Li2CO3 nanoparticles protected vital organs such as the heart and lungs of CBA mice with hepatocellular carcinoma from the damage caused by secondary products of lipid peroxidation and increased macrophages and neutrophils within the tumor, decreased blood micro-vessel density, and increased the tumor necrosis rate [190].
Moreover, nanoparticles of lithium citrate and carbonate suppressed the proliferation of hepatoma-29 cells in lower concentrations than their officinal forms [191]. Previous reports indicated that hepatocarcinoma-29 cells are more sensitive to nanoparticles of lithium salts such as citrate and carbonate in comparison to their original forms [192]. Furthermore, nanoparticles of lithium citrate mainly induce apoptosis, whereas lithium carbonate nanostructures induce apoptosis and autophagic death of hepatocellular carcinoma cells [22]. In addition, nanosized lithium citrate induces apoptosis, whereas nanoparticles of lithium carbonate induce apoptosis and autophagic cell death of HCC cells [22]. Liposomal lithium carbonate nanostructures have a longer half-life, equal efficacy, and better therapeutic dose maintenance in blood compared to their standard-sized counterparts for bipolar disorder treatment [193].
In addition, leaf extract of Zanthoxylum armatum, a tropical medicinal shrub, was used as a reducing agent to synthesize titanium dioxide (TiO2) nanoparticles. The TiO2 nanomaterial exerted cytotoxicity and induced apoptosis through lipid peroxidation in breast cancer cells. The anti-tumor efficacy and safety of TiO2 nanomaterial were evaluated in vivo using a subcutaneous 4T1 breast BALB/c mouse tumor model, displaying significantly less cardiotoxicity and body weight loss compared to doxorubicin [194]. These findings open new possibilities for the preparation of nanosized lithium using plant extracts as reducing agents. Furthermore, a lithium carbonate in situ gelling system appears to be a promising vehicle for Li+ ion administration for bipolar disorders, but its pharmacodynamics and pharmacokinetic properties need to be addressed [195]. However, these nano-drugs also need to be investigated for cancer treatment. To avoid fluctuations in the plasma concentration of lithium, a push-pull osmotic pump was developed for lithium carbonate delivery, to maintain Li+ ion at therapeutic levels [196]. Cellular death results from apoptotic and necrotic processes that have an anticancer effect [197], but apoptosis rather than necrosis is the accepted pathway of cellular death under the immune system control [198]. A mesoporous silica matrix containing Li showed the potential for induction of apoptosis, while reducing necrosis [199].
Lithium formate monohydrate was irradiated by X-rays, electrons, and protons for use as a dosimeter in radiotherapy [200]. In addition, pre-treatment with LiCl induced an increased cellular reactive oxidative species (ROS) level, resulting in a better therapeutic effect of adipose-derived stem cells in intervertebral disc degeneration-related disease [201].
The biocompatibility and multifunctionality of Li-doped bioceramics with polymers provided new biomaterials used in 3D printing for clinical applications of tissue engineering [202]. Furthermore, lithium-ferrite bioactive glasses prepared using the sol-gel methodology were used for hyperthermia therapy. Lithium-ferrite bioactive glasses provide temperatures up to 47.2 °C, making this material a candidate for cancer treatment using hyperthermia [203]. However, no in vitro assays have been published
Furthermore, lithium has been used for the synthesis of new nanomaterials with potential applications in cancer therapy. Layered metal oxides such as MoO3 and WO3 treated with lithium transformed white-colored micrometer-long MoO3 nanobelts into blue-colored short, thin, defective, interlayer gap-expanded MoO3−x nanobelts with strong NIR-II absorption. With this surface modification, the MoO3−x nanobelts could be a promising nanomaterial for photoacoustic imaging-guided photothermal therapy, resulting in an efficient cancer cell ablation and tumor eradication under irradiation from a 1064 nm laser [204]. Lithium intercalation-assisted exfoliation is an approach for the preparation of nanomaterials with applications in noninvasive photothermal therapy [205]. To overcome the tumor hypoxia-induced by oxygen-dependent photodynamic therapy, ultrathin vermiculite nanosheets were synthesized by lithium-ion intercalation for aggregation-induced emission photosensitizer loading, to produce a nanodevice inspired by bonsai [206]. In addition, LiCl alters the sodium and potassium levels, altering the membrane potential in human breast cancer cells (MCF-7), and suggesting that LiCl exerts a dose-dependent biphasic effect on MCF-7 cells by altering the apoptotic/antiapoptotic balance [207].
10. Contradictory Effects of Lithium
Prolonged treatment with lithium causes renal, endocrine, and dermatological side effects. Four weeks of therapy with lithium caused renal tubular concentration defects, polyuria, polydipsia, and nephrogenic diabetes insipidus [208]. Administration of Li2CO3 induced a cutaneous adverse reaction [209] and parathyroid dysfunction [210]. Furthermore, lithium treatment caused a higher prevalence of hypercalcemia in a cohort of bipolar patients [211]. In addition, there is a possibility of teratogenicity activity with lithium treatment [212].
The inhibition of GSK3β using lithium has contradictory effects. On one hand, chemoresistance to apoptosis is observed, but on the other hand, it induces tumor suppressor activity and reduces proliferation. Those effects depend on the type of cancer, the origin of cells, and doses. The anti-cancer effect of lithium has been reported for colorectal cancer, neuroblastoma cells, ovarian cancer cells, medullary thyroid TT cancer cells, esophageal cancer, medulloblastoma, and glioblastoma multiforme, hematological tumors (multiple myeloma), breast cancer cells, MTC, prostate cancer cells, pancreatic ductal adenocarcinoma cells, corneal endothelial cells, and colon cancer cells. However, opposite effects of lithium have been reported for neuroblastoma, hepatoblastoma cells, and malignant glioma cell lines.
Lithium induces apoptosis in promyelocytic leukemia cells, but a small fraction of these cells remain viable and survive the cytotoxic effect of this cation, resulting in altered gene expression patterns [213], suggesting cell tolerance to lithium in culture [214]. Resistance to lithium is a consequence of its activity. Lithium appears to induce apoptosis by increasing the bax/bcl-2 ratio (bax: pro-apoptotic gene and bcl-2: anti-apoptotic gene). Lithium resistance then results when this ratio is reversed, leading to lithium resistance accompanied by DNA fragmentation, and decreasing cell viability [158].
11. Conclusions
The expression of transporters of lithium is linked to various types of cancer, such as NSCLC, colon, stomach, bladder, prostate, breast, ovarian, cervical, head and neck cancer, and gliomas. In both in vivo and in vitro models of cancer, lithium is capable of activating the Wnt/β-catenin pathway. The GSK3β kinase is activated when a secreted glycoprotein (Wnt) binds to its receptor. GSK3β is inhibited by lithium through Ser-9 phosphorylation, stabilizing free β-catenin in the cytoplasm. Therefore, the GSK3β might have anti- or pro-apoptotic activity depending upon the cell type. According to genomics and proteomics data, lithium salts such as citrate, carbonate, chloride, and acetoacetate may stimulate antitumor effects, such as apoptosis, autophagy, cell death, tumor growth, tumor proliferation, invasion, and metastasis and arrest the cell cycle in in vivo and in vitro models (Figure 2). Since Li+ ion has potential for cancer treatment, the selection of the delivery form of lithium, such as nanosized or original forms, plays a key role in triggering the antitumor response, which depends on its transport into the cell, the cancer type, doses, and administration. Li+ ion selectivity for tumor cells, however, needs to be further investigated, as well as doses and administration.
Author Contributions
E.Y.V.-V. and L.I.Q.-G.: collection of data, investigation, resources, images, and writing—original draft preparation; H.C., M.G.-D.C., G.L.-G., M.R.-M., L.P.B.-M. and D.S.-A.: Investigation, formal analysis, data curation, and writing—original draft preparation; C.P.-P. and N.J.-H.: collection of data for tables, data curation—original draft preparation; G.F.-G. and O.D.R.-H.: supervision, project administration, funding acquisition and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UNAM–PAPIIT–IA208422.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
E.Y.V.-V. is grateful to Dirección General de Asuntos del Personal Académico for a postdoctoral scholarship. We thank PAPIIT-IA208422 for academic and financial support and to Lance Wiser and Yuliana León-Wiser for the final English Editing. We are deeply grateful to Leonardo Gabriel Reyes Figueroa for always being an inspiration and pushing us forward.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Warburg, O. The Metabolism of Tumors; Richard R. Smith. Inc.: New York, NY, USA, 1931. [Google Scholar]
- Poff, A.; Koutnik, A.P.; Egan, K.M.; Sahebjam, S.; D’Agostino, D.; Kumar, N.B. Targeting the Warburg effect for cancer treatment: Ketogenic diets for management of glioma. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Won, E.; Kim, Y.-K. An oldie but goodie: Lithium in the treatment of bipolar disorder through neuroprotective and neurotrophic mechanisms. Int. J. Mol. Sci. 2017, 18, 2679. [Google Scholar] [CrossRef] [PubMed]
- Elbe, D. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. J. Can. Acad. Child Adolesc. Psychiatry 2010, 19, 230. [Google Scholar]
- de Freitas, D.M.; Leverson, B.D.; Goossens, J.L. Lithium in medicine: Mechanisms of action. In The Alkali Metal Ions: Their Role for Life; Springer: Berlin/Heidelberg, Germany, 2016; pp. 557–584. [Google Scholar]
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643. [Google Scholar]
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiatry 2015, 72, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.M. Management of bipolar II disorder. Indian J. Psychol. Med. 2011, 33, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kessing, L.V.; Søndergård, L.; Forman, J.L.; Andersen, P.K. Lithium treatment and risk of dementia. Arch. Gen. Psychiatry 2008, 65, 1331–1335. [Google Scholar] [CrossRef]
- Nitrini, R.; Caramelli, P.; Herrera, E.; Bahia, V.S.; Caixeta, L.F.; Radanovic, M.; Anghinah, R.; Charchat-Fichman, H.; Porto, C.S.; Carthery, M.T.; et al. Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Dis. Assoc. Disord. 2004, 18, 241–246. [Google Scholar]
- Köhrle, J. Local activation and inactivation of thyroid hormones: The deiodinase family. Mol. Cell. Endocrinol. 1999, 151, 103–119. [Google Scholar] [CrossRef]
- Berens, S.C.; Bernstein, R.S.; Robbins, J.; Wolff, J. Antithyroid effects of lithium. J. Clin. Investig. 1970, 49, 1357–1367. [Google Scholar] [CrossRef]
- Kurita, M.; Mashiko, H.; Rai, M.; Kumasaka, T.; Kouno, S.-I.; Niwa, S.-I.; Nakahata, N. Lithium chloride at a therapeutic concentration reduces Ca2+ response in protein kinase C down-regulated human astrocytoma cells. Eur. J. Pharmacol. 2002, 442, 17–22. [Google Scholar] [CrossRef]
- Briggs, K.T.; Giulian, G.G.; Li, G.; Kao, J.P.; Marino, J.P. A A Molecular Model for Lithium’s Bioactive Form. Biophys. J. 2016, 111, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, C.; Bschor, T.; Köhler, S. Lithium treatment over the lifespan in bipolar disorders. Front. Psychiatry 2020, 11, 377. [Google Scholar] [CrossRef]
- Ochoa, E.L.M. Lithium as a neuroprotective agent for bipolar disorder: An overview. Cell. Mol. Neurobiol. 2022, 42, 85–97. [Google Scholar] [CrossRef]
- Vidali, S.; Aminzadeh-Gohari, S.; Vatrinet, R.; Iommarini, L.; Porcelli, A.M.; Kofler, B.; Feichtinger, R.G. Lithium and not acetoacetate influences the growth of cells treated with lithium acetoacetate. Int. J. Mol. Sci. 2019, 20, 3104. [Google Scholar] [CrossRef]
- Hossein, G.; Janzamin, E.; Azimian-Zavareh, V. Effect of lithium chloride and antineoplastic drugs on survival and cell cycle of androgen-dependent prostate cancer LNCap cells. Indian J. Pharmacol. 2012, 44, 714. [Google Scholar] [CrossRef]
- Konenkov, V.I.; Borodin, Y.I.; Makarova, O.P.; Bgatova, N.P.; Rachkovskaya, L.N. Effects of Nanosized Lithium Carbonate Particles on the Functional Activity of Macrophages during Development of Hepatocarcinoma 29. Bull. Exp. Biol. Med. 2015, 159, 490–493. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, T.R.; Rajendran, S.; O’Reilly, S.; O’Sullivan, G.C.; McKenna, S.L. Lithium modulates autophagy in esophageal and colorectal cancer cells and enhances the efficacy of therapeutic agents in vitro and in vivo. PLoS ONE 2015, 10, e0134676. [Google Scholar] [CrossRef] [PubMed]
- Bgatova, N.P.; Gavrilova, Y.S.; Lykov, A.P.; Solovieva, A.O.; Makarova, V.; Borodin, Y.I.; Konenkov, V.I. Apoptosis and autophagy in hepatocarcinoma cells induced by different forms of lithium salts. Cell Tissue Biol. 2017, 11, 261–267. [Google Scholar] [CrossRef]
- Han, S.; Meng, L.; Jiang, Y.; Cheng, W.; Tie, X.; Xia, J.; Wu, A. Lithium enhances the antitumour effect of temozolomide against TP53 wild-type glioblastoma cells via NFAT1/FasL signalling. Br. J. Cancer 2017, 116, 1302–1311. [Google Scholar] [CrossRef]
- Yao, R.; Sun, X.; Xie, Y.; Liu, L.; Han, D.; Yao, Y.; Li, H.; Li, Z.; Xu, K. Lithium chloride inhibits cell survival, overcomes drug resistance, and triggers apoptosis in multiple myeloma via activation of the Wnt/β-catenin pathway. Am. J. Transl. Res. 2018, 10, 2610. [Google Scholar]
- Razmi, M.; Rabbani-Chadegani, A.; Hashemi-Niasari, F.; Ghadam, P. Lithium chloride attenuates mitomycin C induced necrotic cell death in MDA-MB-231 breast cancer cells via HMGB1 and Bax signaling. J. Trace Elem. Med. Biol. 2018, 48, 87–96. [Google Scholar] [CrossRef]
- Vijay, G.V.; Na Zhao, N.; Hollander, P.D.; Toneff, M.J.; Joseph, R.; Pietila, M.; Taube, J.H.; Sarkar, T.R.; Ramirez-Pena, E.; Werden, S.J.; et al. GSK3β regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res. 2019, 21, 37. [Google Scholar] [CrossRef]
- Ramshini, S.; Omidi, M. The Psychiatric Drug Lithium Increases DNA Damage and Decreases Cell Survival in MCF-7 and MDA-MB-231 Breast Cancer Cell Lines Expos ed to Ionizing Radiation. Curr. Mol. Pharmacol. 2019, 12, 301–310. [Google Scholar]
- Xue, D.; Yang, P.; Wei, Q.; Li, X.; Lin, L.; Lin, T. IL-21/IL-21R inhibit tumor growth and invasion in non-small cell lung cancer cells via suppressing Wnt/β-catenin signaling and PD-L1 expression. Int. J. Mol. Med. 2019, 44, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shan, H. Keratin 6A gene silencing suppresses cell invasion and metastasis of nasopharyngeal carcinoma via the β-catenin cascade. Mol. Med. Rep. 2019, 19, 3477–3484. [Google Scholar] [CrossRef]
- ohen-Harazi, R.; Hofmann, S.; Kogan, V.; Fulman-Levy, H.; Abaev, K.; Shovman, O.; Brider, T.; Koman, I. Cytotoxicity of Exogenous Acetoacetate in Lithium Salt Form Is Mediated by Lithium and Not Acetoacetate. Anticancer Res. 2020, 40, 3831–3837. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, F.; Conte, A.; Aversano, A.; Muto, P.; Ametrano, G.; Riccio, P.; Turano, M.; Valente, V.; DelRio, P.; Izzo, P.; et al. Lithium chloride increases sensitivity to photon irradiation treatment in primary mesenchymal colon cancer cells. Mol. Med. Rep. 2020, 21, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Zubčić, V.; Rinčić, N.; Kurtović, M.; Trnski, D.; Musani, V.; Ozretić, P.; Levanat, S.; Leović, D.; Sabol, M. GANT61 and lithium chloride inhibit the growth of head and neck cancer cell lines through the regulation of GLI3 processing by GSK3β. Int. J. Mol. Sci. 2020, 21, 6410. [Google Scholar] [CrossRef] [PubMed]
- Taskaeva, Y.S.; Bgatova, N.P.; Dossymbekova, R.S.; Solovieva, A.O.; Miroshnichenko, S.M.; Sharipov, K.O.; Tungushbaeva, Z.B. In vitro effects of lithium carbonate on cell cycle, apoptosis, and autophagy in hepatocellular carcinoma-29 cells. Bull. Exp. Biol. Med. 2020, 170, 246–250. [Google Scholar] [CrossRef]
- Yang, B.; Wang, S.; Xie, H.; Wang, C.; Gao, X.; Rong, Y.; Liu, Z.; Lu, Y. KIF18B promotes hepatocellular carcinoma progression through activating Wnt/β-catenin-signaling pathway. J. Cell. Physiol. 2020, 235, 6507–6514. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, Y.; Zhang, J.; Xie, Y.; Jin, M. Promoting effects of MiR-135b on human multiple myeloma cells via regulation of the Wnt/β-catenin/Versican signaling pathway. Cytokine 2021, 142, 155495. [Google Scholar] [CrossRef]
- Kim, H.; Li, S.; Lee, D.; Park, J.H.; Muramatsu, T.; Harada, H.; Jung, Y.; Jung, H. Activation of Wnt signalling reduces the population of cancer stem cells in ameloblastoma. Cell Prolif. 2021, 54, e13073. [Google Scholar] [CrossRef] [PubMed]
- Taskaeva, I.; Gogaeva, I.; Shatruk, A.; Bgatova, N. Lithium Enhances Autophagy and Cell Death in Skin Melanoma: An Ultrastructural and Immunohistochemical Study. Microsc. Microanal. 2022, 28, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, E.; Argüello-Miranda, O.; Chiu, S.-W.; Fazal, Z.; Kruczek, J.; Nunez-Corrales, S.; Pandit, S.; Pritchet, L. Towards a unified understanding of lithium action in basic biology and its significance for applied biology. J. Membr. Biol. 2017, 250, 587–604. [Google Scholar] [CrossRef] [PubMed]
- de Roos, N.M.; de Vries, J.H.; Katan, M.B. Serum lithium as a compliance marker for food and supplement intake. Am. J. Clin. Nutr. 2001, 73, 75–79. [Google Scholar] [CrossRef]
- Pérez-Escuredo, J.; Van Hée, V.F.; Sboarina, M.; Falces, J.; Payen, V.L.; Pellerin, L.; Sonveaux, P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2481–2497. [Google Scholar] [CrossRef]
- Bröer, S.; Bröer, A.; Schneider, H.-P.; Stegen, C.; Halestrap, A.P.; Deitmer, J.W. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem. J. 1999, 341, 529–535. [Google Scholar] [CrossRef]
- Bröer, S.; Schneider, H.-P.; Bröer, A.; Rahman, B.; Hamprecht, B.; Deitmer, J.W. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 1998, 333, 167–174. [Google Scholar] [CrossRef]
- Halestrap, A.P. The monocarboxylate transporter family—Structure and functional characterization. IUBMB Life 2012, 64, 1–9. [Google Scholar] [CrossRef]
- Garcia, C.K.; Brown, M.S.; Pathak, R.K.; Goldstein, J.L. cDNA Cloning of MCT2, a Second Monocarboxylate Transporter Expressed in Different Cells than MCT1. J. Biol. Chem. 1995, 270, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family—From monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch. 2004, 447, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.K.; Goldstein, J.L.; Pathak, R.K.; Anderson, R.G.; Brown, M.S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: Implications for the Cori cycle. Cell 1994, 76, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Jóhannsson, E.; Nagelhus, E.A.; Mccullagh, K.J.; Sejersted, O.M.; Blackstad, T.W.; Bonen, A.; Ottersen, O.P. Cellular and subcellular expression of the monocarboxylate transporter MCT1 in rat heart: A high-resolution immunogold analysis. Circ. Res. 1997, 80, 400–407. [Google Scholar] [CrossRef]
- Kirat, D.; Inoue, H.; Iwano, H.; Yokota, H.; Taniyama, H.; Kato, S. Monocarboxylate transporter 1 (MCT1) in the liver of pre-ruminant and adult bovines. Vet. J. 2007, 173, 124–130. [Google Scholar] [CrossRef]
- Tamai, I.; Takanaga, H.; Ogihara, T.; Higashida, H.; Maeda, H.; Sai, Y.; Tsuji, A. Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem. Biophys. Res. Commun. 1995, 214, 482–489. [Google Scholar] [CrossRef]
- Ritzhaupt, A.; Wood, I.S.; Ellis, A.; Hosie, K.B.; Shirazi-Beechey, S.P. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: Its potential to transport l-lactate as well as butyrate. J. Physiol. 1998, 513, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Orsenigo, M.N.; Tosco, M.; Bazzini, C.; Laforenza, U.; Faelli, A. A monocarboxylate transporter MCT1 is located at the basolateral pole of rat jejunum. Exp. Physiol. 1999, 84, 1033–1042. [Google Scholar] [CrossRef]
- Kirat, D.; Inoue, H.; Iwano, H.; Hirayama, K.; Yokota, H.; Taniyama, H.; Kato, S. Monocarboxylate transporter 1 gene expression in the ovine gastrointestinal tract. Vet. J. 2006, 171, 462–467. [Google Scholar] [CrossRef]
- Iwanaga, T.; Takebe, K.; Kato, I.; Karaki, S.-I.; Kuwahara, A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed. Res. 2006, 27, 243–254. [Google Scholar] [CrossRef]
- Shimoyama, Y.; Kirat, D.; Akihara, Y.; Kawasako, K.; Komine, M.; Hirayama, K.; Matsuda, K.; Okamoto, M.; Iwano, H.; Kato, S.; et al. Expression of monocarboxylate transporter 1 (MCT1) in the dog intestine. J. Vet. Med. Sci. 2007, 69, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Welter, H.; Claus, R. Expression of the monocarboxylate transporter 1 (MCT1) in cells of the porcine intestine. Cell Biol. Int. 2008, 32, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.C.; Halestrap, A.P. N-terminal protein sequence analysis of the rabbit erythrocyte lactate transporter suggests identity with the cloned monocarboxylate transport protein MCT1. Biochem. J. 1994, 303, 755–759. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Wood, I.S.; Trayhurn, P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflügers Arch.-Eur. J. Physiol. 2010, 459, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Hajduch, E.; Heyes, R.R.; Watt, P.W.; Hundal, H.S. Lactate transport in rat adipocytes: Identification of monocarboxylate transporter 1 (MCT1) and its modulation during streptozotocin-induced diabetes. FEBS Lett. 2000, 479, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Takebe, K.; Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Yajima, T.; Iwanaga, T. Cellular expression of a monocarboxylate transporter (MCT1) in the mammary gland and sebaceous gland of mice. Histochem. Cell Biol. 2009, 131, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Mac, M.; Nałęcz, K.A. Expression of monocarboxylic acid transporters (MCT) in brain cells: Implication for branched chain α-ketoacids transport in neurons. Neurochem. Int. 2003, 43, 305–309. [Google Scholar] [CrossRef]
- Hanu, R.; McKenna, M.; O’Neill, A.; Resneck, W.G.; Bloch, R.J. Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am. J. Physiol. Physiol. 2000, 278, C921–C930. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Moreira, T.J.; Pierre, K.; Maekawa, F.; Repond, C.; Cebere, A.; Liljequist, S.; Pellerin, L. Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J. Cereb. Blood Flow Metab. 2009, 29, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Ainscow, E.K.; Mirshamsi, S.; Tang, T.; Ashford, M.L.; Rutter, G.A. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: Evidence for ATP-independent control of ATP-sensitive K+ channels. J. Physiol. 2002, 544, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Domènech-Estévez, E.; Baloui, H.; Repond, C.; Rosafio, K.; Médard, J.-J.; Tricaud, N.; Pellerin, L.; Chrast, R. Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. J. Neurosci. 2015, 35, 4151–4156. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.M.; Tsingalia, A.; Vidensky, S.; Lee, Y.; Jin, L.; Farah, M.H.; Lengacher, S.; Magistretti, P.J.; Pellerin, L.; Rothstein, J.D. Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Exp. Neurol. 2015, 263, 325–338. [Google Scholar] [CrossRef]
- Boussouar, F.; Mauduit, C.; Tabone, E.; Pellerin, L.; Magistretti, P.J.; Benahmed, M. Developmental and hormonal regulation of the monocarboxylate transporter 2 (MCT2) expression in the mouse germ cells. Biol. Reprod. 2003, 69, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Pierre, K.; Pellerin, L.; Debernardi, R.; Riederer, B.; Magistretti, P.J. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 2000, 100, 617–627. [Google Scholar] [CrossRef]
- Pellerin, L.; Halestrap, A.P.; Pierre, K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J. Neurosci. Res. 2005, 79, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Pierre, K.; Magistretti, P.J.; Pellerin, L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J. Cereb. Blood Flow Metab. 2002, 22, 586–595. [Google Scholar] [CrossRef]
- Reiser, G.; Duhm, J. Transport pathways for lithium ions in neuroblastoma× glioma hybrid cells at ‘therapeutic’concentrations of Li+. Brain Res. 1982, 252, 247–258. [Google Scholar] [CrossRef]
- Sonveaux, P.; Vegran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, F.; Pinheiro, C.; Morais-Santos, F.; Azevedo-Silva, J.; Queiros, O.; Preto, A.; Casal, M. Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol. Histopathol. 2014, 29, 1511–1524. [Google Scholar]
- Pinheiro, C.; Reis, R.M.; Ricardo, S.; Longatto-Filho, A.; Schmitt, F.; Baltazar, F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J. Biomed. Biotechnol. 2010, 2010, 427694. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Dewhirst, M.W. Tumor metabolism of lactate: The influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010, 6, 127–148. [Google Scholar] [CrossRef]
- Pinheiro, C.; Longatto-Filho, A.; Azevedo-Silva, J.; Casal, M.; Schmitt, F.C.; Baltazar, F. Role of monocarboxylate transporters in human cancers: State of the art. J. Bioenerg. Biomembr. 2012, 44, 127–139. [Google Scholar] [CrossRef]
- Miranda-Gonçalves, V.; Honavar, M.; Pinheiro, C.; Martinho, O.; Pires, M.M.; Pinheiro, C.; Cordeiro, M.; Bebiano, G.; Costa, P.; Palmeirim, I.; et al. Monocarboxylate transporters (MCTs) in gliomas: Expression and exploitation as therapeutic targets. Neuro-Oncol. 2013, 15, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.; Santos, L.L.; Miranda-Gonçalves, V.; Morais, A.; Amaro, T.; Longatto-Filho, A.; Baltazar, F. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol. Carcinog. 2015, 54, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Pértega-Gomes, N.; Baltazar, F. Lactate transporters in the context of prostate cancer metabolism: What do we know? Int. J. Mol. Sci. 2014, 15, 18333–18348. [Google Scholar] [CrossRef]
- Eilertsen, M.; Andersen, S.; Al-Saad, S.; Kiselev, Y.; Donnem, T.; Stenvold, H.; Pettersen, I.; Al-Shibli, K.; Richardsen, E.; Busund, L.-T.; et al. Monocarboxylate transporters 1–4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS ONE 2014, 9, e105038. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Takahashi, M.; Uramoto, H.; Nakayama, Y.; Oyama, T.; Wang, K.-Y.; Sasaguri, Y.; Nishizawa, S.; Kohno, K. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 2011, 102, 1007–1013. [Google Scholar] [CrossRef]
- Dhup, S.; Dadhich, R.K.; Porporato, P.E.; Sonveaux, P. Multiple biological activities of lactic acid in cancer: Influences on tumor growth, angiogenesis and metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef]
- Morris, A.A.M. Cerebral ketone body metabolism. J. Inherit. Metab. Dis. 2004, 28, 109–121. [Google Scholar] [CrossRef]
- Bollag, G.; Tsai, J.; Zhang, J.; Zhang, C.; Ibrahim, P.; Nolop, K.; Hirth, P. Vemurafenib: The first drug approved for BRAF-mutant cancer. Nat. Rev. Drug Discov. 2012, 11, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, S.; Payá, A.; Alenda, C.; Bessa, X.; Andreu, M.; Jover, R.; Castells, A.; Llor, X.; Aranda, F.I.; Massutí, B. Detection of BRAF V600E mutation in colorectal cancer: Comparison of automatic sequencing and real-time chemistry methodology. J. Mol. Diagn. 2006, 8, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Arcaini, L.; Zibellini, S.; Boveri, E.; Riboni, R.; Rattotti, S.; Varettoni, M.; Guerrera, M.L.; Lucioni, M.; Tenore, A.; Merli, M.; et al. The BRAF V600E mutation in hairy cell leukemia and other mature B-cell neoplasms. Blood 2012, 119, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Lawrence, M.S.; Keats, J.J.; Cibulskis, K.; Sougnez, C.; Schinzel, A.C.; Harview, C.L.; Brunet, J.-P.; Ahmann, G.J.; Adli, M.; et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011, 471, 467–472. [Google Scholar] [CrossRef]
- Kang, H.-B.; Fan, J.; Lin, R.; Elf, S.; Ji, Q.; Zhao, L.; Jin, L.; Seo, J.H.; Shan, C.; Arbiser, J.L.; et al. Metabolic rewiring by oncogenic BRAF V600E links ketogenesis pathway to BRAF-MEK1 signaling. Mol. Cell 2015, 59, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Ussar, S.; Voss, T. MEK1 and MEK2, different regulators of the G1/S transition. J. Biol. Chem. 2004, 279, 43861–43869. [Google Scholar] [CrossRef]
- De Saedeleer, C.J.; Copetti, T.; Porporato, P.E.; Verrax, J.; Feron, O.; Sonveaux, P. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS ONE 2012, 7, e46571. [Google Scholar] [CrossRef]
- Lu, H.; Dalgard, C.L.; Mohyeldin, A.; McFate, T.; Tait, A.S.; Verma, A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J. Biol. Chem. 2005, 280, 41928–41939. [Google Scholar] [CrossRef]
- Lu, H.; Forbes, R.A.; Verma, A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 2002, 277, 23111–23115. [Google Scholar] [CrossRef]
- Semenza, G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010, 29, 625–634. [Google Scholar] [CrossRef]
- Wang, Z.; Smith, K.S.; Murphy, M.; Piloto, O.; Somervaille, T.C.P.; Cleary, M.L. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature 2008, 455, 1205–1209. [Google Scholar] [PubMed]
- Woodgett, J.R. Regulation and functions of the glycogen synthase kinase-3 subfamily. Semin. Cancer Biol. 1994, 5, 269–275. [Google Scholar] [PubMed]
- Ougolkov, A.V.; Fernandez-Zapico, M.E.; Savoy, D.N.; Urrutia, R.A.; Billadeau, D.D. Glycogen synthase kinase-3β participates in nuclear factor κB–mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005, 65, 2076–2081. [Google Scholar]
- Kotliarova, S.; Pastorino, S.; Kovell, L.C.; Kotliarov, Y.; Song, H.; Zhang, W.; Bailey, R.; Maric, D.; Zenklusen, J.C.; Lee, J.; et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-κB, and glucose regulation. Cancer Res. 2008, 68, 6643–6651. [Google Scholar] [CrossRef]
- Marchand, B.; Tremblay, I.; Cagnol, S.; Boucher, M.-J. Inhibition of glycogen synthase kinase-3 activity triggers an apoptotic response in pancreatic cancer cells through JNK-dependent mechanisms. Carcinogenesis 2012, 33, 529–537. [Google Scholar] [PubMed]
- Shakoori, A.; Ougolkov, A.; Yu, Z.W.; Zhang, B.; Modarressi, M.H.; Billadeau, D.D.; Mai, M.; Takahashi, Y.; Minamoto, T. Deregulated GSK3β activity in colorectal cancer: Its association with tumor cell survival and proliferation. Biochem. Biophys. Res. Commun. 2005, 334, 1365–1373. [Google Scholar] [CrossRef]
- Mazor, M.; Kawano, Y.; Zhu, H.; Waxman, J.; Kypta, R.M. Inhibition of glycogen synthase kinase-3 represses androgen receptor activity and prostate cancer cell growth. Oncogene 2004, 23, 7882–7892. [Google Scholar] [CrossRef]
- Ryves, W.; Harwood, A.J. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem. Biophys. Res. Commun. 2001, 280, 720–725. [Google Scholar] [CrossRef]
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Friedman, A.B.; Zhu, W.; Wang, L.; Boswell, S.; May, R.S.; Davis, L.L.; Jope, R.S. Lithium regulates glycogen synthase kinase-3β in human peripheral blood mononuclear cells: Implication in the treatment of bipolar disorder. Biol. Psychiatry 2007, 61, 216–222. [Google Scholar] [CrossRef]
- Qu, L.; Huang, S.; Baltzis, D.; Rivas-Estilla, A.-M.; Pluquet, O.; Hatzoglou, M.; Koumenis, C.; Taya, Y.; Yoshimura, A.; Koromilas, A.E. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3β. Genes Dev. 2004, 18, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Watcharasit, P.; Bijur, G.N.; Song, L.; Zhu, J.; Chen, X.; Jope, R.S. Glycogen synthase kinase-3β (GSK3β) binds to and promotes the actions of p53. J. Biol. Chem. 2003, 278, 48872–48879. [Google Scholar] [CrossRef] [PubMed]
- Watcharasit, P.; Bijur, G.N.; Zmijewski, J.W.; Song, L.; Zmijewska, A.; Chen, X.; Johnson, G.V.W.; Jope, R.S. Direct, activating interaction between glycogen synthase kinase-3β and p53 after DNA damage. Proc. Natl. Acad. Sci. USA 2002, 99, 7951–7955. [Google Scholar] [CrossRef]
- Beurel, E.; Kornprobst, M.; Eggelpoël, M.-J.B.-V.; Ruiz-Ruiz, C.; Cadoret, A.; Capeau, J.; Desbois-Mouthon, C. GSK-3β inhibition by lithium confers resistance to chemotherapy-induced apoptosis through the repression of CD95 (Fas/APO-1) expression. Exp. Cell Res. 2004, 300, 354–364. [Google Scholar] [CrossRef]
- Cao, Q.; Lu, X.; Feng, Y.-J. Glycogen synthase kinase-3β positively regulates the proliferation of human ovarian cancer cells. Cell Res. 2006, 16, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Kunnimalaiyaan, M.; Vaccaro, A.M.; Ndiaye, M.A.; Chen, H. Inactivation of glycogen synthase kinase-3β, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol. Cancer Ther. 2007, 6, 1151–1158. [Google Scholar] [CrossRef]
- Wang, J.S.; Wang, C.L.; Wen, J.F.; Wang, Y.J.; Hu, Y.B.; Ren, H.Z. Lithium inhibits proliferation of human esophageal cancer cell line Eca-109 by inducing a G2/M cell cycle arrest. J. Gastroenterol. 2008, 14, 3982. [Google Scholar] [CrossRef]
- Korur, S.; Huber, R.M.; Sivasankaran, B.; Petrich, M.; Morin, P., Jr.; Hemmings, B.A.; Merlo, A.; Lino, M.M. GSK3β regulates differentiation and growth arrest in glioblastoma. PLoS ONE 2009, 4, e7443. [Google Scholar] [CrossRef]
- Maurer, G.D.; Brucker, D.P.; Bähr, O.; Harter, P.N.; Hattingen, E.; Walenta, S.; Mueller-Klieser, W.; Steinbach, J.P.; Rieger, J. Differential utilization of ketone bodies by neurons and glioma cell lines: A rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 2011, 11, 315. [Google Scholar] [CrossRef]
- Bijur, G.N.; De Sarno, P.; Jope, R.S. Glycogen synthase kinase-3β facilitates staurosporine-and heat shock-induced apoptosis: Protection by lithium. J. Biol. Chem. 2000, 275, 7583–7590. [Google Scholar] [CrossRef]
- Chen, R.-W.; Chuang, D.-M. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression: A prominent role in neuroprotection against excitotoxicity. J. Biol. Chem. 1999, 274, 6039–6042. [Google Scholar] [CrossRef]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar]
- King, T.D.; Bijur, G.N.; Jope, R.S. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3β and attenuated by lithium. Brain Res. 2001, 919, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Gustin, J.P.; Karakas, B.; Weiss, M.B.; Abukhdeir, A.M.; Lauring, J.; Garay, J.P.; Cosgrove, D.; Tamaki, A.; Konishi, H.; Konishi, Y.; et al. Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 2835–2840. [Google Scholar] [CrossRef] [PubMed]
- Karlovic, D.; Jakopec, S.; Dubravcic, K.; Batinic, D.; Buljan, D.; Osmak, M. Lithium increases expression of p21WAF/Cip1 and survivin in human glioblastoma cells. Cell Biol. Toxicol. 2007, 23, 83–90. [Google Scholar] [CrossRef]
- Mora, A.; Sabio, G.; Alonso, J.C.; Soler, G.; Centeno, F. Different dependence of lithium and valproate on PI3K/PKB pathway. Bipolar Disord. 2002, 4, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.T.; Hottinger, D.G.; Kunnimalaiyaan, M.; Chen, H. Inhibition of growth in medullary thyroid cancer cells with histone deacetylase inhibitors and lithium chloride. J. Surg. Res. 2010, 159, 640–644. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, L.; Thrasher, J.B.; Du, J.; Li, B. Glycogen synthase kinase-3β suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Mol. Cancer Ther. 2003, 2, 1215–1222. [Google Scholar] [PubMed]
- Ozaki, N.; Chuang, D.M. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J. Neurochem. 1997, 69, 2336–2344. [Google Scholar] [CrossRef]
- Chen, R.W.; Qin, Z.H.; Ren, M.; Kanai, H.; Chalecka-Franaszek, E.; Leeds, P.; Chuang, D.M. Regulation of c-Jun N-terminal kinase, p38 kinase and AP-1 DNA binding in cultured brain neurons: Roles in glutamate excitotoxicity and lithium neuroprotection. J. Neurochem. 2003, 84, 566–575. [Google Scholar] [CrossRef]
- Wang, M.J.; Huang, H.Y.; Chen, W.F.; Chang, H.F.; Kuo, J.S. Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-α production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades. J. Neuroinflamm. 2010, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ji, Z.; Mei, F.; Lu, M.; Ou, Y.; Cheng, X. Lithium inhibits tumorigenic potential of PDA cells through targeting hedgehog-GLI signaling pathway. PLoS ONE 2013, 8, e61457. [Google Scholar] [CrossRef]
- Li, H.; Huang, K.; Liu, X.; Liu, J.; Lu, X.; Tao, K.; Wang, G.; Wang, J. Lithium chloride suppresses colorectal cancer cell survival and proliferation through ROS/GSK-3β/NF-κB signaling pathway. Oxidative Med. Cell. Longev. 2014, 2014, 241864. [Google Scholar] [CrossRef]
- Sarkar, S.; Rubinsztein, D.C. Inositol and IP3 levels regulate autophagy—Biology and therapeutic speculations. Autophagy 2006, 2, 132–134. [Google Scholar] [CrossRef]
- Sarkar, S.; Floto, R.A.; Berger, Z.; Imarisio, S.; Cordenier, A.; Pasco, M.; Cook, L.J.; Rubinsztein, D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005, 170, 1101–1111. [Google Scholar] [CrossRef]
- Chiu, C.-T.; Chuang, D.-M. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol. Ther. 2010, 128, 281–304. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Perlstein, E.O.; Imarisio, S.; Pineau, S.; Cordenier, A.; Maglathlin, R.L.; Webster, J.A.; Lewis, T.A.; O’Kane, C.J.; Schreiber, S.L.; et al. Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat. Chem. Biol. 2007, 3, 331–338. [Google Scholar] [CrossRef]
- Heiseke, A.; Aguib, Y.; Riemer, C.; Baier, M.; Schätzl, H.M. Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J. Neurochem. 2009, 109, 25–34. [Google Scholar] [CrossRef]
- Bgatova, N.; Taskaeva, I.; Makarova, V. Influence of distant tumor growth and lithium treatment on ultrastructural organization of kidney proximal tubules. Ultrastruct. Pathol. 2021, 45, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Taskaeva, I.; Bgatova, N. Ultrastructural and immunofluorescent analysis of lithium effects on autophagy in hepatocellular carcinoma cells. Asian Pac. J. Cancer Biol. 2018, 3, 83–87. [Google Scholar] [CrossRef]
- Taskaeva, I.S.; Bgatova, N.P.; Solovieva, A.O. Autophagy in Hepatocellular Carcinoma-29 after Single or Combined Administration of Lithium Carbonate and Rapamycin. Cell Tissue Biol. 2019, 13, 353–359. [Google Scholar] [CrossRef]
- Kim, E.C.; Meng, H.; Jun, A.S. Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br. J. Ophthalmol. 2013, 97, 1068–1073. [Google Scholar] [CrossRef]
- Yang, J.; Takahashi, Y.; Cheng, E.; Liu, J.; Terranova, P.F.; Zhao, B.; Thrasher, J.B.; Wang, H.-G.; Li, B. GSK-3β promotes cell survival by modulating Bif-1-dependent autophagy and cell death. J. Cell Sci. 2010, 123, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, H.; Zhong, L.; Yang, R.; Yang, X.-Q.; Jiang, K.-L.; Liu, B.-Z. Lithium chloride promotes apoptosis in human leukemia NB4 cells by inhibiting glycogen synthase kinase-3 beta. Int. J. Med Sci. 2015, 12, 805. [Google Scholar] [CrossRef]
- Fine, E.J.; Miller, A.; Quadros, E.V.; Sequeira, J.M.; Feinman, R.D. Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2. Cancer Cell Int. 2009, 9, 14. [Google Scholar] [PubMed]
- Franklin, S.; Young, J.; Nonnecke, B. Effects of ketones, acetate, butyrate, and glucose on bovine lymphocyte proliferation. J. Dairy Sci. 1991, 74, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Bartmann, C.; Raman, S.R.J.; Flöter, J.; Schulze, A.; Bahlke, K.; Willingstorfer, J.; Strunz, M.; Wöckel, A.; Klement, R.J.; Kapp, M.; et al. Beta-hydroxybutyrate (3-OHB) can influence the energetic phenotype of breast cancer cells, but does not impact their proliferation and the response to chemotherapy or radiation. Cancer Metab. 2018, 6, 8. [Google Scholar] [CrossRef]
- Liu, J.; Ju, P.; Zhou, Y.; Zhao, Y.; Xie, Y.; Long, Y.; Gu, Y.; Ni, D.; Lyv, Z.; Mao, Z.; et al. Six2 is a coordinator of LiCl-induced cell proliferation and apoptosis. Int. J. Mol. Sci. 2016, 17, 1504. [Google Scholar]
- Zanni, G.; Di Martino, E.; Omelyanenko, A.; Andäng, M.; Delle, U.; Elmroth, K.; Blomgren, K. Lithium increases proliferation of hippocampal neural stem/progenitor cells and rescues irradiation-induced cell cycle arrest in vitro. Oncotarget 2015, 6, 37083. [Google Scholar] [CrossRef] [PubMed]
- Smits, V.A.; Essers, M.A.; Loomans, D.S.; Klompmaker, R.; Rijksen, G.; Medema, R.H. Inhibition of cell proliferation by lithium is associated with interference in cdc2 activation. FEBS Lett. 1999, 457, 23–27. [Google Scholar] [CrossRef]
- Fu, Y.; Zheng, Y.; Chan, K.G.; Liang, A.; Hu, F. Lithium chloride decreases proliferation and migration of C6 glioma cells harboring isocitrate dehydrogenase 2 mutant via GSK-3β. Mol. Biol. Rep. 2014, 41, 3907–3913. [Google Scholar] [CrossRef]
- Grimes, C.A.; Jope, R.S. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog. Neurobiol. 2001, 65, 391–426. [Google Scholar] [CrossRef]
- Ding, Q.; Xia, W.; Liu, J.-C.; Yang, J.-Y.; Lee, D.-F.; Xia, J.; Bartholomeusz, G.; Li, Y.; Pan, Y.; Li, Z.; et al. associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol. Cell 2005, 19, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Vidal, F.; Cruz, A.L.; De Araujo, W.M.; Tanaka, M.N.; Viola, J.P.; Morgado-Diaz, J.A. Lithium reduces tumorigenic potential in response to EGF signaling in human colorectal cancer cells. Int. J. Oncol. 2011, 38, 1365–1373. [Google Scholar]
- Farina, A.K.; Bong, Y.-S.; Feltes, C.M.; Byers, S.W. Post-transcriptional regulation of cadherin-11 expression by GSK-3 and β-catenin in prostate and breast cancer cells. PLoS ONE 2009, 4, e4797. [Google Scholar] [CrossRef]
- Ohira, T.; Gemmill, R.M.; Ferguson, K.; Kusy, S.; Roche, J.; Brambilla, E.; Zeng, C.; Baron, A.; Bemis, L.; Erickson, P.; et al. WNT7a induces E-cadherin in lung cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 10429–10434. [Google Scholar] [CrossRef] [PubMed]
- Maeng, Y.-S.; Lee, R.; Lee, B.; Choi, S.-I.; Kim, E.K. Lithium inhibits tumor lymphangiogenesis and metastasis through the inhibition of TGFBIp expression in cancer cells. Sci. Rep. 2016, 6, 20739. [Google Scholar] [CrossRef]
- Nowicki, M.O.; Dmitrieva, N.; Stein, A.M.; Cutter, J.L.; Godlewski, J.; Saeki, Y.; Nita, M.; Berens, M.E.; Sander, L.M.; Newton, H.B.; et al. Lithium inhibits invasion of glioma cells; possible involvement of glycogen synthase kinase-3. Neuro-Oncology 2008, 10, 690–699. [Google Scholar] [CrossRef]
- Mao, C.D.; Hoang, P.; DiCorleto, P.E. Lithium inhibits cell cycle progression and induces stabilization of p53 in bovine aortic endothelial cells. J. Biol. Chem. 2001, 276, 26180–26188. [Google Scholar] [CrossRef]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar] [CrossRef]
- Gradl, D.; Kühl, M.; Wedlich, D. The Wnt/Wg signal transducer β-catenin controls fibronectin expression. Mol. Cell. Biol. 1999, 19, 5576–5587. [Google Scholar] [CrossRef]
- Tetsu, O.; McCormick, F.J.N. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Mol. Cell. Biol. 1999, 398, 422–426. [Google Scholar]
- He, T.-C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Shanmugam, I.; Song, J.; Terranova, P.F.; Thrasher, J.B.; Li, B. Lithium suppresses cell proliferation by interrupting E2F–DNA interaction and subsequently reducing S–phase gene expression in prostate cancer. Prostate 2007, 67, 976–988. [Google Scholar] [CrossRef]
- Matsebatlela, T.; Gallicchio, V.; Becker, R. Lithium modulates cancer cell growth, apoptosis, gene expression and cytokine production in HL-60 promyelocytic leukaemia cells and their drug-resistant sub-clones. Biol. Trace Element Res. 2012, 149, 323–330. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, S.-W.; Kim, J.-H.; Kim, H.-J.; Um, J.; Jung, D.-W.; Williams, D. Lithium chloride protects against sepsis-induced skeletal muscle atrophy and cancer cachexia. Cells 2021, 10, 1017. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lin, Y.-S.; Wang, C.-Y.; Tsai, C.-C.; Tseng, H.-C.; Chen, C.-L.; Lu, P.-J.; Chen, P.-S.; Qian, L.; Hong, J.-S.; et al. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology 2009, 128, e275–e286. [Google Scholar] [CrossRef]
- Nahman, S.; Belmaker, R.; Azab, A.N. Effects of lithium on lipopolysaccharide-induced inflammation in rat primary glia cells. Endotoxin Res. 2012, 18, 447–458. [Google Scholar] [CrossRef]
- Nassar, A.; Azab, A.N. Effects of lithium on inflammation. ACS Chem. Neurosci. 2014, 5, 451–458. [Google Scholar] [CrossRef]
- Scholtz, W. IL-6 in disease: Cause or cure. Immunopathology 1996, 3, 131–150. [Google Scholar]
- Bgatova, N.; Taskaeva, I. Ultrastructure of the kidney filtration barrier in conditions of distant tumor growth and lithium treatment. Ultrastruct. Pathol. 2020, 44, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Novetsky, A.P.; Thompson, D.M.; Zighelboim, I.; Thaker, P.H.; Powell, M.A.; Mutch, D.G.; Goodfellow, P.J. Lithium chloride and inhibition of glycogen synthase kinase 3β as a potential therapy for serous ovarian cancer. Int. J. Gynecol. Cancer 2013, 23, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Lubner, S.J.; Kunnimalaiyaan, M.; Holen, K.D.; Ning, L.; Ndiaye, M.; LoConte, N.K.; Mulkerin, D.L.; Schelman, W.R.; Chen, H. A preclinical and clinical study of lithium in low-grade neuroendocrine tumors. Oncology 2011, 16, 452–457. [Google Scholar] [CrossRef]
- Khasraw, M.; Ashley, D.; Wheeler, G.; Berk, M. Using lithium as a neuroprotective agent in patients with cancer. BMC Med. 2012, 10, 131. [Google Scholar] [CrossRef]
- Rouhani, M.; Goliaei, B.; Khodagholi, F.; Nikoofar, A. Lithium increases radiosensitivity by abrogating DNA repair in breast cancer spheroid culture. Arch. Iran. Med. 2014, 17, 352–360. [Google Scholar]
- Bilir, A.; Aynacioğlu, A.; Tuna, M.Y. The possible interactions and therapeutic roles of lithium chloride and midkine on cancer treatment. Crit. Rev. Oncog. 2019, 24, 35–45. [Google Scholar] [CrossRef]
- Huo, K.; Sun, Y.; Li, H.; Du, X.; Wang, X.; Karlsson, N.; Zhu, C.; Blomgren, K. Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Mol. Cell. Neurosci. 2012, 51, 32–42. [Google Scholar] [CrossRef]
- Malaterre, J.; McPherson, C.S.; Denoyer, D.; Lai, E.; Hagekyriakou, J.; Lightowler, S.; Shudo, K.; Ernst, M.; Ashley, D.M.; Short, J.L.; et al. Enhanced lithium-induced brain recovery following cranial irradiation is not impeded by inflammation. Stem Cells Transl. Med. 2012, 1, 469–479. [Google Scholar] [CrossRef]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Martin, D.C.; Castelo-Branco, P.; Zhang, C.H.; Fraser, M.; Tse, K.; Poon, R.; Shih, D.J.; et al. WNT activation by lithium abrogates TP53 mutation associated radiation resistance in medulloblastoma. Acta Neuropathol. Commun. 2014, 2, 174. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.; Zhu, K.; Liu, W.; Luo, Y.; Yuan, X.; Wang, J.; Cheng, T.; Zhang, X. Lithium chloride with immunomodulatory function for regulating titanium nanoparticle-stimulated inflammatory response and accelerating osteogenesis through suppression of MAPK signaling pathway. Int. J. Nanomed. 2019, 14, 7475. [Google Scholar] [CrossRef]
- Yavari, S.; Geramifar, P.; Fallahpoor, M.; Changizi, V.; Gholami, M.; Meysamie, A.; Farzanehfar, S.; Abbasi, M. The effect of lithium on radioiodine thyroid tissue ablation. Intern. J. Radiat. Res. 2021, 19, 1045–1048. [Google Scholar] [CrossRef]
- Boehmerle, W.; Zhang, K.; Sivula, M.; Heidrich, F.M.; Lee, Y.; Jordt, S.-E.; Ehrlich, B.E. Chronic exposure to paclitaxel diminishes phosphoinositide signaling by calpain-mediated neuronal calcium sensor-1 degradation. Proc. Natl. Acad. Sci. USA 2007, 104, 11103–11108. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.Y.; Ehrlich, B.E. Prevention of chemotherapy-induced peripheral neuropathy: A review of recent findings. Crit. Rev. Oncol./Hematol. 2020, 145, 102831. [Google Scholar] [CrossRef]
- Sánchez, J.C.; Muñoz, L.V.; Ehrlich, B.E. Modulating TRPV4 channels with paclitaxel and lithium. Cell Calcium 2020, 91, 102266. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Jakobsson, E. Systems biology understanding of the effects of lithium on cancer. Front. Oncol. 2019, 9, 296. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Hsieh, K.-P.; Huang, W.-W.; Yang, Y.-H. of lithium and cancer risk in patients with bipolar disorder: Population-based cohort study. Br. J. Psychiatry 2016, 209, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Heidarali, Z.; Rajabi, M.; Omidi, Z.; Zayeri, F.; Salehi, M.; Haghighat, S. Lithium and preventing chemotherapy-induced peripheral neuropathy in breast cancer patients: A placebo-controlled randomized clinical trial. Trials 2021, 22, 835. [Google Scholar] [CrossRef]
- Ueda, M.; Stefan, T.; Stetson, L.; Ignatz-Hoover, J.J.; Tomlinson, B.; Creger, R.J.; Cooper, B.; Lazarus, H.M.; de Lima, M.; Wald, D.N.; et al. Phase I Trial of Lithium and Tretinoin for Treatment of Relapsed and Refractory Non-promyelocytic Acute Myeloid Leukemia. Front Oncol. 2020, 10, 327. [Google Scholar] [CrossRef]
- Sherman, S.I. Thyroid carcinoma. Lancet 2003, 361, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Steinbach, G.; Hockenbery, D.M.; Huls, G.; Furlong, T.; Myerson, D.; Loeb, K.R.; Fann, J.R.; Castilla-Llorente, C.; McDonald, G.B.; Martin, P.J. Pilot study of lithium to restore intestinal barrier function in severe graft-versus-host disease. PLoS ONE 2017, 12, e0183284. [Google Scholar] [CrossRef] [PubMed]
- Linssen, J.D.G.; van Neerven, S.M.; Aelvoet, A.S.; Elbers, C.C.; Vermeulen, L.; Dekker, E. The CHAMP-study: The CHemopreventive effect of lithium in familial AdenoMatous Polyposis; study protocol of a phase II trial. BMC Gastroenterol 2022, 22, 383. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Patrizio, M.P.; Magagnoli, F.; Luppi, S.; Serra, M. An update on emerging drugs in osteosarcoma: Towards tailored therapies? Expert Opin. Emerg. Drugs 2019, 24, 153–171. [Google Scholar] [CrossRef]
- Bgatova, N.P.; Borodin, Y.I.; Makarova, V.; Pozhidaeva, A.A.; Rachkovskaya, L.N.; Konenkov, V.I. Effects of Nanosized Lithium Carbonate Particles on Intact Muscle Tissue and Tumor Growth. Bull. Exp. Biol. Med. 2014, 157, 89–94. [Google Scholar] [CrossRef]
- Bgatova, N.P.; Makarova, O.P.; Pozhidayeva, A.A.; Borodin, Y.I.; Rachkovskaya, L.N.; Konenkov, V.I. Effects of Lithium Nano-Scaled Particles on Local and Systemic Structural and Functional Organism Transformations Under Tumour Growth. Achiev. Life Sci. 2014, 8, 101–111. [Google Scholar] [CrossRef]
- Lykov, A.P.; Poveshchenko, O.V.; Bondarenko, N.A.; Bogatova, N.P.; Makarova, O.P.; Konenkov, V.I. Antiproliferative potential of officinal forms and nanoparticles of lithium salts. Bull. Exp. Biol. Med. 2016, 160, 827. [Google Scholar] [CrossRef]
- Gavrilova, Y.S.; Bgatova, N.P.; Solov’Eva, A.O.; Trifonova, K.E.; Lykov, A.; Borodin, Y.I.; Konenkov, V.I. Target cells for lithium in different forms within a heterogeneous hepatocarcinoma-29 population. Cell Tissue Biol. 2016, 10, 284–289. [Google Scholar] [CrossRef]
- Hosseini, Y.; Alavi, S.E.; Akbarzadeh, A.; Heidarinasab, A. Improving lithium carbonate therapeutics by pegylated liposomal technology: An in vivo study. Comp. Clin. Pathol. 2016, 25, 211–218. [Google Scholar] [CrossRef]
- Iqbal, H.; Razzaq, A.; Uzair, B.; Ain, N.U.; Sajjad, S.; Althobaiti, N.A.; Albalawi, A.E.; Menaa, B.; Haroon, M.; Khan, M.; et al. Breast cancer inhibition by biosynthesized titanium dioxide nanoparticles is comparable to free doxorubicin but appeared safer in BALB/c mice. Materials 2021, 14, 3155. [Google Scholar] [CrossRef] [PubMed]
- Özer, S.; Şenel, B.; Yazan, Y. Preparation and in vitro evaluation of in situ gelling system containing lithium carbonate for parenteral administration. Polym. Bull. 2020, 77, 599–622. [Google Scholar] [CrossRef]
- Patel, V.; Chudasama, A.; Nivsarkar, M.; Vasu, K.; Shishoo, C. Push-pull osmotic pump for zero order delivery of lithium carbonate: Development and in vitro characterization. Pharm. Dev. Technol. 2012, 17, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Hashimoto, S.; Sawa, T.; Amaya, F. Tumor necrosis factor-alpha induces expression of C/EBP-beta in primary afferent neurons following nerve injury. Neuroscience 2014, 279, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gambelli, F.; Di, Y.P.; Niu, X.; Friedman, M.; Hammond, T.; Riches, D.W.; Ortiz, L. Phosphorylation of tumor necrosis factor receptor 1 (p55) protects macrophages from silica-induced apoptosis. J. Biol. Chem. 2004, 279, 2020–2029. [Google Scholar] [CrossRef]
- Saleh, K.A.; Aldulmani, S.A.A.; Awwad, N.S.; Ibrahium, H.A.; Asiri, T.H.; Hamdy, M.S. Utilization of lithium incorporated mesoporous silica for preventing necrosis and increase apoptosis in different cancer cells. BMC Chem. 2019, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Belahmar, A.; Mikou, M.; Hoehr, C.; El Ghalmi, M. Cumulative dose experiments on Lithium formate monohydrate as an EPR-dosimeter for use in different radiation therapy scenarios. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2022, 532, 1–6. [Google Scholar] [CrossRef]
- Zhu, Z.; Xing, H.; Tang, R.; Qian, S.; He, S.; Hu, Q.; Zhang, N. The preconditioning of lithium promotes mesenchymal stem cell-based therapy for the degenerated intervertebral disc via upregulating cellular ROS. Stem Cell Res. Ther. 2021, 12, 239. [Google Scholar] [CrossRef]
- Farmani, A.R.; Salmeh, M.A.; Golkar, Z.; Moeinzadeh, A.; Ghiasi, F.F.; Amirabad, S.Z.; Shoormeij, M.H.; Mahdavinezhad, F.; Momeni, S.; Moradbeygi, F.; et al. Li-Doped Bioactive Ceramics: Promising Biomaterials for Tissue Engineering and Regenerative Medicine. J. Funct. Biomater. 2022, 13, 162. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Moztarzadeh, F.; Arabyazdi, S. A heat-generating lithium-ferrite doped bioactive glass for cancer hyperthermia. Phys. B Condens. Matter 2020, 593, 412298. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Zhang, H.; Huang, H.; Sun, L.; Ma, L.; Du, Y.; Pei, C.; Zhang, Q.; Li, H.; et al. Activating layered metal oxide nanomaterials via structural engineering as biodegradable nanoagents for photothermal cancer therapy. Small 2021, 17, 2007486. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Hu, T.; Xue, B.; Chen, H.; Ma, L.; Liang, R.; Tan, C. Molybdenum-Based Nanomaterials for Photothermal Cancer Therapy. Adv. NanoBiomed Res. 2022, 2, 2200065. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Y.-C.; Yang, X.; Huang, Z.; Zhang, T.; Yang, L.; Meng, W.; Liu, X.; Gong, P.; Forni, A.; et al. Bonsai-inspired AIE nanohybrid photosensitizer based on vermiculite nanosheets for ferroptosis-assisted oxygen self-sufficient photodynamic cancer therapy. Nano Today 2022, 44, 101477. [Google Scholar] [CrossRef]
- Suganthi, M.; Sangeetha, G.; Gayathri, G.; Sankar, B.R. Biphasic dose-dependent effect of lithium chloride on survival of human hormone-dependent breast cancer cells (MCF-7). Biol. Trace Elem. Res. 2012, 150, 477–486. [Google Scholar] [CrossRef]
- Walker, R.J.; Weggery, S.; Bedford, J.J.; Mcdonald, F.J.; Ellis, G.; Leader, J.P. Lithium-induced reduction in urinary concentrating ability and urinary aquaporin 2 (AQP2) excretion in healthy volunteers. Kidney Int. 2005, 67, 291–294. [Google Scholar] [CrossRef]
- Pinna, M.; Manchia, M.; Puddu, S.; Minnai, G.; Tondo, L.; Salis, P. Cutaneous adverse reaction during lithium treatment: A case report and updated systematic review with meta-analysis. Int. J. Bipolar Disord. 2017, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Nair, C.G.; Menon, R.; Jacob, P.; Babu, M. Lithium-induced parathyroid dysfunction: A new case. Indian J. Endocrinol. Metab. 2013, 17, 930–932. [Google Scholar] [CrossRef]
- Meehan, A.D.; Udumyan, R.; Kardell, M.; Landén, M.; Järhult, J.; Wallin, G. Lithium-associated hypercalcemia: Pathophysiology, prevalence, management. World J. Surg. 2018, 42, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Poels, E.M.P.; Bijma, H.H.; Galbally, M.; Bergink, V. Lithium during pregnancy and after delivery: A review. Int. J. Bipolar Disord. 2018, 6, 26. [Google Scholar] [CrossRef]
- Matsebatlela, T.M.; Mogodiri, R.K.; Hart, D.A.; Gallicchio, V.S.; Becker, R.W. Resistance to lithium-induced apoptosis in a lithium tolerant clone of HL-60 promyelocytes. J. Trace Microprobe Tech. 2000, 18, 163–170. [Google Scholar]
- Lucas, K.C.; Hart, D.A.; Becker, R.W. Porcine proximal tubular cells (LLC-PK1) are able to tolerate high levels of lithium chloride in vitro: Assessment of the influence of 1–20 mM LiCl on cell death and alterations in cell biology and biochemistry. Cell Biol. Int. 2010, 34, 225–233. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).