Silybin Showed Higher Cytotoxic, Antiproliferative, and Anti-Inflammatory Activities in the CaCo Cancer Cell Line while Retaining Viability and Proliferation in Normal Intestinal IPEC-1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Silybin A/B
2.2. Cell Cultures
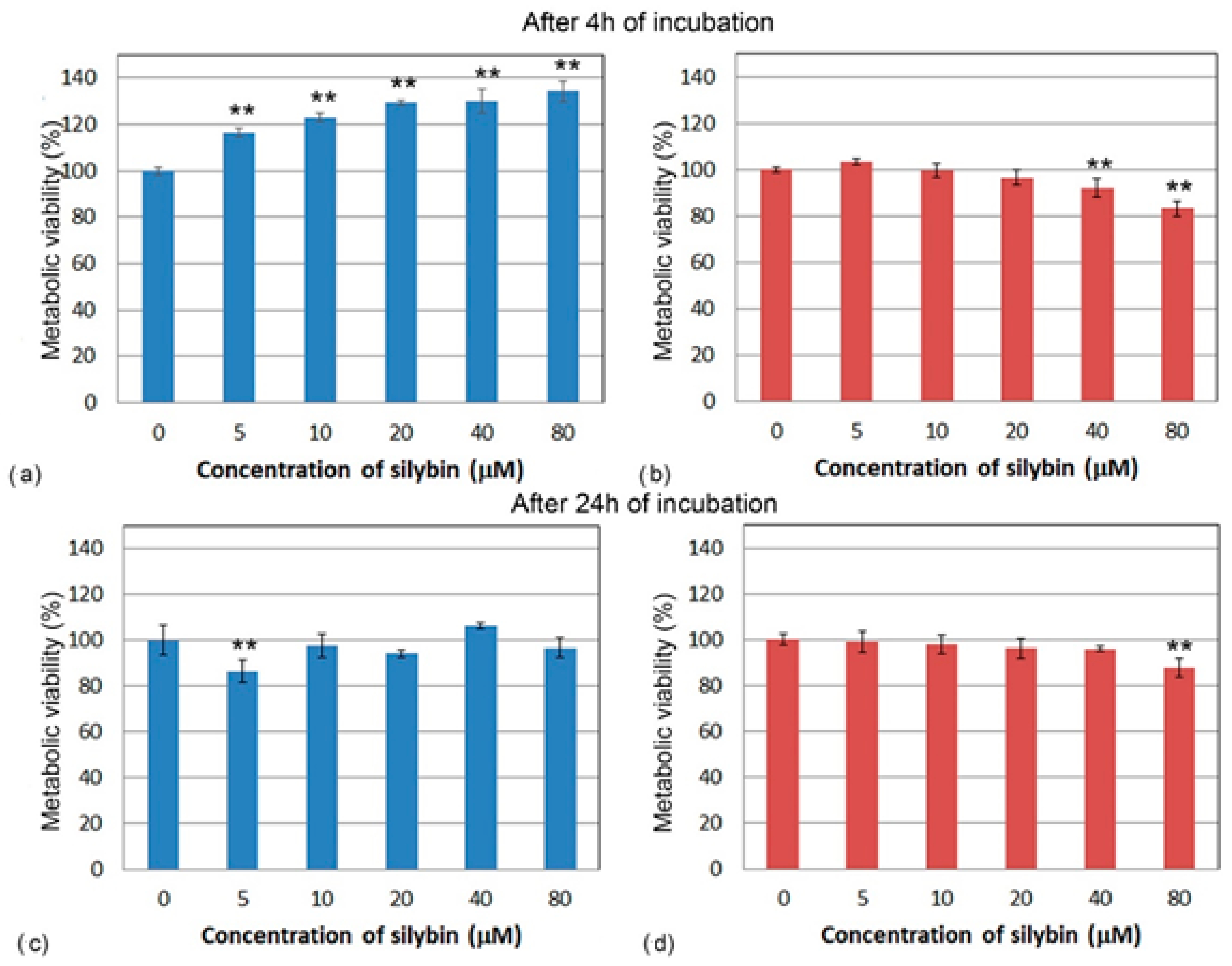
2.3. Metabolic Viability Assay
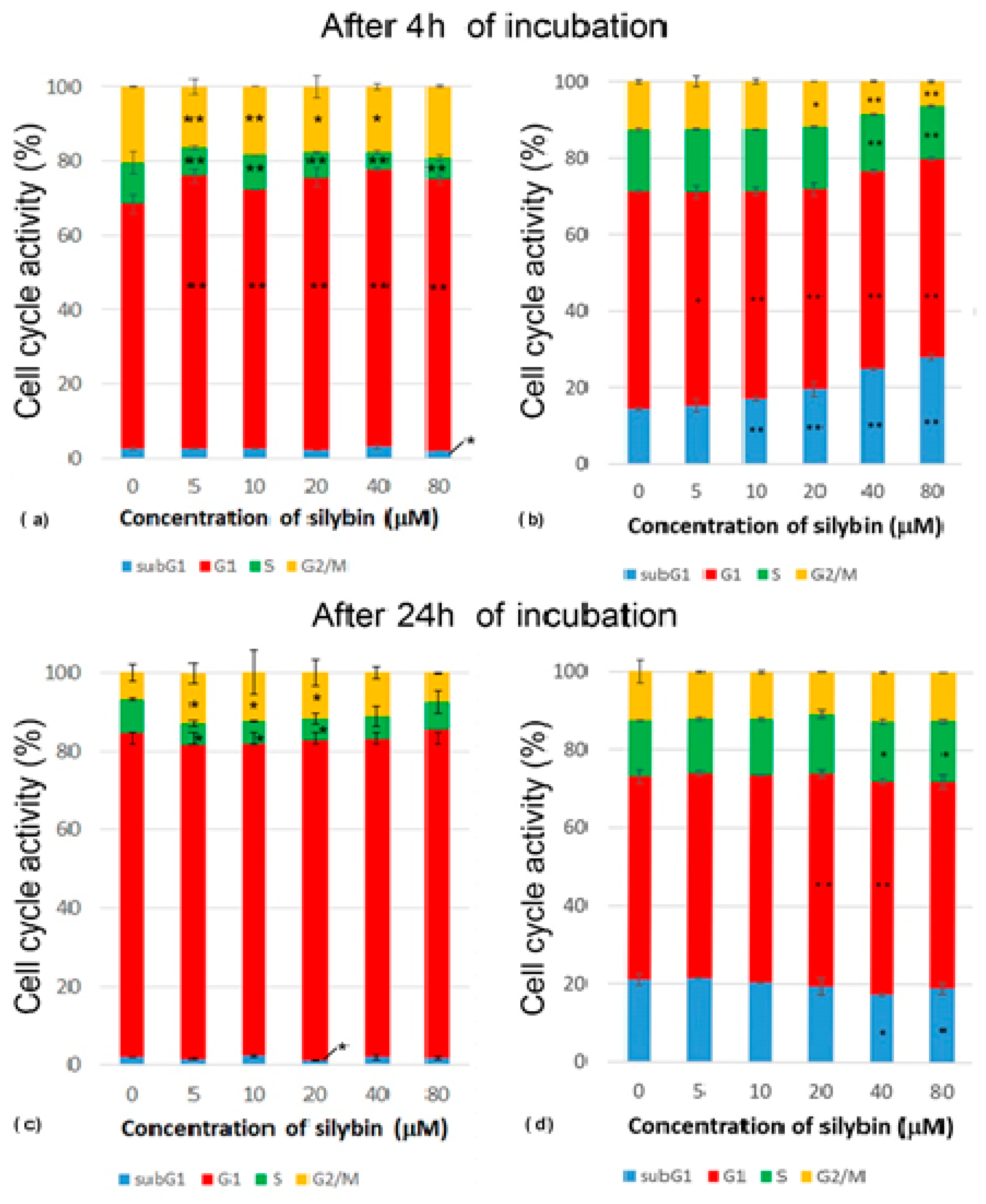
2.4. Cell Cycle Analysis
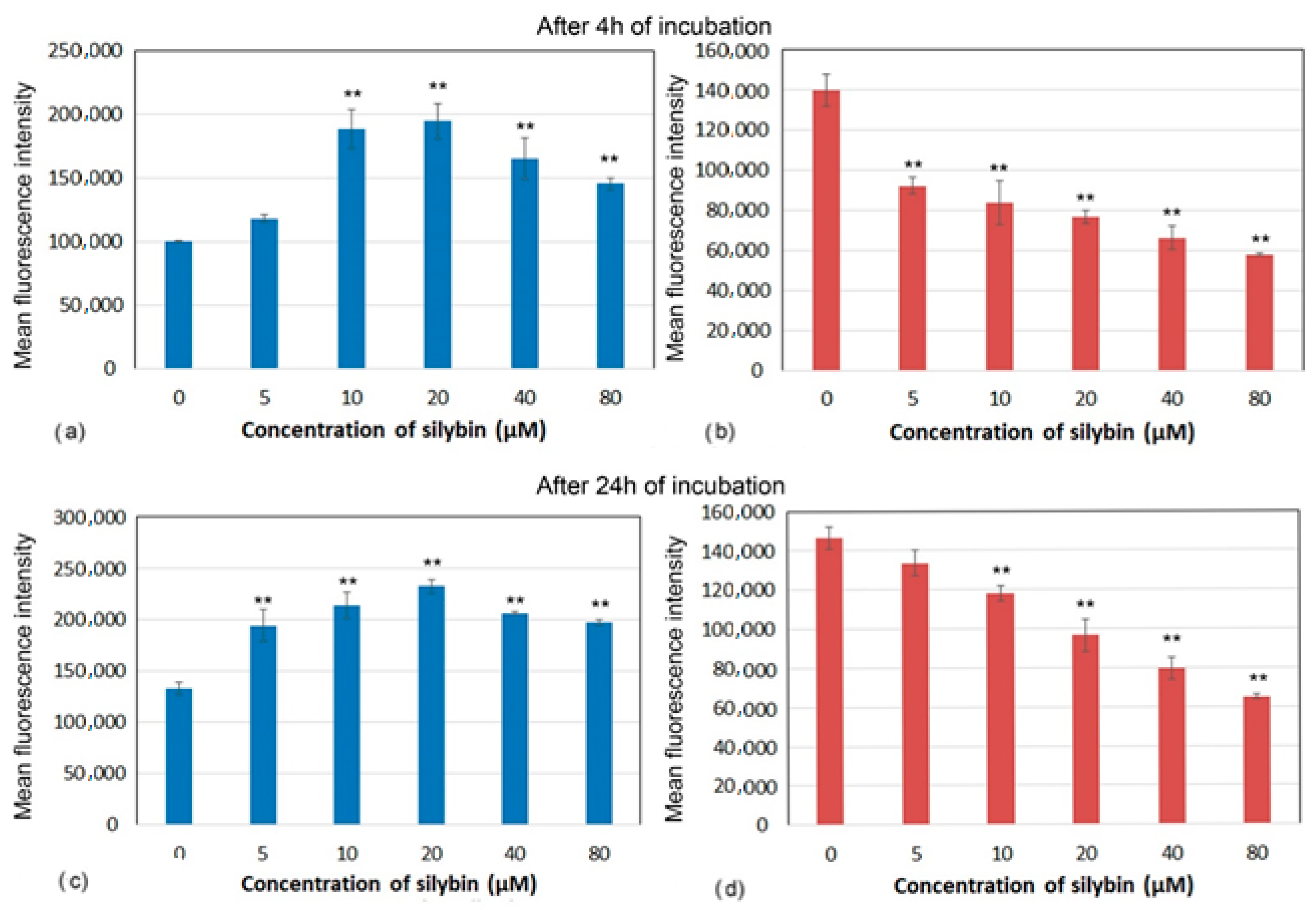
2.5. Mitochondrial Membrane Potential
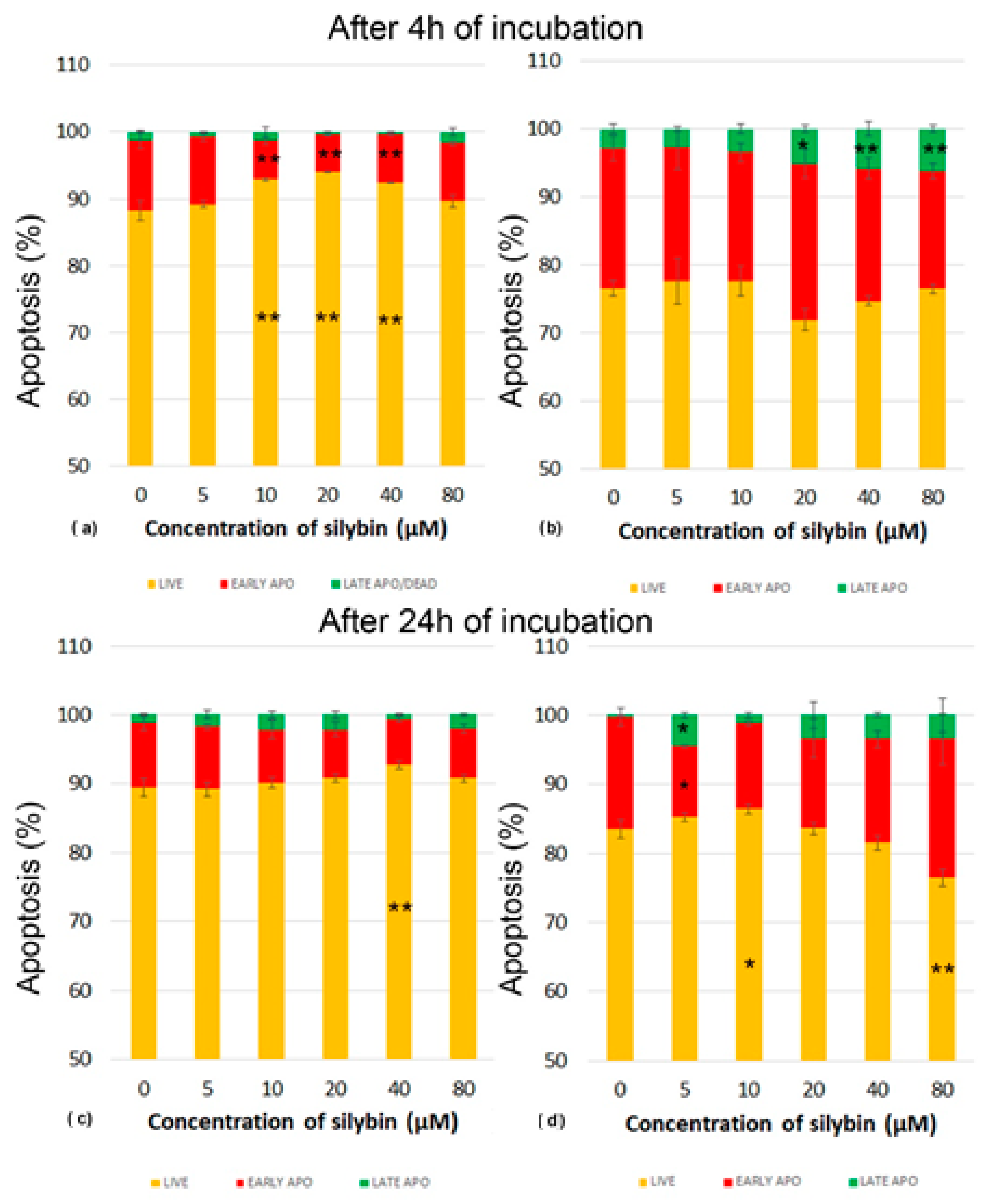
2.6. Annexin V/Propidium Iodide Apoptosis Assay
2.7. RNA Isolation and cDNA Synthesis
2.8. Gene Expression Analysis (qPCR)
2.9. Statistical Analysis
3. Results
3.1. Metabolic Viability Assessment
3.2. Analysis of Cell Cycle
3.3. The Effect of Silybin on the Induction of Apoptosis
3.4. Mitochondrial Membrane Potential
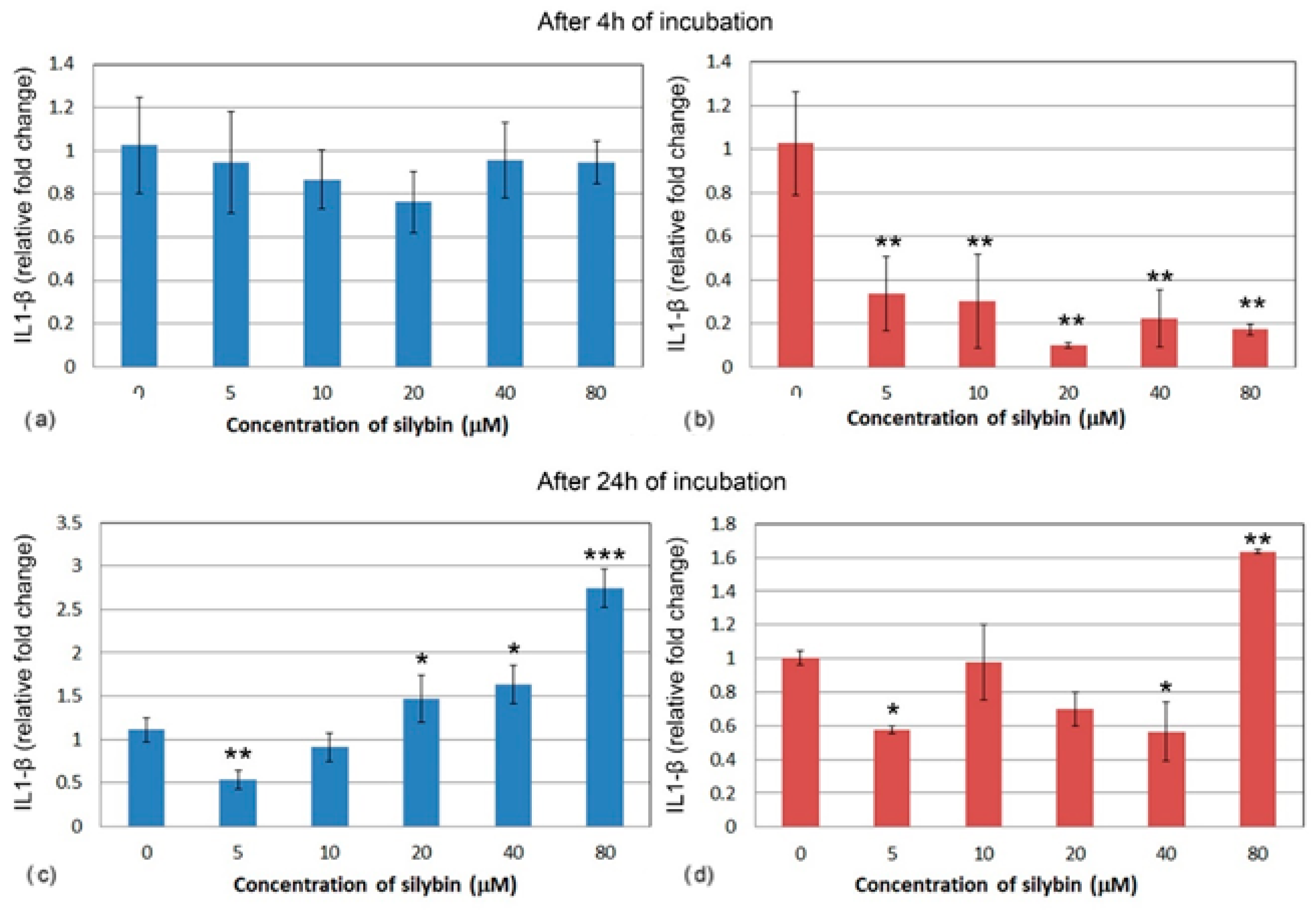
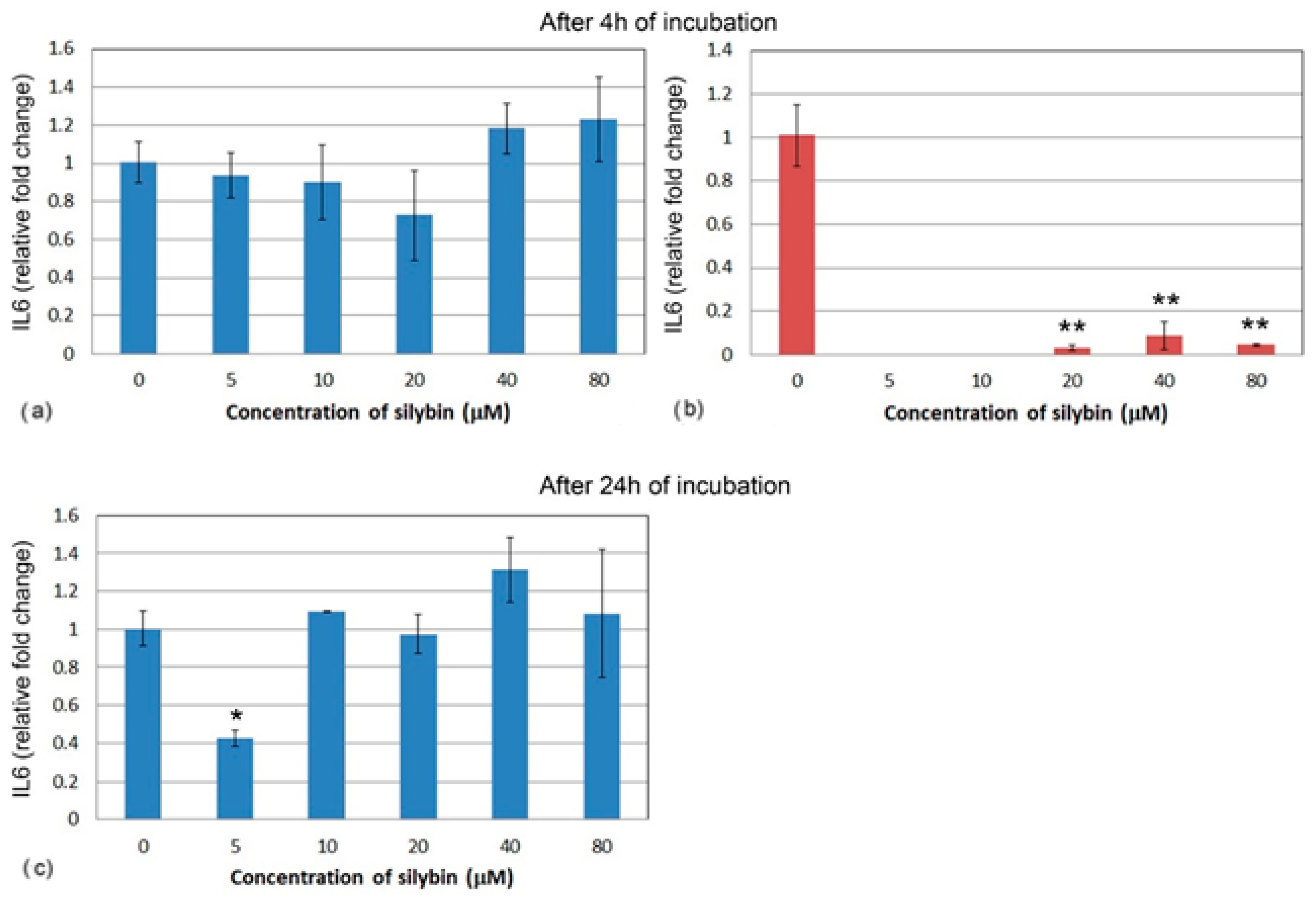
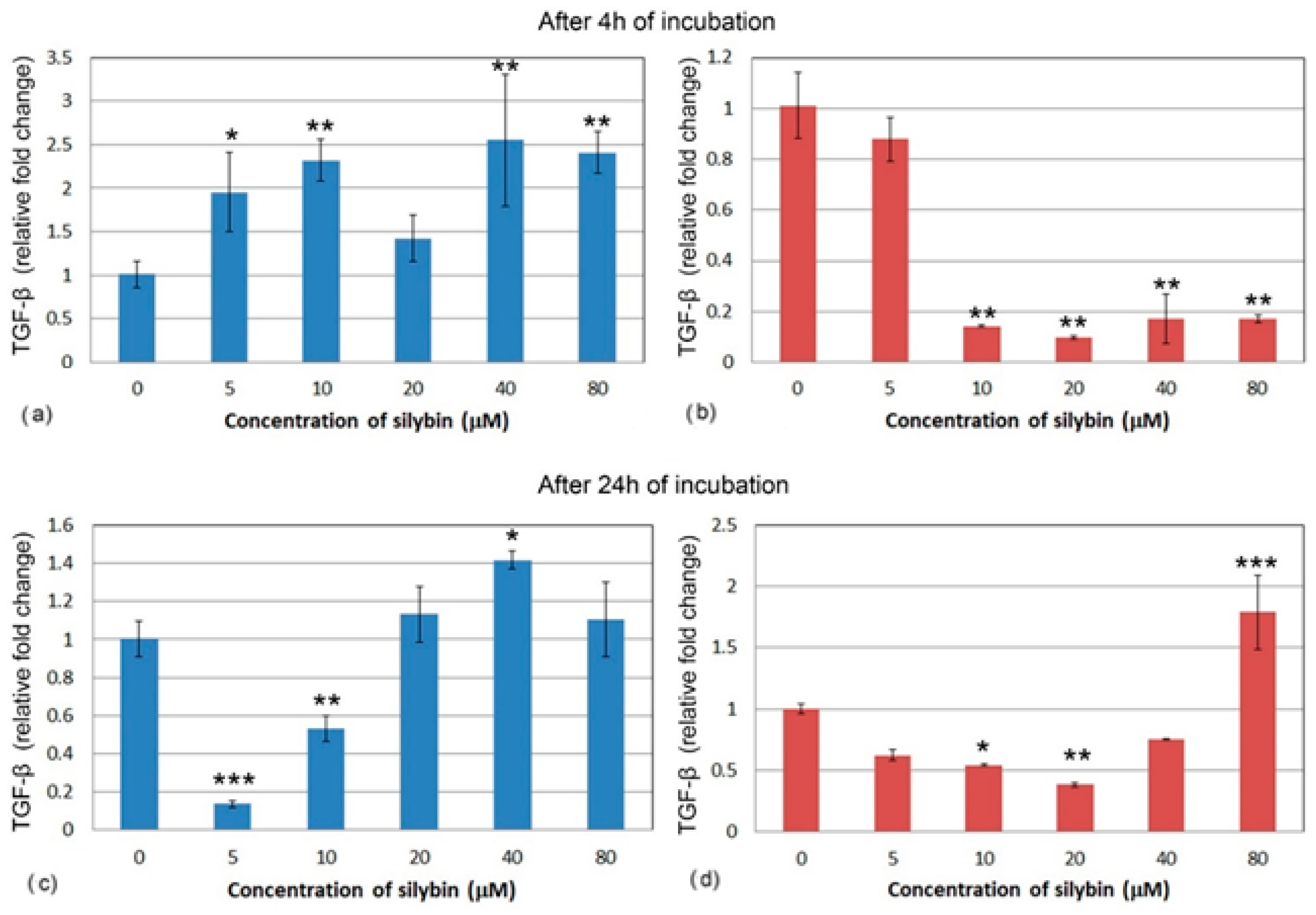
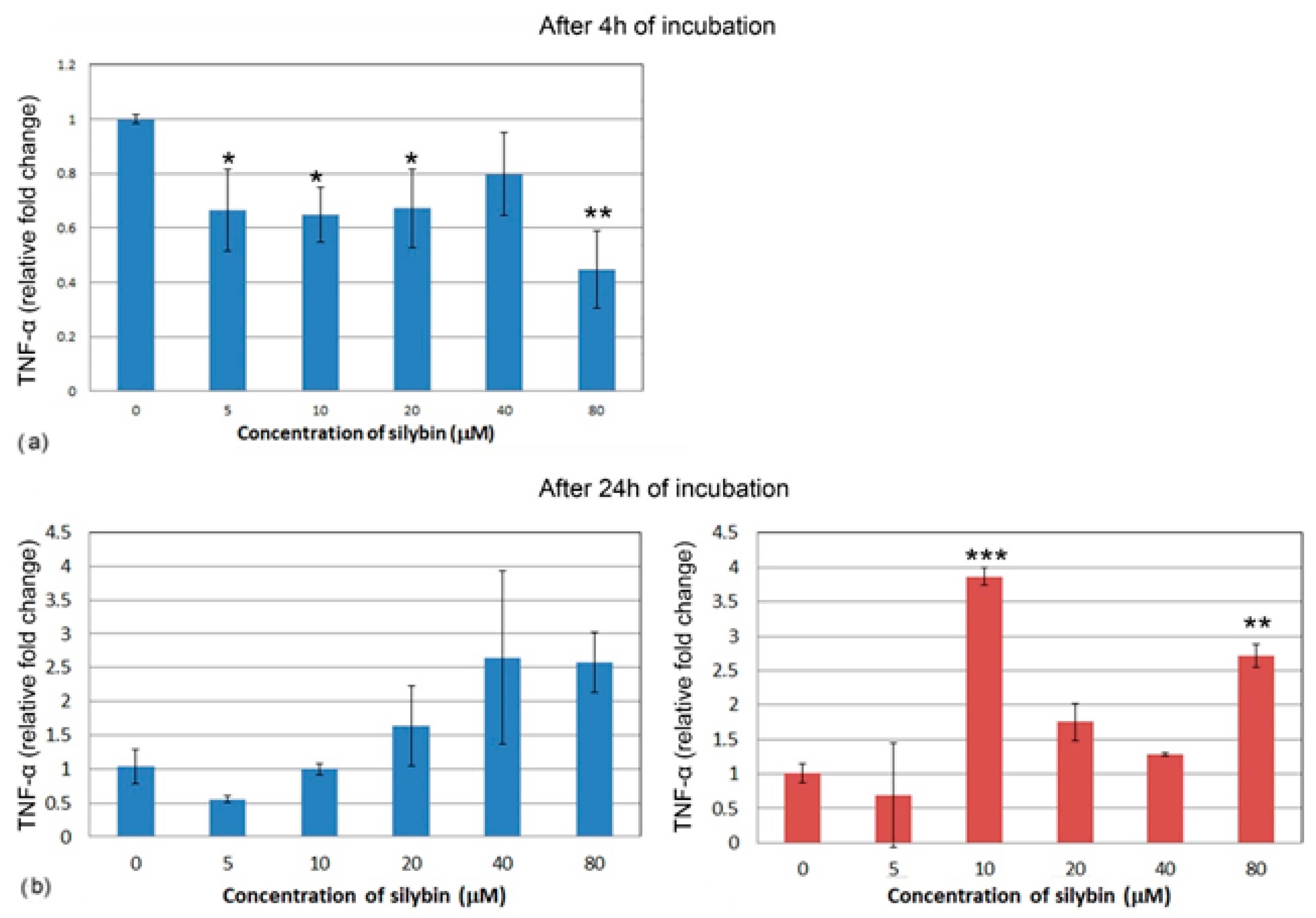
3.5. The Effect of Silybin on the Gene Expression of Selected Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abenavoli, L.; Caspasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef]
- Wesolowska, O.; Lania-Pietrzak, B.; Kuzdzal, K. Influence of silybin on biophysical properties of phospholipid bilayers. Acta Pharmacol. Sin. 2007, 28, 296–306. [Google Scholar] [CrossRef]
- Bijak, B. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef]
- Milić, N.; Milošević, N.; Suvajdžić, L.; Žarkov, M.; Abenavoli, L. New Therapeutic Potentials of Milk Thistle (Silybum marianum). Nat. Prod. Commun. 2013, 8, 1801–1810. [Google Scholar]
- Anwar, S.; Madkor, H.R.; Ahmed, N.; Wagih, M.E. In vivo anticlastogenic effect of silymarin from milk thistle Silybum marianum L. Indian J. Pharmacol. 2018, 50, 108–115. [Google Scholar] [CrossRef]
- Johnson, V.J.; Osuchowski, M.F.; He, Q.; Sharma, R.P. Physiological responses to a natural antioxidant flavonoid mixture, silymarin, in BALB/c mice: II. alterations in thymic differentiation correlate with changes in c-myc gene expression. Planta Med. 2002, 68, 961–965. [Google Scholar] [CrossRef]
- Johnson, V.J.; He, Q.; Osuchowski, M.F.; Sharma, R.P. Physiological responses of a natural antioxidant flavonoid mixture, silymarin, in BALB/c mice: III. Silymarin inhibits T-lymphocyte function at low doses but stimulates inflammatory processes at high doses. Planta Med. 2003, 69, 44–49. [Google Scholar] [CrossRef]
- Agarwal, R.; Agarwal, C.; Ichikawa, H.; Singh, R.P.; Agarwal, B.B. Anticancer Potential of Silymarin: From Bench to Bed Side. Anticancer Res. 2006, 26, 4457–4498. [Google Scholar]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gažák, R.; Křen, V.; Agarwal, R. Anti-cancer efficacy of silybin derivatives—A structure-activity relationship. PLoS ONE 2013, 8, e60074. [Google Scholar] [CrossRef]
- Maihesuti, L.; Gao, H.; Wang, Q.; Cao, J.; Aisa, H.A.; Huang, G. Structural Modification of Sylibin to Derivatives of Sylibin/Hydnocarpin D/Silandrin, and Evaluation of their Cytotoxicity against Cancer Cells. Curr. Top. Med. Chem. 2021, 21, 1398–1417. [Google Scholar] [CrossRef]
- Agarwal, C.; Singh, R.P.; Dhanalakshmi, S.; Tyagi, A.K.; Tecklenburg, M.; Sclafani, R.A.; Agarwal, R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene 2003, 22, 8271–8282. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Khoshdel, Z.; Amirghofran, Z. The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: A comparison with desferrioxamine. Eur. J. Pharmacol. 2008, 589, 1–7. [Google Scholar] [CrossRef]
- Hrčková, G.; Kubašková, T.M.; Benada, O.; Kofroňová, O.; Tumová, L.; Biedermann, D. Differential Effects of the Flavonolignans Silybin, Silychristin and 2,3-Dehydrosilybin on Mesocestoides vogae Larvae (Cestoda) under Hypoxic and Aerobic In Vitro Conditions. Molecules 2018, 23, 2999. [Google Scholar] [CrossRef]
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of mycoplasma incell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Traganos, F.; Staiano-Coico, L.; Kapuscinski, J.; Melamed, M.R. Interaction of rhodamine 123 with living cells studied by flow cytometry. Cancer Res. 1982, 42, 799–806. [Google Scholar]
- Rajalakshmi, S.; Vimalraj, S.; Saravanan, S.; Raj Preeth, D.; Shairam, M.; Anuradha, D. Synthesis and characterization of silibinin/phenanthroline/neocuproine copper(II) complexes for augmenting bone tissue regeneration: An in vitro analysis. J. Biol. Inorg. Chem. 2018, 23, 753–762. [Google Scholar] [CrossRef]
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal complexes of flavonoids: Their synthesis, characterization and enhanced antioxidant and anticancer activities. Future Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef]
- Deep, G.; Oberlies, N.H.; Kroll, D.J.; Agarwal, R. Identifying the differential effects of silymarin constituents on cell growth and cell cycle regulatory molecules in human prostate cancer cells. Int. J. Cancer 2008, 123, 41–50. [Google Scholar] [CrossRef]
- Salucci, M.; Stivala, L.A.; Maiani, G.; Bugianesi, R.; Vannini, V. Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2). Br. J. Cancer 2002, 86, 1645–1651. [Google Scholar] [CrossRef]
- Marchetti, P.; Castedo, M.; Susin, S.A.; Zamzami, N.; Hirsch, T.; Macho, A.; Haeffner, A.; Hirsch, F.; Geuskens, M.; Kroemer, G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J. Exp. Med. 1996, 184, 1155–1160. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. 2003: The mitochondrial membrane potential (∆ψm) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-Modified Cancer Cell Metabolism. Front. Cell Dev. Biol. 2019, 29, 4. [Google Scholar] [CrossRef]
- Esselun, C.; Bruns, B.; Hagl, S.; Grewal, R.; Eckert, G.P. Differential Effects of Silibinin A on Mitochondrial Function in Neuronal PC12 and HepG2 Liver Cells. Oxid. Med. Cell Longev. 2019, 2019, 1652609. [Google Scholar] [CrossRef]
- Kang, J.S.; Jeon, Y.J.; Park, S.K.; Yang, K.H.; Kim, H.M. Protection against lipopolysaccharide-induced sepsis and inhibition of interleukin-1beta and prostaglandin E2 synthesis by silymarin. Biochem. Pharmacol. 2004, 67, 175–181. [Google Scholar] [CrossRef]
- Katiyar, S.K. Silymarin and skin cancer prevention: Anti-inflammatory, antioxidant and immunomodulatory effects. Int. J. Oncol. 2005, 26, 169–176. [Google Scholar] [CrossRef]
- Zhao, J.; Sharma, Y.; Agarwal, R. Significant inhibition by the flavonoid antioxidant silymarin against 12-O-tetradecanoylphorbol 13-acetate-caused modulation of antioxidant and inflammatory enzymes, and cyclooxygenase 2 and interleukin-1alpha expression in SENCAR mouse epidermis: Implications in the prevention of stage I tumor promotion. Mol. Carcinog. 1999, 26, 321–333. [Google Scholar]
- Amin, M.M.; Arbid, M.S. Estimation of the novel antipyretic, anti-inflammatory, antinociceptive and antihyperlipidemic effects of silymarin in Albino rats and mice. Asian Pac. J. Trop. Biomed. 2015, 5, 619–623. [Google Scholar] [CrossRef]
- Thakare, V.N.; Aswar, M.K.; Kulkarni, Y.P.; Patil, R.R.; Patel, B.M. Silymarin ameliorates experimentally induced depressive like behavior in rats: Involvement of hippocampal BDNF signaling, inflammatory cytokines and oxidative stress response. Physiol. Behav. 2017, 179, 401–410. [Google Scholar] [CrossRef]
- Zheng, R.; Ma, J.; Wang, D.; Dong, W.; Wang, S.; Liu, T.; Xie, R.; Liu, L.; Wang, B.; Cao, H. Chemopreventive Effects of Silibinin on Colitis-Associated Tumorigenesis by Inhibiting IL-6/STAT3 Signaling Pathway. Mediat. Inflamm. 2018, 25, 1562010. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P.; Sharma, J.; Dixit, A. Flavonoids modulate tight junction barrier functions in hyperglycemic human intestinal Caco-2 cells. Nutrition 2020, 78, 110792. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.; Sun, K.; Su, S.; Geng, T.; Sun, H. Hippophae rhamnoides polysaccharides protect IPEC-J2 cells from LPS-induced inflammation, apoptosis, and barrier dysfunction in vitro via inhibiting TLR4/NF-κB signaling pathway. Int. J. Biol. Macromol. 2020, 155, 1202–1215. [Google Scholar] [CrossRef]
- Pomothy, J.M.; Barna, R.F.; Pászti, E.A.; Babiczky, Á.; Szóládi, Á.; Jerzsele, Á.; Pásztiné, G.E. Beneficial Effects of Rosmarinic Acid on IPEC-J2 Cells Exposed to the Combination of Deoxynivalenol and T-2 Toxin. Mediators Inflamm. 2020, 2020, 8880651. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, L.; Zhou, H.; Hou, G. Chemical composition and anti-inflammatory activity of n-butanol extract of Piper sarmentosum Roxb. In the intestinal porcine epithelial cells (IPEC-J2). J. Ethnopharmacol. 2021, 269, 113723. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Wang, X.; Mai, K.; He, G. Effects of silymarin on growth performance, antioxidant capacity and immune response in turbot (Scophthalmus maximus L.). J. World Aquacult. Soc. 2019, 50, 1168–1181. [Google Scholar] [CrossRef]
- Eraky, S.M.; El-Mesery, M.; El-Karef, A.; Eissa, L.A.; El-Gayar, A.M.; Salma, M. Silymarin, and caffeine combination ameliorates experimentally-induced hepatic fibrosis through down-regulation of LPAR1 expression. Biomed. Pharmacother. 2018, 101, 49–57. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Yang, T. Silymarin nanoliposomes attenuate renal injury on diabetic nephropathy rats via co-suppressing TGF-β/Smad and JAK2/STAT3/SOCS1 pathway. Life Sci. 2021, 271, 119197. [Google Scholar] [CrossRef]
- Meng, S.; Yang, F.; Wang, Y.; Qin, Y.; Xian, H.; Che, H.; Wang, L. Silymarin ameliorates diabetic cardiomyopathy via inhibiting TGF-β1/Smad signaling. Cell Biol. Int. 2019, 43, 65–72. [Google Scholar] [CrossRef]
- Hosseini, S.Y.; Kalantar, K.; Shahin, K.; Ghayour, M.; Rajabi Bazl, M.; Fattahi, M.; Moini, M.; Amirghofran, Z. Comparison of the In Vitro Antifibrogenic Effects of Silymarin, Silybin A and 18α-Glycyrrhizin on Activated Hepatic Stellate Cells. Jundishapur J. Nat. Pharm. Prod. 2017, 12, e40285. [Google Scholar] [CrossRef]
- Wu, J.; Huang, C.; Kan, G.; Xiao, H.; Zhang, X.; Yang, J. Silymarin Regulates Tgf-[beta]1/Smad3 Signaling Pathway and Improves Renal Tubular Interstitial Fibrosis Caused by Obstructive Nephropathy. Curr. Top. Nutraceutical Res. 2021, 19, 508–513. [Google Scholar]
- Wang, M.J.; Lin, W.W.; Chen, H.L.; Chang, Y.H.; Ou, H.C.; Kuo, J.S.; Hong, J.S.; Jeng, K.C.G. Silymarin protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity by inhibiting microglia activation. Eur. J. Neurosci. 2002, 16, 2103–2112. [Google Scholar] [CrossRef]
| IPEC-1 | Forward | Reverse |
| GAPDH | CCTGCTTCACCACCTTCTTGA | CCCCAACGTGTCGGTTGT |
| IL1 β | TGGATGGGCGGCTGATTTGAAG | CGAACCCGTGTTGCTGAAGGAG |
| IL6 | CAGGGTCTGGATCAGTGCTT | AGCAAGGAGGTACTGGCAGA |
| TNF-α | TAGACCTGCCCAGATTCAGC | AACCTCCTCTCTGCCATCAA |
| TGF-β | ACAACTCCGGTGACATCAAAGG | ACGTGGAGCTATACCAGAAATACAG |
| CaCo-2 | Forward | Reverse |
| GAPDH | GACAAGCTTCCCGTTCTCAG | TCACCAGGGCTGCTTTTAAC |
| IL1 β | TCTTTCAACACGCAGGACAG | TCCAGGGACAGGATATGGAG |
| IL6 | AGTGCCTCTTTGCTGCTTTC | TACCCCCAGGAGAAGATTCC |
| TNF-α | GCCAGAGGGCTGATTAGAGA | TCAGCCTCTTCTCCTTCCTG |
| TGF-β | GCGGAAGTCAATGTACAGCTGCCGC | TGAACCGGCCTTTCCTGCTTCTCATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faixová, D.; Ratvaj, M.; Maruščáková, I.C.; Hrčková, G.; Karaffová, V.; Faixová, Z.; Mudroňová, D. Silybin Showed Higher Cytotoxic, Antiproliferative, and Anti-Inflammatory Activities in the CaCo Cancer Cell Line while Retaining Viability and Proliferation in Normal Intestinal IPEC-1 Cells. Life 2023, 13, 492. https://doi.org/10.3390/life13020492
Faixová D, Ratvaj M, Maruščáková IC, Hrčková G, Karaffová V, Faixová Z, Mudroňová D. Silybin Showed Higher Cytotoxic, Antiproliferative, and Anti-Inflammatory Activities in the CaCo Cancer Cell Line while Retaining Viability and Proliferation in Normal Intestinal IPEC-1 Cells. Life. 2023; 13(2):492. https://doi.org/10.3390/life13020492
Chicago/Turabian StyleFaixová, Dominika, Marek Ratvaj, Ivana Cingeľová Maruščáková, Gabriela Hrčková, Viera Karaffová, Zita Faixová, and Dagmar Mudroňová. 2023. "Silybin Showed Higher Cytotoxic, Antiproliferative, and Anti-Inflammatory Activities in the CaCo Cancer Cell Line while Retaining Viability and Proliferation in Normal Intestinal IPEC-1 Cells" Life 13, no. 2: 492. https://doi.org/10.3390/life13020492
APA StyleFaixová, D., Ratvaj, M., Maruščáková, I. C., Hrčková, G., Karaffová, V., Faixová, Z., & Mudroňová, D. (2023). Silybin Showed Higher Cytotoxic, Antiproliferative, and Anti-Inflammatory Activities in the CaCo Cancer Cell Line while Retaining Viability and Proliferation in Normal Intestinal IPEC-1 Cells. Life, 13(2), 492. https://doi.org/10.3390/life13020492







