Novel ELAC2 Mutations in Individuals Presenting with Variably Severe Neurological Disease in the Presence or Absence of Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Exome Sequencing and Genetic Analyses
2.2. Cell Culture
2.3. Whole-Cell Protein Extracts
2.4. SDS-PAGE and Immunoblotting
2.5. RNA Isolation and Northern Blot Analysis
2.6. Measurement of OXPHOS Activities
2.7. Statistical Analysis
3. Results
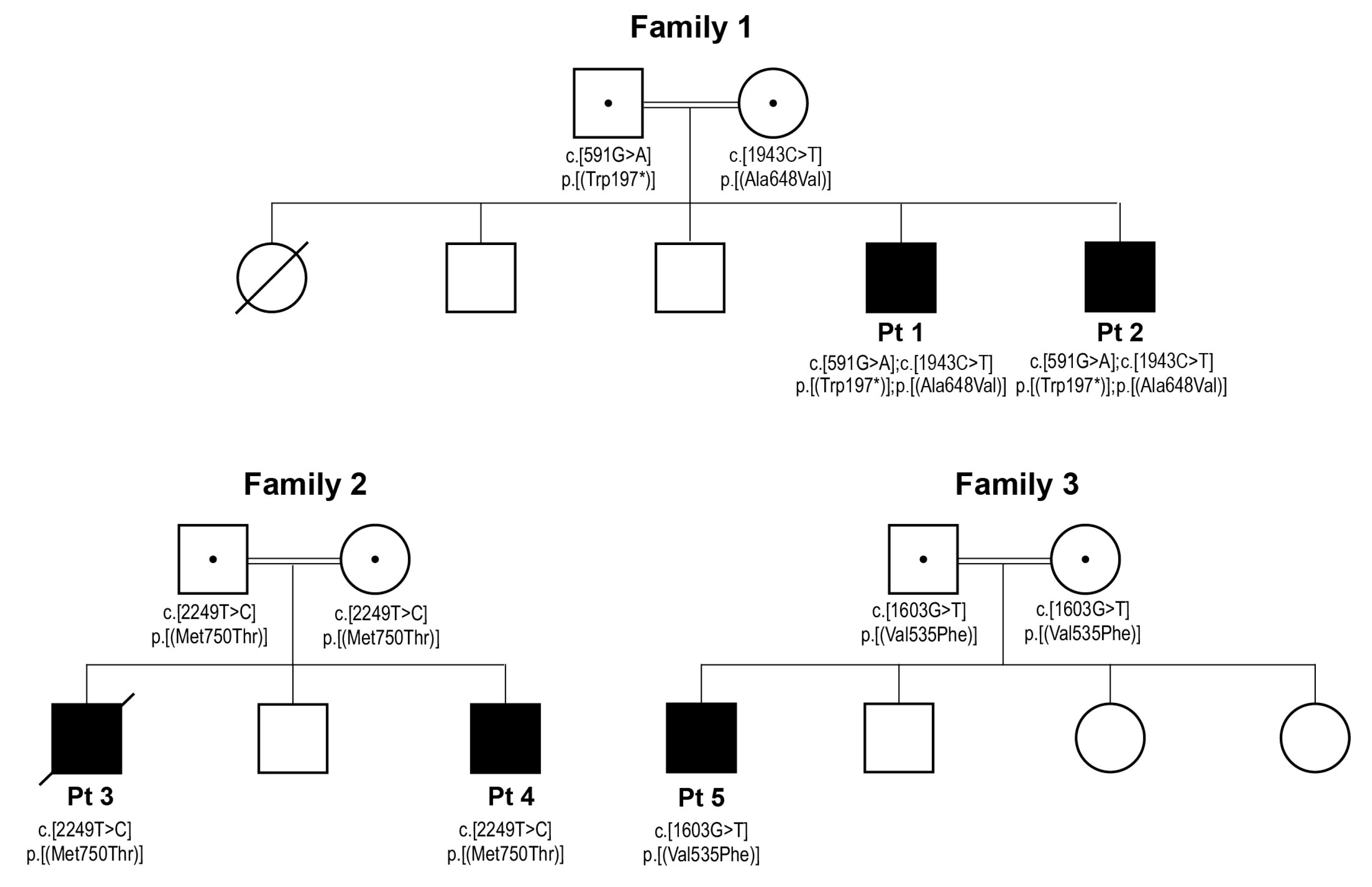
3.1. Case Reports
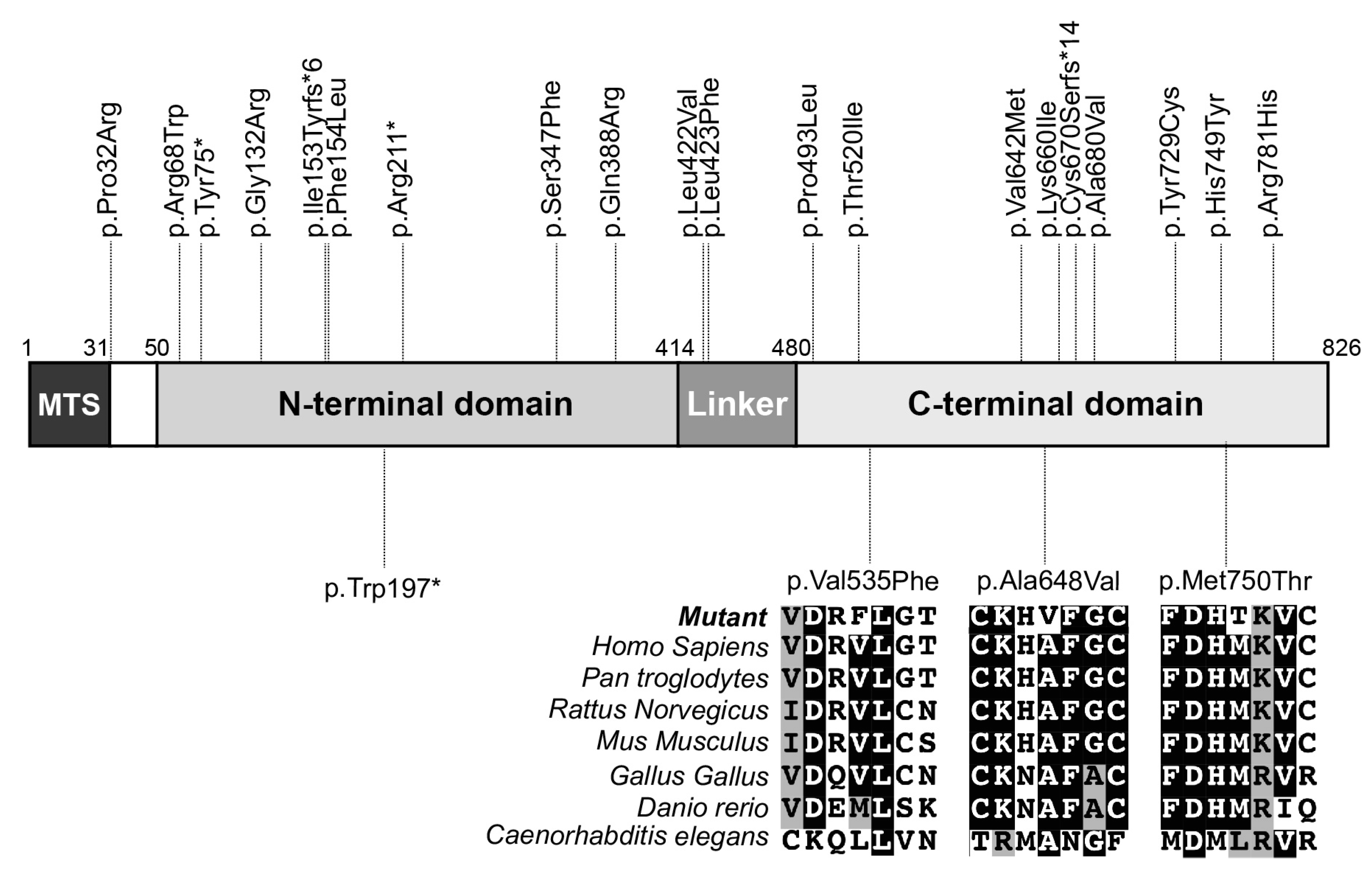
3.2. Genetic Studies
3.3. Decreased ELAC2 Abundance and an Accumulation of mt-tRNAVal-16S rRNA Precursor in Patient Fibroblasts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.A. Replication and transcription of vertebrate mitochondrial DNA. Annu. Rev. Cell Biol. 1991, 7, 453–478. [Google Scholar] [CrossRef]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, J.; Frank, P.; Loffler, E.; Bennett, K.L.; Gerner, C.; Rossmanith, W. RNase P without RNA: Identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell 2008, 135, 462–474. [Google Scholar] [CrossRef]
- Sanchez, M.I.; Mercer, T.R.; Davies, S.M.; Shearwood, A.M.; Nygard, K.K.; Richman, T.R.; Mattick, J.S.; Rackham, O.; Filipovska, A. RNA processing in human mitochondria. Cell Cycle 2011, 10, 2904–2916. [Google Scholar] [CrossRef]
- Reinhard, L.; Sridhara, S.; Hallberg, B.M. The MRPP1/MRPP2 complex is a tRNA-maturation platform in human mitochondria. Nucleic Acids Res. 2017, 45, 12469–12480. [Google Scholar] [CrossRef]
- Yang, S.Y.; He, X.Y.; Schulz, H. Multiple functions of type 10 17beta-hydroxysteroid dehydrogenase. Trends Endocrinol. Metab. TEM 2005, 16, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Takaku, H.; Minagawa, A.; Takagi, M.; Nashimoto, M. A candidate prostate cancer susceptibility gene encodes tRNA 3’ processing endoribonuclease. Nucleic Acids Res. 2003, 31, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- Rossmanith, W. Localization of human RNase Z isoforms: Dual nuclear/mitochondrial targeting of the ELAC2 gene product by alternative translation initiation. PLoS ONE 2011, 6, e19152. [Google Scholar] [CrossRef]
- Brzezniak, L.K.; Bijata, M.; Szczesny, R.J.; Stepien, P.P. Involvement of human ELAC2 gene product in 3’ end processing of mitochondrial tRNAs. RNA Biol. 2011, 8, 616–626. [Google Scholar] [CrossRef]
- Siira, S.J.; Rossetti, G.; Richman, T.R.; Perks, K.; Ermer, J.A.; Kuznetsova, I.; Hughes, L.; Shearwood, A.J.; Viola, H.M.; Hool, L.C.; et al. Concerted regulation of mitochondrial and nuclear non-coding RNAs by a dual-targeted RNase Z. EMBO Rep. 2018, 19, e46198. [Google Scholar] [CrossRef]
- Xavier, V.J.; Martinou, J.C. RNA Granules in the Mitochondria and Their Organization under Mitochondrial Stresses. Int. J. Mol. Sci. 2021, 22, 9502. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, A.J.; Amberger, A.; Zavadil, C.; Steinbeisser, H.; Mayr, J.A.; Feichtinger, R.G.; Oerum, S.; Yue, W.W.; Zschocke, J. Mutation or knock-down of 17beta-hydroxysteroid dehydrogenase type 10 cause loss of MRPP1 and impaired processing of mitochondrial heavy strand transcripts. Hum. Mol. Genet. 2014, 23, 3618–3628. [Google Scholar] [CrossRef]
- Metodiev, M.D.; Thompson, K.; Alston, C.L.; Morris, A.A.M.; He, L.; Assouline, Z.; Rio, M.; Bahi-Buisson, N.; Pyle, A.; Griffin, H.; et al. Recessive Mutations in TRMT10C Cause Defects in Mitochondrial RNA Processing and Multiple Respiratory Chain Deficiencies. Am. J. Hum. Genet. 2016, 98, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Oerum, S.; Roovers, M.; Leichsenring, M.; Acquaviva-Bourdain, C.; Beermann, F.; Gemperle-Britschgi, C.; Fouilhoux, A.; Korwitz-Reichelt, A.; Bailey, H.J.; Droogmans, L.; et al. Novel patient missense mutations in the HSD17B10 gene affect dehydrogenase and mitochondrial tRNA modification functions of the encoded protein. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.J.; Lace, B.; Buhas, D.; Gravel, S.; Cyr, D.; Boucher, R.M.; Bernard, G.; Levesque, S.; Maranda, B. HSD10 mitochondrial disease: P.Leu122Val variant, mild clinical phenotype, and founder effect in French-Canadian patients from Quebec. Mol. Genet. Genom. Med. 2019, 7, e1000. [Google Scholar] [CrossRef]
- Haack, T.B.; Kopajtich, R.; Freisinger, P.; Wieland, T.; Rorbach, J.; Nicholls, T.J.; Baruffini, E.; Walther, A.; Danhauser, K.; Zimmermann, F.A.; et al. ELAC2 mutations cause a mitochondrial RNA processing defect associated with hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2013, 93, 211–223. [Google Scholar] [CrossRef]
- Akawi, N.A.; Ben-Salem, S.; Hertecant, J.; John, A.; Pramathan, T.; Kizhakkedath, P.; Ali, B.R.; Al-Gazali, L. A homozygous splicing mutation in ELAC2 suggests phenotypic variability including intellectual disability with minimal cardiac involvement. Orphanet J. Rare Dis. 2016, 11, 139. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kim, Y.M.; Lee, Y.J.; Cheon, C.K. The First Korean case of combined oxidative phosphorylation deficiency-17 diagnosed by clinical and molecular investigation. Korean J. Pediatr. 2017, 60, 408–412. [Google Scholar] [CrossRef]
- Shinwari, Z.M.A.; Almesned, A.; Alakhfash, A.; Al-Rashdan, A.M.; Faqeih, E.; Al-Humaidi, Z.; Alomrani, A.; Alghamdi, M.; Colak, D.; Alwadai, A.; et al. The Phenotype and Outcome of Infantile Cardiomyopathy Caused by a Homozygous ELAC2 Mutation. Cardiology 2017, 137, 188–192. [Google Scholar] [CrossRef]
- Paucar, M.; Pajak, A.; Freyer, C.; Bergendal, A.; Dory, M.; Laffita-Mesa, J.M.; Stranneheim, H.; Lagerstedt-Robinson, K.; Savitcheva, I.; Walker, R.H.; et al. Chorea, psychosis, acanthocytosis, and prolonged survival associated with ELAC2 mutations. Neurology 2018, 91, 710–712. [Google Scholar] [CrossRef]
- Saoura, M.; Powell, C.A.; Kopajtich, R.; Alahmad, A.; Al-Balool, H.H.; Albash, B.; Alfadhel, M.; Alston, C.L.; Bertini, E.; Bonnen, P.E.; et al. Mutations in ELAC2 associated with hypertrophic cardiomyopathy impair mitochondrial tRNA 3’-end processing. Hum. Mutat. 2019, 40, 1731–1748. [Google Scholar] [CrossRef]
- Mendes, L.C.; de Oliveira Magalhaes, R.; Pereira Dos Santos, R.K.; Araujo, R.S. Pseudohypoaldosteronism associated with hypertrophic cardiomyopathy, hypertension and thrombocytosis due to mutation in the ELAC2 gene: A case report. J. Pediatr. Endocrinol. Metab. 2022, 35, 1437–1442. [Google Scholar] [CrossRef]
- Barcia, G.; Rio, M.; Assouline, Z.; Zangarelli, C.; Gueguen, N.; Dumas, V.D.; Marcorelles, P.; Schiff, M.; Slama, A.; Barth, M.; et al. Clinical, neuroimaging and biochemical findings in patients and patient fibroblasts expressing ten novel GFM1 mutations. Hum. Mutat. 2020, 41, 397–402. [Google Scholar] [CrossRef]
- Gardeitchik, T.; Mohamed, M.; Ruzzenente, B.; Karall, D.; Guerrero-Castillo, S.; Dalloyaux, D.; van den Brand, M.; van Kraaij, S.; van Asbeck, E.; Assouline, Z.; et al. Bi-allelic Mutations in the Mitochondrial Ribosomal Protein MRPS2 Cause Sensorineural Hearing Loss, Hypoglycemia, and Multiple OXPHOS Complex Deficiencies. Am. J. Hum. Genet. 2018, 102, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Ruzzenente, B.; Assouline, Z.; Barcia, G.; Rio, M.; Boddaert, N.; Munnich, A.; Rotig, A.; Metodiev, M.D. Inhibition of mitochondrial translation in fibroblasts from a patient expressing the KARS p.(Pro228Leu) variant and presenting with sensorineural deafness, developmental delay, and lactic acidosis. Hum. Mutat. 2018, 39, 2047–2059. [Google Scholar] [CrossRef] [PubMed]
- Rustin, P.; Chretien, D.; Bourgeron, T.; Gerard, B.; Rotig, A.; Saudubray, J.M.; Munnich, A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta 1994, 228, 35–51. [Google Scholar] [CrossRef]
- Chretien, D.; Benit, P.; Chol, M.; Lebon, S.; Rotig, A.; Munnich, A.; Rustin, P. Assay of mitochondrial respiratory chain complex I in human lymphocytes and cultured skin fibroblasts. Biochem. Biophys. Res. Commun. 2003, 301, 222–224. [Google Scholar] [CrossRef]
- Migunova, E.; Theophilopoulos, J.; Mercadante, M.; Men, J.; Zhou, C.; Dubrovsky, E.B. ELAC2/RNaseZ-linked cardiac hypertrophy in Drosophila melanogaster. Dis. Model. Mech. 2021, 14, dmm048931. [Google Scholar] [CrossRef] [PubMed]

| Patient 1 | Patient 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle | Liver | Muscle | Heart | ||||||
| Pt | Control Range | Pt | Control Range | Pt | Control Range | Pt | Control Range | ||
| Absolute enzyme activity | CI | 32↓ | 39–100 | 83.3 | 15–31.7 | 4↓ | 13–29 | 21↓ | 64–134 |
| CII | 116 | 61–153 | 212.9 | 107–75 | 27 | 24–49 | 406 | 97–192 | |
| CIII | 557↓ | 714–1821 | 204.3 | 275–488 | 204↓ | 231–445 | 1575 | 1084–1872 | |
| CIV | 404 | 388–1237 | 260.6 | 130–237.4 | 70↓ | 111–252 | 186↓ | 459–863 | |
| CV | 372 | 202–506 | 347.8 | 59.7–128.8 | 42↓ | 55–128 | 82↓ | 139–327 | |
| Relative enzyme activity | CI/CS | n.p. | n.p. | 1.01 | 0.26–0.51 | 0.04↓ | 0.14–0.22 | 0.04↓ | 0.22–0.32 |
| CII/CS | n.p. | n.p. | 2.58 | 1.69–2.70 | 0.30 | 0.24–0.36 | 0.69 | 0.35–0.45 | |
| CIII/CS | n.p. | n.p. | 2.48↓ | 4.89–7.74 | 2.29~ | 2.30–3.30 | 2.69↓ | 3.20–4.27 | |
| CIV/CS | n.p. | n.p. | 3.16 | 2.27–3.81 | 0.79↓ | 1.26–1.98 | 0.32↓ | 1.51–2.10 | |
| CV/CS | n.p. | n.p. | 4.22 | 1.19–2.14 | 0.47↓ | 0.50–0.90 | 0.14↓ | 0.46–0.68 | |
| Family | Patient | Variant 1 | Amino Acid 2 | Exon | SIFT (Score) | Polyphen (Score) |
|---|---|---|---|---|---|---|
| 1 | Pt 1, Pt 2 | c.1943C > T | p.(Ala648Val) | 21 | deleterious (0.03) | probably damaging (0.998) |
| 2 | Pt 3, Pt 4 | c.2249T > C | p.(Met750Thr) | 23 | deleterious (0.00) | probably damaging (0.996) |
| 3 | Pt 5 | c.1603G > T | p.(Val535Phe) | 17 | deleterious (0.01) | probably damaging (0.979) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cafournet, C.; Zanin, S.; Guimier, A.; Hully, M.; Assouline, Z.; Barcia, G.; de Lonlay, P.; Steffann, J.; Munnich, A.; Bonnefont, J.-P.; et al. Novel ELAC2 Mutations in Individuals Presenting with Variably Severe Neurological Disease in the Presence or Absence of Cardiomyopathy. Life 2023, 13, 445. https://doi.org/10.3390/life13020445
Cafournet C, Zanin S, Guimier A, Hully M, Assouline Z, Barcia G, de Lonlay P, Steffann J, Munnich A, Bonnefont J-P, et al. Novel ELAC2 Mutations in Individuals Presenting with Variably Severe Neurological Disease in the Presence or Absence of Cardiomyopathy. Life. 2023; 13(2):445. https://doi.org/10.3390/life13020445
Chicago/Turabian StyleCafournet, Cérane, Sofia Zanin, Anne Guimier, Marie Hully, Zahra Assouline, Giulia Barcia, Pascale de Lonlay, Julie Steffann, Arnold Munnich, Jean-Paul Bonnefont, and et al. 2023. "Novel ELAC2 Mutations in Individuals Presenting with Variably Severe Neurological Disease in the Presence or Absence of Cardiomyopathy" Life 13, no. 2: 445. https://doi.org/10.3390/life13020445
APA StyleCafournet, C., Zanin, S., Guimier, A., Hully, M., Assouline, Z., Barcia, G., de Lonlay, P., Steffann, J., Munnich, A., Bonnefont, J.-P., Rötig, A., Ruzzenente, B., & Metodiev, M. D. (2023). Novel ELAC2 Mutations in Individuals Presenting with Variably Severe Neurological Disease in the Presence or Absence of Cardiomyopathy. Life, 13(2), 445. https://doi.org/10.3390/life13020445







