The Efficacy of Pulmonary Rehabilitation in Patients with Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
2. Materials and Methods
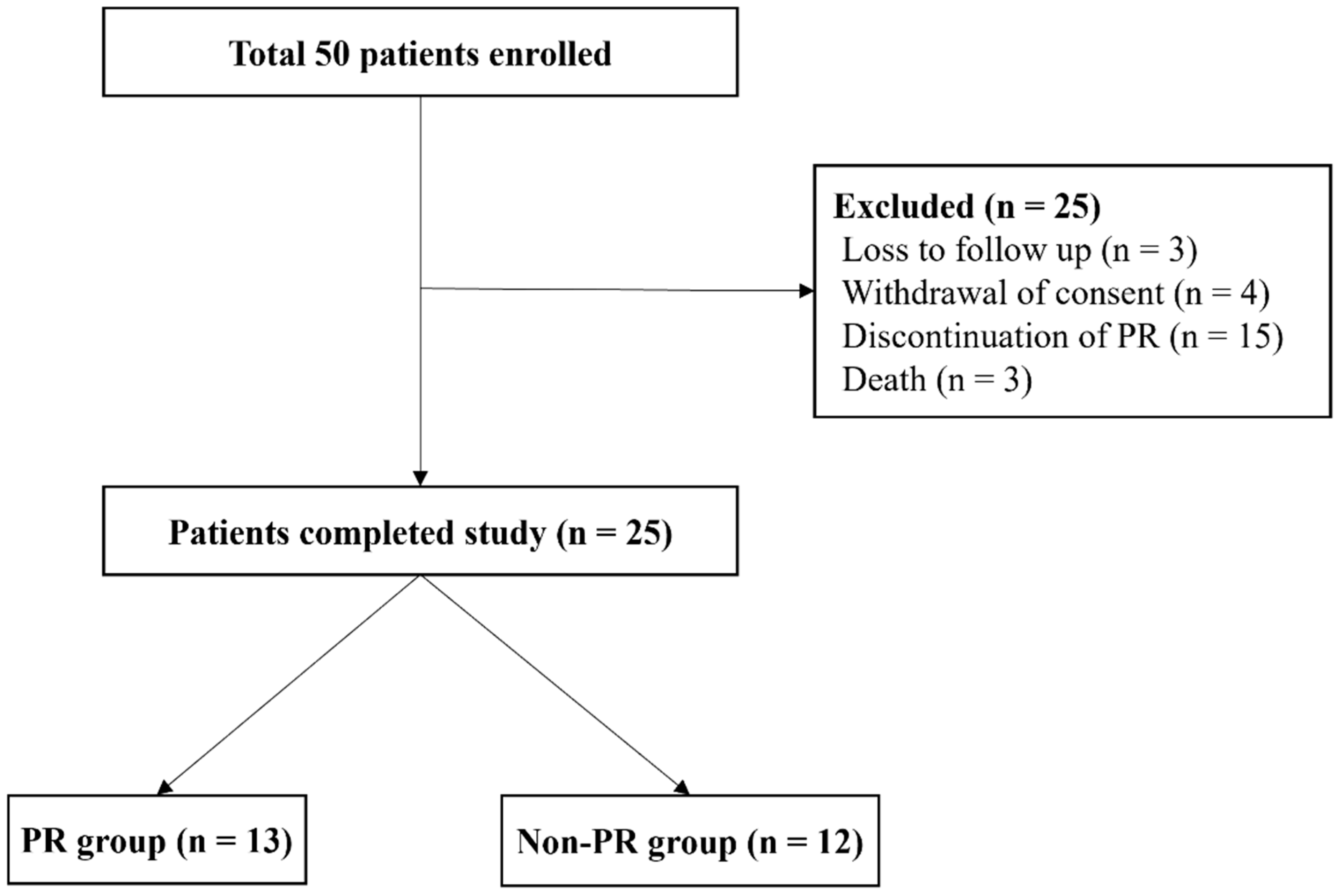
2.1. Subjects
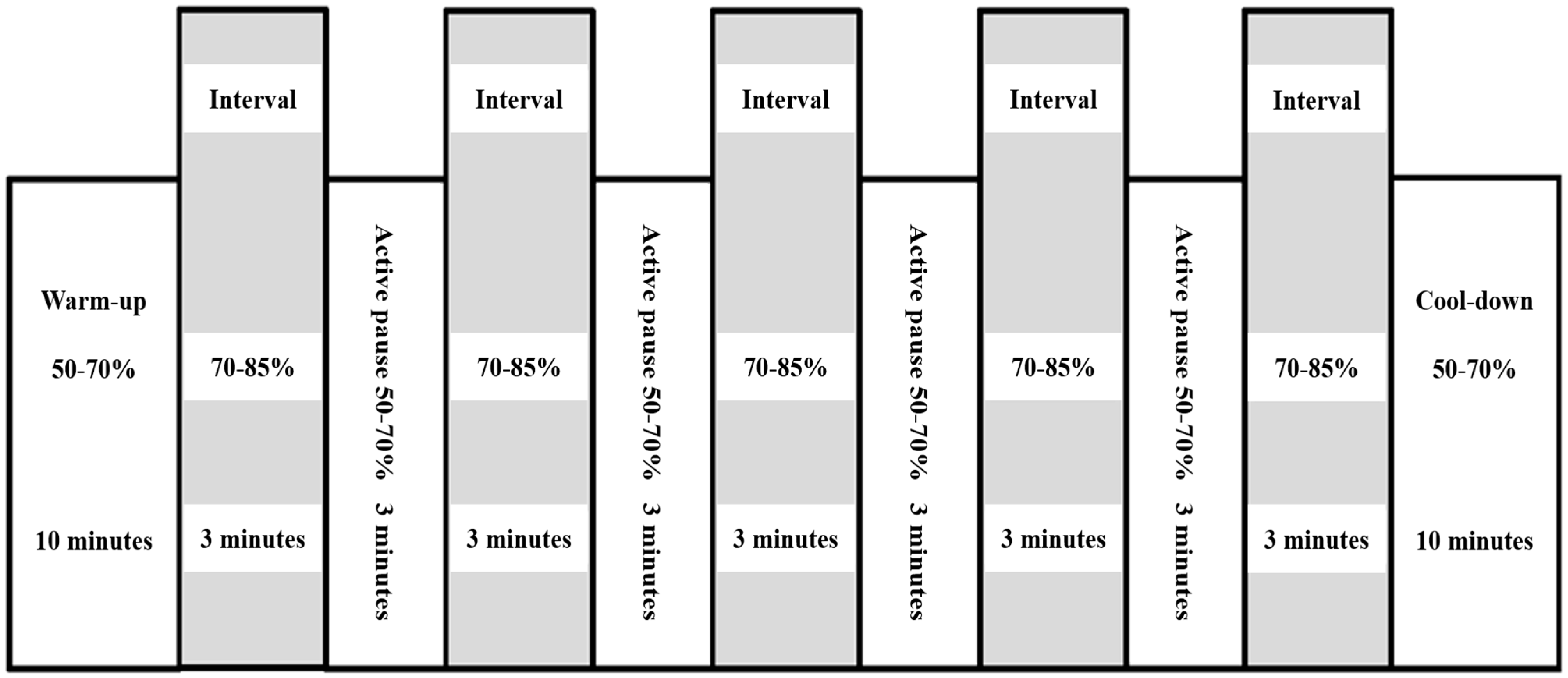
2.2. Exercise Training
2.3. Primary and Secondary Outcomes
2.4. Exercise Capacity
2.4.1. Cardiopulmonary Exercise Test
2.4.2. Six-Minute Walk Test
2.5. Pulmonary Function Test
2.6. Health-Related Quality of Life Assessment
2.7. Muscle Strength Test and Bio-Electrical Impedance Analysis
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
PR, Pulmonary Rehabilitation
3.2. Cardiopulmonary Exercise Test
3.3. Six-Minute Walk Test
3.4. Pulmonary Function Test
3.5. Saint George’s Respiratory Questionnaire Scores
3.6. Muscle Strength Test and Bio-Electrical Impedance Analysis
3.7. Safety Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Shea, B.S.; Tager, A.M. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am. J. Respir. Crit. Care Med. 2014, 190, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.M.; Gómez-Marín, O.W.; Ramos, C.F.; Sol, C.M.; Cohen, M.I.; Gaunaurd, I.A.; Cahalin, L.P.; Cardenas, D.D. Exercise Limitation in IPF Patients: A Randomized Trial of Pulmonary Rehabilitation. Lung 2014, 192, 367–376. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Wang, L.; Liu, R.; Xie, Y.; Li, S.; Li, J. Pulmonary Rehabilitation for Exercise Tolerance and Quality of Life in IPF Patients: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 2019, 8498603. [Google Scholar] [CrossRef]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Garcia, C.A.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Nici, L.; Donner, C.; Wouters, E.; Zuwallack, R.; Ambrosino, N.; Bourbeau, J.; Carone, M.; Celli, B.; Engelen, M.; Fahy, B.; et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2006, 173, 1390–1413. [Google Scholar] [CrossRef]
- Perrotta, F.; Bianco, A.; Cioffi, G.; Cennamo, A.; Mazzarella, G. Benefits of Pulmonary Rehabilitation in Idiopathic Pulmonary Fibrosis: A CASE REPORT. J. Cardiopulm. Rehabil. Prev. 2018, 38, E16–E18. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2018, 39, 1144–1161. [Google Scholar] [CrossRef] [PubMed]
- Herdy, A.H.; Ritt, L.E.; Stein, R.; Araujo, C.G.; Milani, M.; Meneghelo, R.S.; Ferraz, A.S.; Hossri, C.; Almeida, A.E.; Fernandes-Silva, M.M.; et al. Cardiopulmonary Exercise Test: Background, Applicability and Interpretation. Arq. Bras. Cardiol. 2016, 107, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- El Batrawy, S.; Elassal, G. Is there a role for cough peak flow in assessment of patients with severe COPD? Egypt. J. Chest Dis. Tuberc. 2014, 63, 837–841. [Google Scholar] [CrossRef]
- Gomes-Neto, M.; Silva, C.M.; Ezequiel, D.; Conceicao, C.S.; Saquetto, M.; Machado, A.S. Impact of pulmonary rehabilitation on exercise tolerance and quality of life in patients with idiopathic pulmonary fibrosis: A systematic review and meta-analysis. J. Cardiopulm. Rehabil. Prev. 2018, 38, 273–278. [Google Scholar] [CrossRef]
- Swigris, J.J.; Esser, D.; Conoscenti, C.S.; Brown, K.K. The psychometric properties of the St George's Respiratory Questionnaire (SGRQ) in patients with idiopathic pulmonary fibrosis: A literature review. Health Qual. Life Outcomes 2014, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, S.J.; Han, Y.; Ryu, Y.J.; Lee, J.H.; Chang, J.H. Hand grip strength and chronic obstructive pulmonary disease in Korea: An analysis in KNHANES VI. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2313–2321. [Google Scholar] [CrossRef]
- Chamorro, C.; Armijo-Olivo, S.; De la Fuente, C.; Fuentes, J.; Javier Chirosa, L. Absolute Reliability and Concurrent Validity of Hand Held Dynamometry and Isokinetic Dynamometry in the Hip, Knee and Ankle Joint: Systematic Review and Meta-analysis. Open. Med. 2017, 12, 359–375. [Google Scholar] [CrossRef]
- Ballarin, G.; Valerio, G.; Alicante, P.; Di Vincenzo, O.; Scalfi, L. Bioelectrical Impedance Analysis (BIA)- Derived Phase Angle in Children and Adolescents: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 120–130. [Google Scholar] [CrossRef]
- Jones, P.W. St. George’s Respiratory Questionnaire: MCID. COPD 2005, 2, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, Y.; Wong, E.; Guyatt, G.H.; King, D.; Cook, D.J.; Goldstein, R.S. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet 1996, 348, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Thomas, H.R. Pulmonary rehabilitation in lung disease other than chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1990, 141, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Erikssen, G.; Liestøl, K.; Bjørnholt, J.; Thaulow, E.; Sandvik, L.; Erikssen, J. Changes in physical fitness and changes in mortality. Lancet 1998, 352, 759–762. [Google Scholar] [CrossRef]
- Keteyian, S.J.; Brawner, C.A.; Savage, P.D.; Ehrman, J.K.; Schairer, J.; Divine, G.; Aldred, H.; Ophaug, K.; Ades, P.A. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am. Heart J. 2008, 156, 292–300. [Google Scholar] [CrossRef]
- Holland, A.E.; Hill, C.J.; Conron, M.; Munro, P.; McDonald, C.F. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax 2008, 63, 549–554. [Google Scholar] [CrossRef]
- Nishiyama, O.; Kondoh, Y.; Kimura, T.; Kato, K.; Kataoka, K.; Ogawa, T.; Watanabe, F.; Arizono, S.; Nishimura, K.; Taniguchi, H. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 2008, 13, 394–399. [Google Scholar] [CrossRef]
- Vainshelboim, B.; Oliveira, J.; Yehoshua, L.; Weiss, I.; Fox, B.D.; Fruchter, O.; Kramer, M.R. Exercise training-based pulmonary rehabilitation program is clinically beneficial for idiopathic pulmonary fibrosis. Respiration 2014, 88, 378–388. [Google Scholar] [CrossRef]
- Dowman, L.M.; McDonald, C.F.; Hill, C.J.; Lee, A.L.; Barker, K.; Boote, C.; Glaspole, I.; Goh, N.S.L.; Southcott, A.M.; Burge, A.T.; et al. The evidence of benefits of exercise training in interstitial lung disease: A randomised controlled trial. Thorax 2017, 72, 610–619. [Google Scholar] [CrossRef]
- Gupta, R.; Ruppel, G.L.; Espiritu, J.R.D. Exercise-Induced Oxygen Desaturation during the 6-Minute Walk Test. Med. Sci. 2020, 8, 8. [Google Scholar] [CrossRef]
- Dowman, L.; Hill, C.J.; May, A.; Holland, A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst. Rev. 2021, 2, CD006322. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.H. Utility of the six-minute walk test in patients with idiopathic pulmonary fibrosis. Multidiscip. Respir. Med. 2018, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Sciriha, A.; Lungaro-Mifsud, S.; Scerri, J.; Magro, R.; Camilleri, L.; Montefort, S. Health status of COPD patients undergoing pulmonary rehabilitation: A comparative responsiveness of the CAT and SGRQ. Chron. Respir. Dis. 2017, 14, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yi, X.; Tang, D. Aerobic Exercise Improves Pulmonary Fibrosis by Improving Insulin Resistance and Inflammation in Obese Mice. Front. Physiol. 2021, 12, 785117. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 25) | PR Group (n = 13) | Non-PR Group (n = 12) | p-Value | |
|---|---|---|---|---|
| Male | 23(92%) | 11(85%) | 12(100%) | 0.480 |
| Age (year) | 68 ± 5.5 | 68 ± 5.3 | 69 ± 5.9 | 0.430 |
| Ever smoker | 23(92%) | 11(85%) | 12(100%) | 0.480 |
| BMI (kg/m2) | 25 ± 3.4 | 25 ± 4.4 | 25 ± 2.2 | 0.915 |
| FVC, % predicted | 78.3 (69.5–88.5) | 73.7 (63.5–80.5) | 83.2 (76.2–91.5) | 0.062 |
| FEV1, % predicted | 85.1 (74.5–94.5) | 82.6 (72.0–89.5) | 87.8 (76.5–98.5) | 0.361 |
| FEV1/FVC, % | 77.1 (71.5–84.0) | 79.9 (77.0–85.0) | 74.0 (70.2–79.7) | 0.059 |
| DLco, % predicted | 63.2 (57.5–70.0) | 64.3 (56.5–71.5) | 62.0 (57.2–68.7) | 0.642 |
| VO2max, mL·kg−1·min−1 | 21.3 (18.9–24.5) | 22.6 (20.1–24.9) | 19.8 (15.4–23.3) | 0.071 |
| LVEF (%) | 64.0 (61.2–68.0) | 66.2 (63.0–69.0) | 62.8 (58.5–67.0) | 0.259 |
| mMRC | 1.0 (1.0–2.0) | 1.1 (1.0–2.0) | 1.0 (0.2–1.7) | 0.591 |
| SGRQ_Total | 27.0 (16.1–33.5) | 23.9 (16.4–30.7) | 30.3 (15.9–40.6) | 0.339 |
| Anti-fibrotic drug use | 25(100%) | 13(100%) | 12(100%) | - |
| Hazardous Substance Exposure | 3(12%) | 3(23%) | 0(%) | 0.220 |
| Family history | 0(0.0%) | 0(0.0%) | 0(0.0%) | - |
| Variables | PR Group (n = 13) | Non-PR Group (n = 12) | Inter-Group p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | |
| VO2max mL·kg−1·min−1 | 23 (20–25) | 25 (23–28) | 10 (2–19) | 0.006 | 20 (15–23) | 21 (16–25) | 7 (−3–8) | 0.508 | 0.071 | 0.041 | 0.115 |
| VE/VCO2 slope | 37 (32–39) | 32 (27–37) | −12 (−26–1) | 0.020 | 40 (32–49) | 39 (34–42) | −1 (−9–9) | 0.679 | 0.271 | 0.054 | 0.088 |
| RPP stage 3 | 21491 (18,868–23,998) | 17240 (14,457–20,039) | −20 (−26–−15) | 0.003 | 19,619 (17,718–20,925) | 17,448 (13,342–19,892) | −4 (−18–3) | 0.459 | 0.341 | 0.916 | 0.051 |
| HRR at 1 min | 8 (4–10) | 10 (5–14) | 60 (−11–133) | 0.460 | 13 (5–14) | 14 (5–16) | 51 (−52–49) | 0.720 | 0.340 | 0.827 | 0.149 |
| HRR at 3 min | 22 (11–31) | 34 (25–45) | 79 (−4–124) | 0.015 | 26 (4–39) | 29 (19–38) | 0 (−17–17) | 0.391 | 0.596 | 0.459 | 0.015 |
| Total exercise time | 557 (471–642) | 809 (740–844) | 76 (24–59) | 0.001 | 576 (316–754) | 637 (445–778) | 22 (−7–22) | 0.346 | 0.821 | 0.017 | 0.005 |
| HR rest | 81 (65–94) | 79 (66–87) | −1 (−5–9) | 0.454 | 78 (68–79) | 79 (67–84) | 2 (−8–12) | 0.642 | 0.430 | 0.890 | 0.509 |
| HR max | 139 (127–158) | 144 (132–160) | 4 (0–10) | 0.042 | 131 (106–150) | 135 (126–152) | 4 (−3–7) | 0.270 | 0.461 | 0.386 | 0.974 |
| RER at VO2max | 0.9 (0.9–1) | 1 (1–1.1) | 11 (3–15) | 0.003 | 0.9 (0.8–1) | 0.9 (0.8–1) | 2 (−10–21) | 0.640 | 0.230 | 0.056 | 0.193 |
| Variables | PR Group (n = 13) | Non-PR Group (n = 12) | Inter-Group p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | |
| 6MWD | 491 (441–541) | 537 (499–568) | 10 (−1–21) | 0.013 | 532 (505–553) | 492 (447–544) | −7 (−12–1) | 0.075 | 0.057 | 0.067 | 0.002 |
| Initial SpO2 | 96 (94–98) | 95 (94–96) | −1 (−2–1) | 0.086 | 97 (96–98) | 97 (96–99) | 0 (0–1) | 0.666 | 0.142 | 0.029 | 0.237 |
| Lowest SpO2 | 91 (88–95) | 89 (85–94) | −3 (−5–0) | 0.027 | 92 (92–96) | 93 (92–96) | 0 (−1–2) | 0.435 | 0.269 | 0.080 | 0.017 |
| SpO2 difference | 4 (3–6) | 6 (3–9) | 43 (−12–75) | 0.035 | 5 (1–5) | 4 (2–4) | −6 (−50–0) | 0.437 | 0.227 | 0.114 | 0.051 |
| Variables | PR Group (n = 13) | Non-PR Group (n = 12) | Inter-Group p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | |
| FVC, % predicted | 73.7 (63.5–80.5) | 72.8 (63.5–81.5) | −0.3 (−8.5–5.8) | 0.651 | 83.2 (76.2–91.5) | 83.9 (73.7–93.7) | 0.9 (−3.5–5.1) | 0.643 | 0.062 | 0.018 | 0.717 |
| FEV1, % predicted | 82.6 (72.0–89.5) | 80.9 (74.0–86.0) | −1.6 (−7.2–4.1) | 0.367 | 87.8 (76.5–98.5) | 88.6 (79.2–97.5) | 1.0 (−5.0–6.6) | 0.622 | 0.361 | 0.170 | 0.401 |
| FEV1/FVC | 79.9 (77.0–85.0) | 78.3 (76.0–83.0) | −1.8 (−4.1–0.6) | 0.062 | 74.0 (70.2–79.7) | 73.5 (68.5–78.7) | −0.6 (−3.5–2.2) | 0.430 | 0.059 | 0.085 | 0.358 |
| DLco, % predicted | 64.3 (56.5–71.5) | 61.8 (54.5–72.0) | −3.5 (−12.0–1.3) | 0.135 | 62.0 (57.2–68.7) | 61.9 (51.2–73.2) | 0.7 (−11.2–9.7) | 0.940 | 0.642 | 0.988 | 0.183 |
| PCF | 323.0 (250.0–395.0) | 426.1 (350.0–475.0) | 35.5 (16.6–57.1) | 0.001 | 414.1 (325.0–485.0) | 456.6 (367.5–510.0) | 18.7 (−4.2–16.1) | 0.208 | 0.039 | 0.427 | 0.006 |
| mMRC | 1.1 (1.0–2.0) | 0.9 (1.0–1.0) | −13.6 (−50.0–0.0) | 0.083 | 1.0 (0.2–1.7) | 1.0 (0.2–1.7) | 0.0 (0.0–0.0) | - | 0.591 | 0.774 | 0.098 |
| Variables | PR Group (n = 13) | Non-PR Group (n = 12) | Inter-Group p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | |
| SGRQ_Symptom | 32.7 (18.5–49.5) | 32.3 (11.9–51.3) | 10.0 (−23.5–33.7) | 0.861 | 34.5 (15.1–44.8) | 31.4 (21.5–41.3) | 3.4 (−46.5–55.7) | 0.466 | 1.000 | 0.910 | 0.786 |
| SGRQ_Activity | 39.6 (29.6–55.5) | 34.7 (17.8–50.6) | −21.5 (−37.6–0.0) | 0.250 | 46.0 (29.8–66.1) | 47.4 (31.2–65.4) | 3.0 (−16.6–20.7) | 0.660 | 0.500 | 0.167 | 0.083 |
| SGRQ_Impact | 12.2 (6.4–17.8) | 12.0 (2.9–19.7) | 5.7 (−67.4–63.5) | 0.924 | 20.0 (5.0–27.9) | 18.5 (4.9–26.6) | 37.1 (−43.5–64.9) | 0.790 | 0.683 | 0.237 | 0.862 |
| SGRQ_Total | 23.9 (16.4–30.7) | 22.3 (11.5–33.4) | −4.6 (−26.8–12.0) | 0.274 | 30.3 (15.9–40.6) | 29.5 (14.8–38.4) | 8.1 (−28.1–43.1) | 0.796 | 0.339 | 0.239 | 0.386 |
| Variables | PR Group (n = 13) | Non-PR Group (n = 12) | Inter-Group p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | p-Value | Baseline | 8 Weeks Later | Rate of Change (%) | |
| Grip power (Right) | 30.3 (26.5–35.9) | 33.1 (28.05–40.10) | 8.4 (0.72–20.45) | 0.144 | 35.1 (31.25–41.20) | 34.1 (31.0–41.6) | −2.9 (−13.1–5.1) | 0.406 | 0.112 | 0.739 | 0.065 |
| Grip power (Left) | 28.3 (23.8–33.8) | 32.0 (27.25–36.65) | 16.1 (4.92–32.10) | 0.005 | 33.6 (24.13–41.00) | 31.8 (24.6–38.5) | −2.8 (−12.0–8.7) | 0.335 | 0.096 | 0.928 | 0.007 |
| SMM_Total | 26.5 (23.1–30.1) | 26.6 (22.80–30.40) | 0.4 (−1.25–2.14) | 0.670 | 28.4 (24.93–31.98) | 28.7 (25.0–31.9) | 2.2 (−2.9–3.3) | 0.959 | 0.320 | 0.216 | 0.513 |
| SMM_Right UEx | 2.6 (2.2–3.1) | 2.6 (2.23–3.01) | −2.1 (−4.41–0.74) | 0.078 | 2.9 (2.62–3.46) | 3.0 (2.5–3.5) | 6.0 (−2.8–10.4) | 0.656 | 0.197 | 0.025 | 0.242 |
| SMM_Left UEx | 2.6 (2.2–3.0) | 2.5 (2.19–2.95) | −2.1 (−3.92–0.18) | 0.028 | 2.9 (2.56–3.25) | 3.0 (2.5–3.4) | 4.8 (−3.8–8.7) | 0.534 | 0.221 | 0.097 | 0.341 |
| SMM_Right LEx | 7.5 (6.4–8.4) | 7.6 (6.45–8.80) | 2.2 (−0.32–4.43) | 0.023 | 7.6 (6.8–8.6) | 8.1 (7.1–9.3) | 7.8 (−1.0–6.2) | 0.213 | 0.769 | 0.404 | 1.000 |
| SMM Left LEx | 7.4 (6.5–8.5) | 7.6 (6.49–8.94) | 2.5 (0.93–4.74) | 0.009 | 7.6 (6.7–8.5) | 8.1 (7.2–9.1) | 8.2 (−2.1–6.0) | 0.286 | 0.735 | 0.374 | 0.828 |
| PhA Right UEx | 5.6 (5.1–6.0) | 5.5 (5.10–6.00) | −0.3 (−3.44–2.05) | 0.660 | 5.8 (5.5–6.3) | 5.6 (5.2–6.0) | −3.3 (−6.5–1.3) | 0.073 | 0.319 | 0.796 | 0.216 |
| PhA Left UEx | 5.4 (5.1–5.7) | 5.3 (4.95–6.00) | −1.1 (−4.81–1.80) | 0.387 | 5.6 (5.2–6.2) | 5.4 (4.9–6.0) | −3.1 (−8.3–2.4) | 0.076 | 0.535 | 0.854 | 0.397 |
| PhA Right LEx | 5.9 (5.0–6.9) | 5.9 (5.00–6.80) | 0.0 (−6.95–5.49) | 0.787 | 5.8 (5.0–6.5) | 5.4 (4.7–6.1) | −6.3 (−9.4–0.0) | 0.024 | 0.751 | 0.187 | 0.056 |
| PhA Left LEx | 5.9 (5.1–6.9) | 5.8 (5.15–6.70) | 0.2 (−4.35–4.90) | 0.865 | 5.7 (5.0–6.4) | 5.2 (4.6–6.1) | −7.5 (−10.6–−1.7) | 0.006 | 0.672 | 0.076 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.E.; Kim, T.H.; Jang, J.H.; Jang, H.-J.; Yi, J.; Jung, S.Y.; Kim, D.-W.; Lee, J.H. The Efficacy of Pulmonary Rehabilitation in Patients with Idiopathic Pulmonary Fibrosis. Life 2023, 13, 403. https://doi.org/10.3390/life13020403
Choi HE, Kim TH, Jang JH, Jang H-J, Yi J, Jung SY, Kim D-W, Lee JH. The Efficacy of Pulmonary Rehabilitation in Patients with Idiopathic Pulmonary Fibrosis. Life. 2023; 13(2):403. https://doi.org/10.3390/life13020403
Chicago/Turabian StyleChoi, Hee Eun, Tae Hoon Kim, Ji Hoon Jang, Hang-Jea Jang, Jisook Yi, So Young Jung, Dae-Wook Kim, and Jae Ha Lee. 2023. "The Efficacy of Pulmonary Rehabilitation in Patients with Idiopathic Pulmonary Fibrosis" Life 13, no. 2: 403. https://doi.org/10.3390/life13020403
APA StyleChoi, H. E., Kim, T. H., Jang, J. H., Jang, H.-J., Yi, J., Jung, S. Y., Kim, D.-W., & Lee, J. H. (2023). The Efficacy of Pulmonary Rehabilitation in Patients with Idiopathic Pulmonary Fibrosis. Life, 13(2), 403. https://doi.org/10.3390/life13020403





