Clinical Application of a Customized 3D-Printed Bolus in Radiation Therapy for Distal Extremities
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatments
2.2. Manufacturing Workflow for the Customized 3D-Printed Boluses
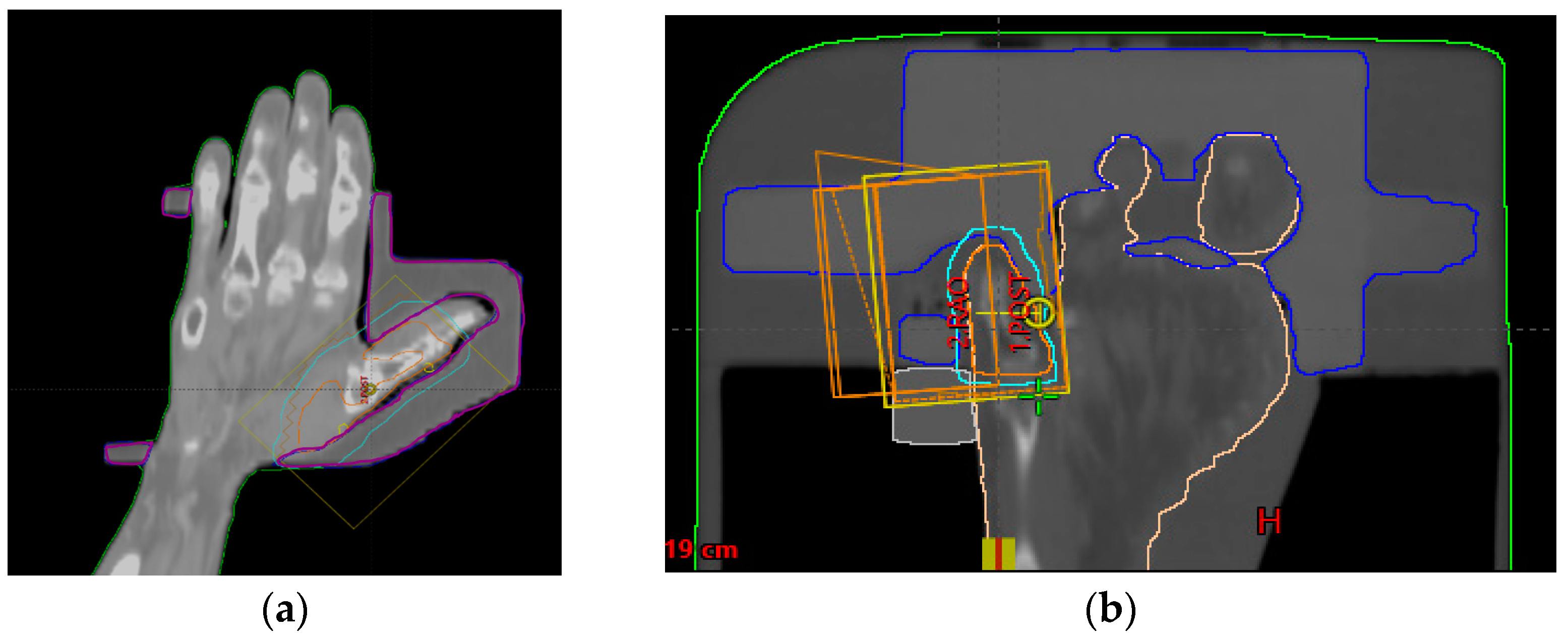
2.3. Dose Evaluation
3. Results
3.1. Dose Evaluation
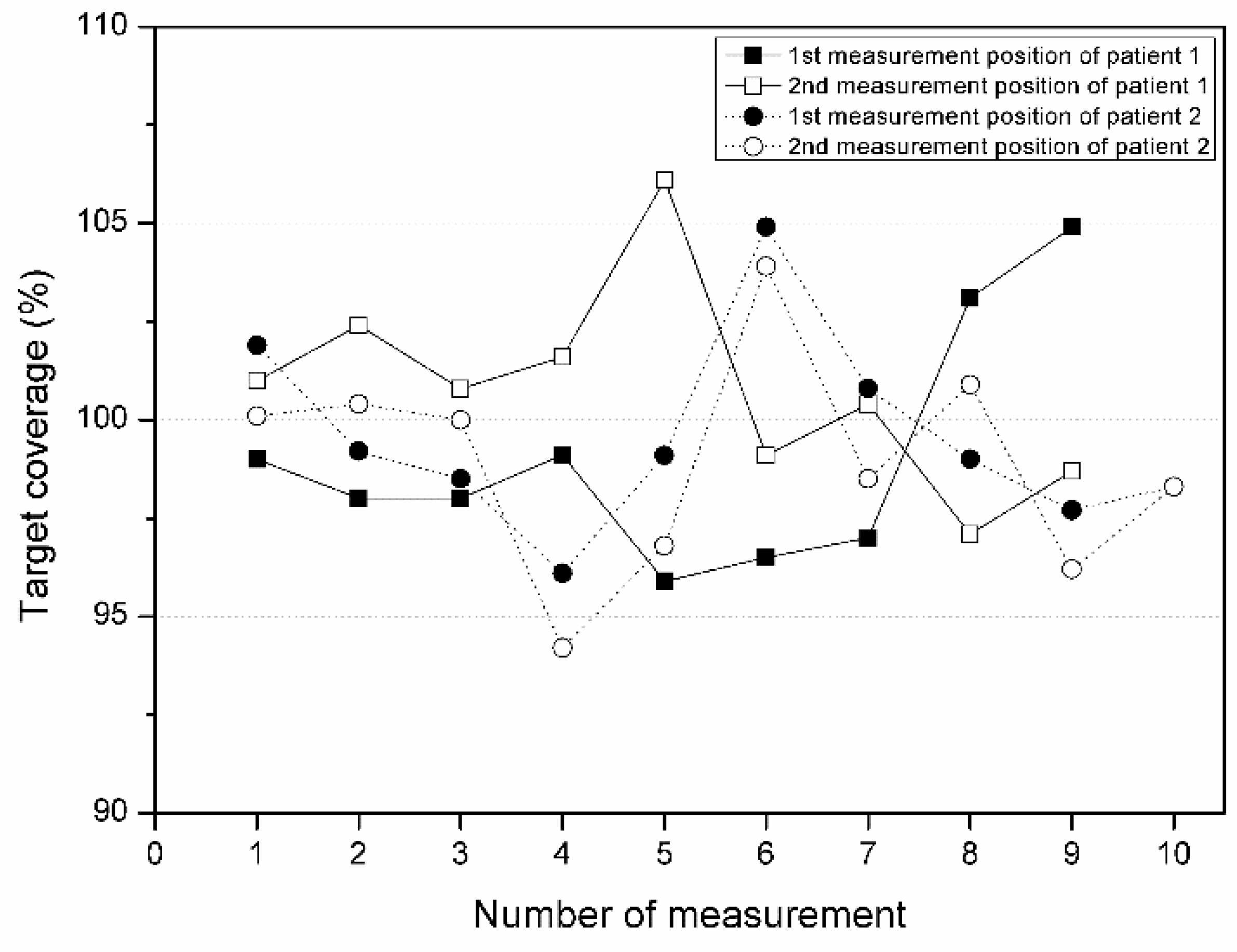
3.2. Target Coverage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pashazadeh, A.; Boese, A.; Friebe, M. Radiation therapy techniques in the treatment of skin cancer: An overview of the current status and outlook. J. Dermatol. Treat. 2019, 30, 831–839. [Google Scholar] [CrossRef]
- Ferro, M.; Deodato, F.; Ferro, M.; Panza, G.; Buwenge, M.; Pezzulla, D.; Cilla, S.; Boccardi, M.; Romano, C.; Arcelli, A.; et al. A SHort course Accelerated RadiatiON therapy (SHARON) dose-escalation trial in older adults head and neck non-melanoma skin cancer. Br. J. Radiol. 2022, 95, 20211347. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kwak, J.; Cho, B.; Song, S.Y.; Lee, S.-W.; Jeong, C. Clinical Implementation of 3D Printing in the Construction of Patient Specific Bolus for Photon Beam Radiotherapy for Mycosis Fungoides. Prog. Med. Phys. 2017, 28, 33–38. [Google Scholar] [CrossRef]
- Al-sudani, T.A.; Biasi, G.; Wilkinson, D.; Davis, J.A.; Kearnan, R.; Matar, F.S.; Custajar, D.L.; Metcalfe, P.; Rosenfeld, A.B. eXaSkin: A novel high-density bolus for 6MV X-rays radiotherapy. Phys. Med. 2020, 80, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yea, J. Three-dimensional customized bolus for intensity-modulated radiotherapy in a patient with Kimura’s disease involving the auricle. Cancer Radiother. 2016, 20, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Jreije, A.; Keshelava, L.; Ilickas, M.; Laurikaitiene, J.; Urbonavicius, B.G.; Adliene, D. Development of Patient Specific Conformal 3D-Printed Devices for Dose Verification in Radiotherapy. Appl. Sci. 2021, 11, 8657. [Google Scholar] [CrossRef]
- Muramatsu, N.; Ito, S.; Hanmura, M.; Nishimura, T. Development of a transparent and flexible patient-specific bolus for total scalp irradiation. Radiol. Phys. Technol. 2021, 14, 82–92. [Google Scholar] [CrossRef]
- Ricotti, R.; Ciardo, D.; Pansini, F.; Bazani, A.; Comi, S.; Spoto, R.; Noris, S.; Cattani, F.; Baroni, G.; Orecchia, R.; et al. Dosimetric characterization of 3D printed bolus at different infill percentage for external photon beam radiotherapy. Phys. Med. 2017, 39, 25–32. [Google Scholar] [CrossRef]
- Khan, Y.; Villarreal-Barajas, J.E.; Udowicz, M.; Sinha, R.; Muhammad, W.; Abbasi, A.N.; Hussain, A. Clinical and Dosimetric Implications of Air Gaps between Bolus and Skin Surface during Radiation Therapy. J. Cancer Ther. 2013, 04, 1251–1255. [Google Scholar] [CrossRef]
- Dyer, B.A.; Campos, D.D.; Hernandez, D.D.; Wright, C.L.; Perks, J.R.; Lucero, S.A.; Bewley, A.F.; Yamamoto, T.; Zhu, X.; Rao, S.S. Characterization and clinical validation of patient-specific three-dimensional printed tissue-equivalent bolus for radiotherapy of head and neck malignancies involving skin. Phys. Med. 2020, 77, 138–145. [Google Scholar] [CrossRef]
- Lee, H.; Mauceri, T.C.; Bhagwat, M.S.; Patel, C.G. Water Bath Radiation for Extensive, Extremity-Based Cutaneous Disease of Mycosis Fungoides. Adv. Radiat. Oncol. 2020, 5, 1370–1374. [Google Scholar] [CrossRef]
- Fujimoto, K.; Shiinoki, T.; Yuasa, Y.; Hanazawa, H.; Shibuya, K. Efficacy of patient-specific bolus created using three-dimensional printing technique in photon radiotherapy. Phys. Med. 2017, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chatchumnan, N.; Kingkaew, S.; Aumnate, C.; Sanghangthum, T. Development and dosimetric verification of 3D customized bolus in head and neck radiotherapy. J. Radiat. Res. 2022, 63, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Ahn, S.; Ju, E.; Jung, N.H. Customized 3D Bolus Applied to the Oral Cavity and Supraclavicular Area for Head and Neck Cancer. In Vivo 2021, 35, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Yan, T.; Sun, Y.; Qian, J.; Zhou, G.; Cai, S.; Tian, Y. A dosimetric study on the use of 3D-printed customized boluses in photon therapy: A hydrogel and silica gel study. J. Appl. Clin. Med. Phys. 2018, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Robar, J.L.; Moran, K.; Allan, J.; Clancey, J.; Joseph, T.; Chytyk-Praznik, K.; MacDonald, R.L.; Lincoln, J.; Sadeghi, P.; Rutledge, R. Intrapatient study comparing 3D printed bolus versus standard vinyl gel sheet bolus for postmastectomy chest wall radiation therapy. Pract. Radiat. Oncol. 2018, 8, 221–229. [Google Scholar] [CrossRef]
- Mahdavi, S.R.; Tutuni, M.; Farhood, B.; Nafisi, N.; Ghasemi, S.; Mirzaee, H.; Ahmadi, S.; Alizadeh, A. Measurement of peripheral dose to the pelvic region and the associated risk for cancer development after breast intraoperative electron radiation therapy. J. Radiol. Prot. 2019, 39, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.F.; Veen, S.; van Egmond, J.; van Hameren, M.; van Oorschot, T.; de Vet, S.; van Santvoort, J.P.; Wiggenraad, R.G.; Mast, M.E. The influence of a six degrees of freedom couch and an individual head support in patient positioning in radiotherapy of head and neck cancer. Phys. Imaging Radiat. Oncol. 2019, 11, 30–33. [Google Scholar] [CrossRef]
- Dias, A.G.; Pinto, D.F.S.; Borges, M.F.; Pereira, M.H.; Santos, J.A.M.; Cunha, L.T.; Lencart, J. Optimization of skin dose using in-vivo MOSFET dose measurements in bolus/non-bolus fraction ratio: A VMAT and a 3DCRT study. J. Appl. Clin. Med. Phys. 2019, 20, 63–70. [Google Scholar] [CrossRef]
- Tyran, M.; Tallet, A.; Resbeut, M.; Ferre, M.; Favrel, V.; Fau, P.; Moureau-Zabotto, L.; Darreon, J.; Gonzague, L.; Benkemouche, A.; et al. Safety and benefit of using a virtual bolus during treatment planning for breast cancer treated with arc therapy. J. Appl. Clin. Med. Phys. 2018, 19, 463–472. [Google Scholar] [CrossRef]
- Kim, S.-W.; Shin-Wook, K.; Kay, C.S.; Son, S.H. A Customized Bolus Produced Using a 3-Dimensional Printer for Radiotherapy. PLoS ONE 2014, 9, e110746. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kang, S.-W.; Shin, D.-S.; Kim, M.-S.; Suh, T.S.; Chung, J.-B.; Kang, S.-H. Characteristics of Megavoltage Electron Beams Directed through Silicone for Bolus Electron Therapy. J. Korean Phys. Soc. 2020, 76, 182–189. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Xiang, Z.; Zeng, Y.; Liu, F.; Shao, B.; He, T.; Ma, J.; Yu, S.; Liu, L. The Clinical Application of 3D-Printed Boluses in Superficial Tumor Radiotherapy. Front. Oncol. 2021, 11, 698773. [Google Scholar] [CrossRef]

| Patient 1 | Patient 2 | |
|---|---|---|
| Gender | Female | Male |
| Age | 66 y | 68 y |
| Prescribed dose | 60.4 Gy/33 fx (50.4 Gy/28 fx, 10 Gy/5 fx) | 54 Gy/30 fx |
| MU | 240, 265 | 235 |
| Treatment site | Left 1st metacarpal phalangeal joint | Right 5th toe |
| Beam energy | 6 MeV | 6 MeV |
| OSLD Measurement Mean Dose (cGy) | Difference to Prescription | Difference to TPS | |
|---|---|---|---|
| Upper 3D bolus (Pos1) | 193.2 ± 4.1 | 7.4% | −0.7% |
| Target region (Pos2) | 187.6 ± 2.1 | 4.2% | −2.0% |
| Mean dose (cGy) | 190.4 ± 3.1 | 5.8% | −1.4% |
| OSLD Measurement Mean Dose (cGy) | Difference to Prescription | Difference to TPS | |
|---|---|---|---|
| Base of the 5th toe (Pos1) | 189.2 ± 3.7 | 5.1% | −0.9% |
| Left side of the 5th toe (Pos2) | 187.7 ± 4.9 | 4.3% | −1.4% |
| Mean dose (cGy) | 188.5 ± 4.3 | 4.7% | −1.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Ahn, S.H.; Choi, S.H.; Ahn, W.S.; Jung, I.-h. Clinical Application of a Customized 3D-Printed Bolus in Radiation Therapy for Distal Extremities. Life 2023, 13, 362. https://doi.org/10.3390/life13020362
Yu S, Ahn SH, Choi SH, Ahn WS, Jung I-h. Clinical Application of a Customized 3D-Printed Bolus in Radiation Therapy for Distal Extremities. Life. 2023; 13(2):362. https://doi.org/10.3390/life13020362
Chicago/Turabian StyleYu, Suah, So Hyun Ahn, Sang Hyoun Choi, Woo Sang Ahn, and In-hye Jung. 2023. "Clinical Application of a Customized 3D-Printed Bolus in Radiation Therapy for Distal Extremities" Life 13, no. 2: 362. https://doi.org/10.3390/life13020362
APA StyleYu, S., Ahn, S. H., Choi, S. H., Ahn, W. S., & Jung, I.-h. (2023). Clinical Application of a Customized 3D-Printed Bolus in Radiation Therapy for Distal Extremities. Life, 13(2), 362. https://doi.org/10.3390/life13020362






