Spark of Life: Role of Electrotrophy in the Emergence of Life
Abstract
1. Introduction
2. The Cradle of Life
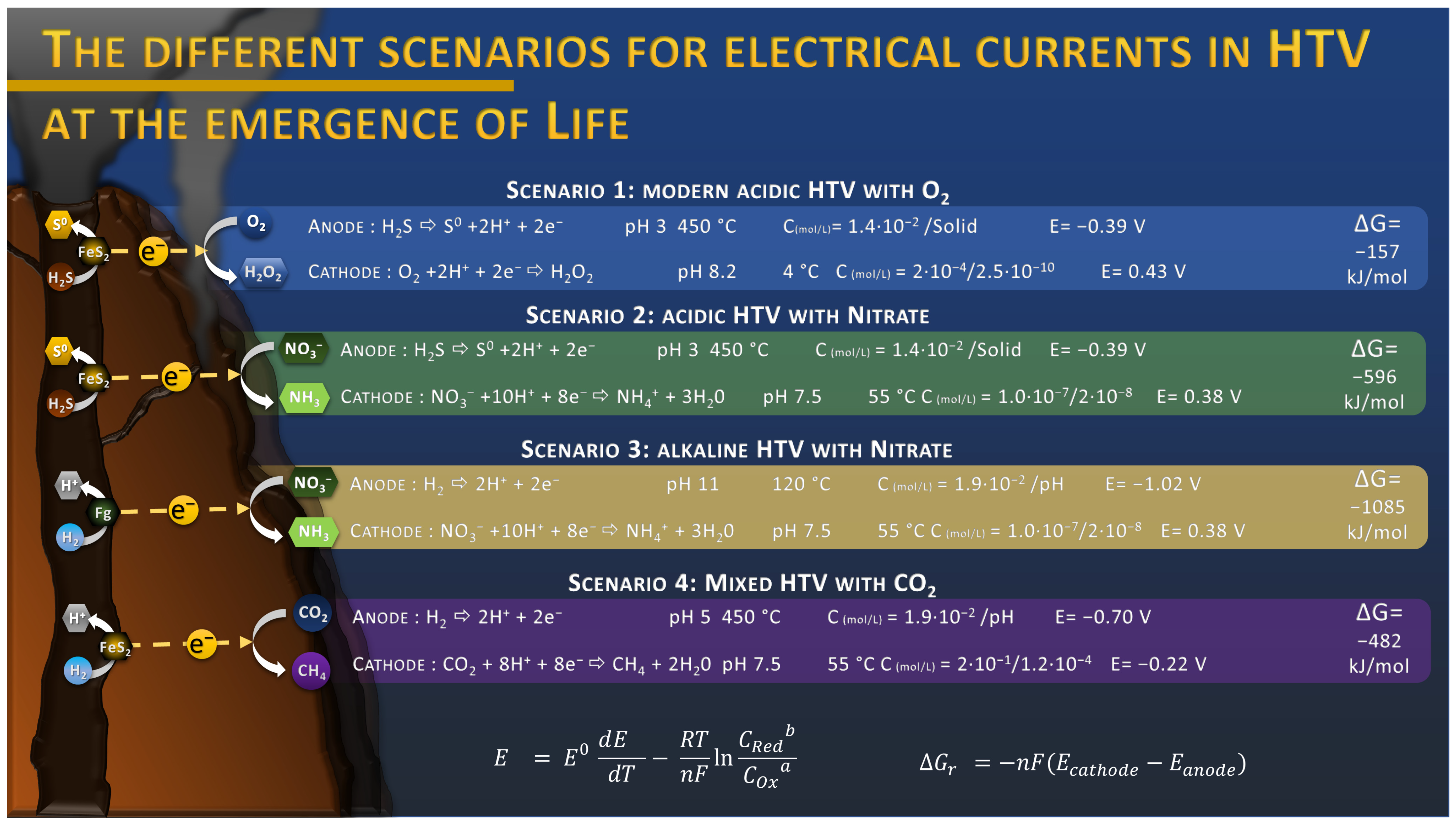
3. Natural Electrical Current in Hydrothermal Chimneys
4. Electrotrophy in Modern Hydrothermal Vents
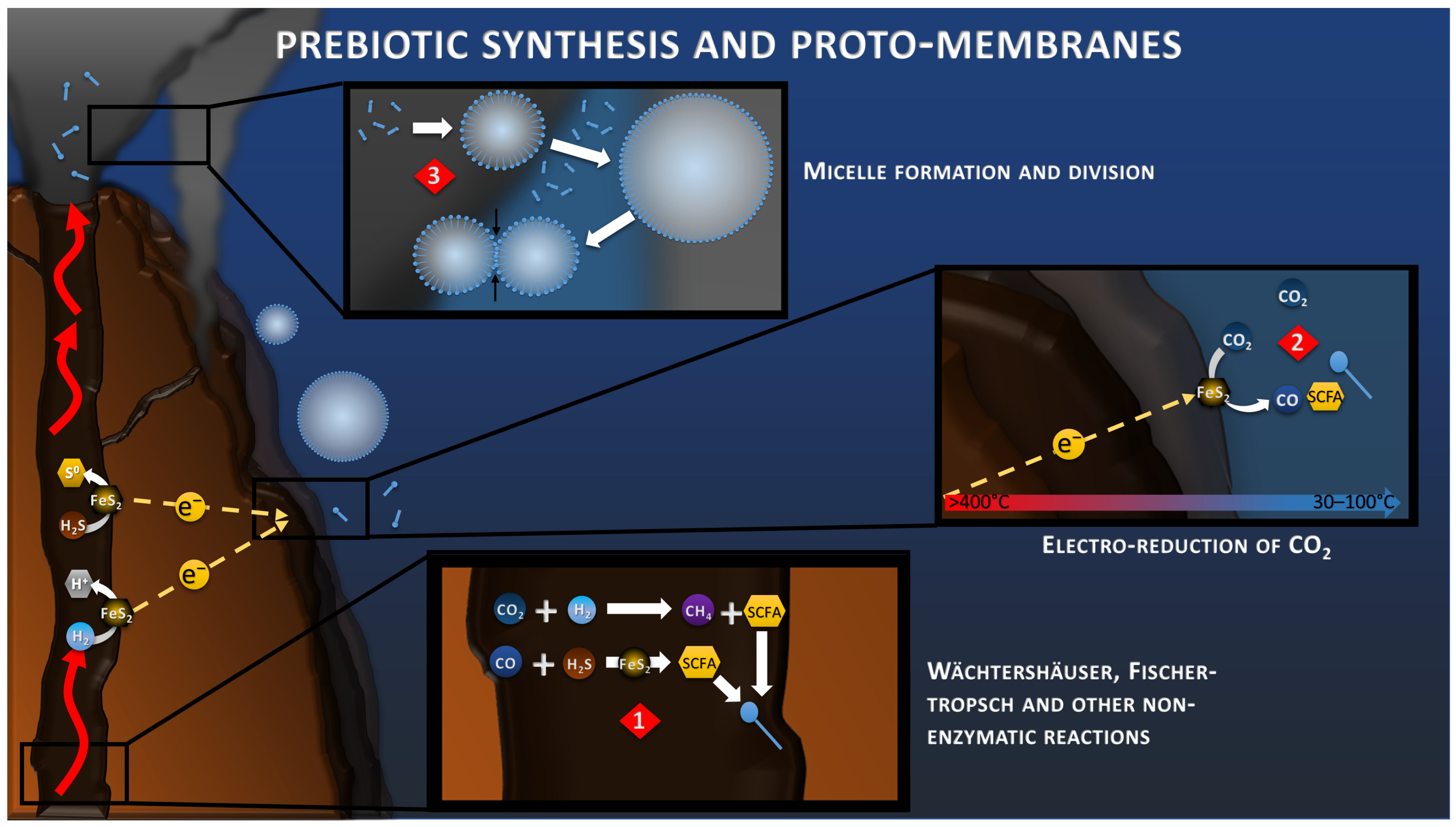
5. Prebiotic Synthesis and CO2 Electroreduction
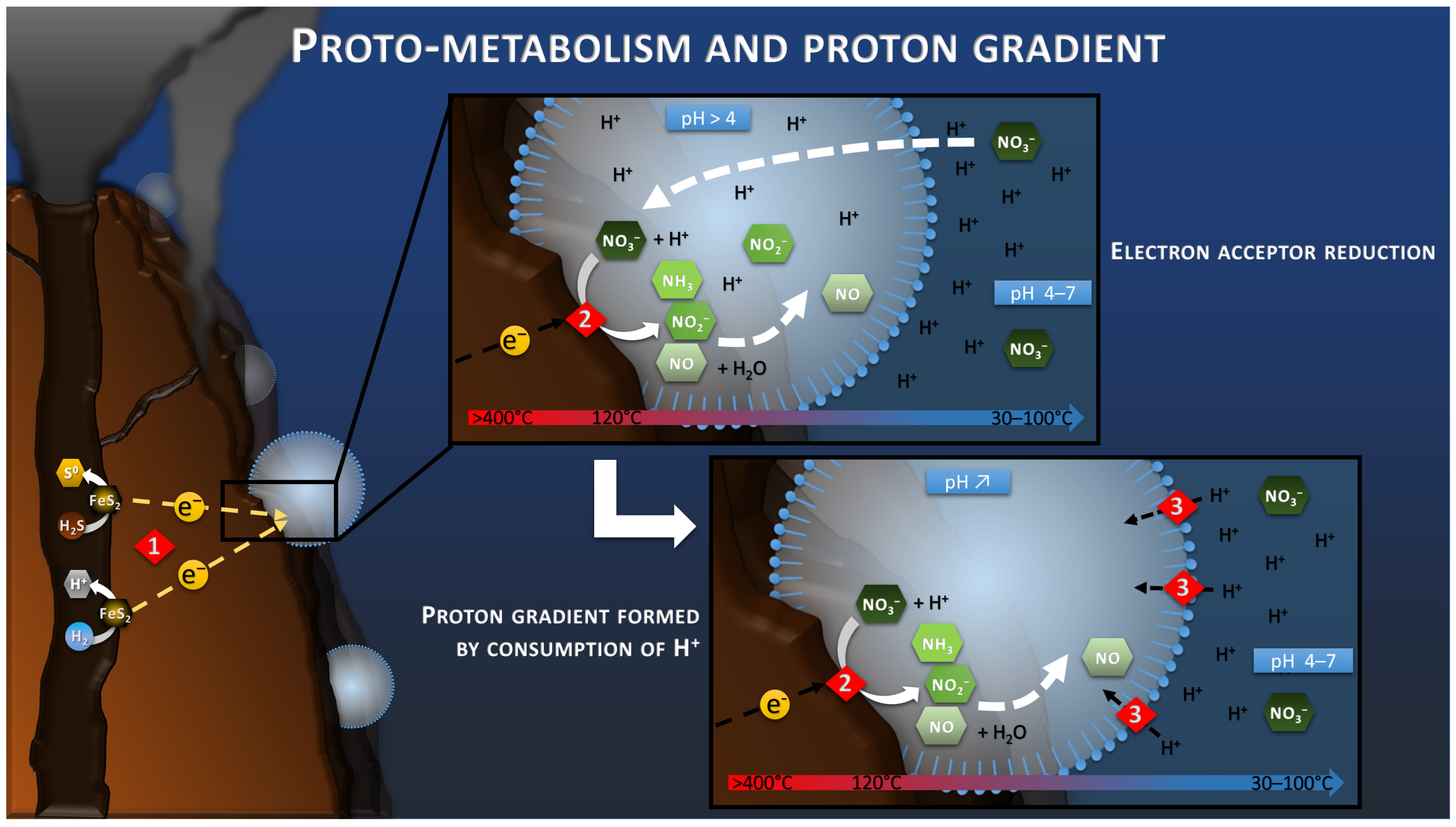
6. Energetic Proto-Metabolism
7. Proton Gradient
8. From CO2 Electroreduction to Autotrophic Pathway
8.1. Autotrophic Pathway of Electrotrophs
8.2. Autotrophic Pathways at the Emergence of Life
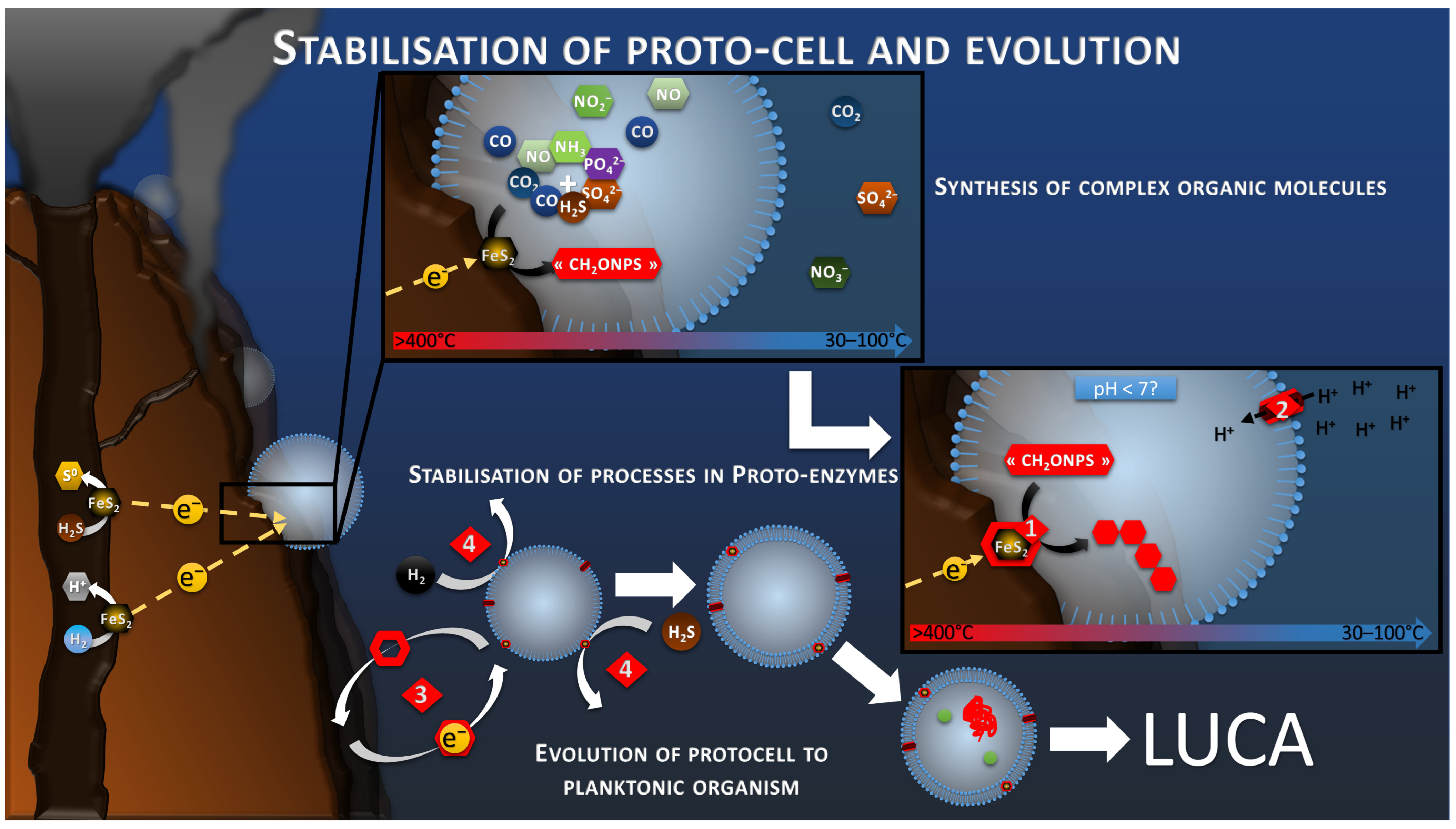
8.3. Anabolism, and Further Synthesis
8.4. Polymerization
8.5. Electron Donor Mediation for Planktonic Migration
9. Electrotrophy, Serpentinization and Iron–Sulfur World
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonfio, C.; Valer, L.; Scintilla, S.; Shah, S.; Evans, D.J.; Jin, L.; Szostak, J.W.; Sasselov, D.D.; Sutherland, J.D.; Mansy, S.S. UV-Light-Driven Prebiotic Synthesis of Iron–Sulfur Clusters. Nat. Chem. 2017, 9, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Fahrenbach, A.C.; Giurgiu, C.; Tam, C.P.; Li, L.; Hongo, Y.; Aono, M.; Szostak, J.W. Common and Potentially Prebiotic Origin for Precursors of Nucleotide Synthesis and Activation. J. Am. Chem. Soc. 2017, 139, 8780–8783. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, E.A.; Kratzer, J.T.; Randall, R.N. Deep Phylogeny—How a Tree Can Help Characterize Early Life on Earth. Cold Spring Harb. Perspect. Biol. 2010, 2, a002238. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The Physiology and Habitat of the Last Universal Common Ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef] [PubMed]
- Gesteland, R.; Atkins, J. The RNA World; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 1993. [Google Scholar]
- Crisp, A.; Carell, T. Rethinking the Tools of the RNA World. eLife 2018, 7, e38297. [Google Scholar] [CrossRef] [PubMed]
- Dodd, M.S.; Papineau, D.; Grenne, T.; Slack, J.F.; Rittner, M.; Pirajno, F.; O’Neil, J.; Little, C.T.S. Evidence for Early Life in Earth’s Oldest Hydrothermal Vent Precipitates. Nature 2017, 543, 60–64. [Google Scholar] [CrossRef]
- Follmann, H.; Brownson, C. Darwin’s Warm Little Pond Revisited: From Molecules to the Origin of Life. Naturwissenschaften 2009, 96, 1265–1292. [Google Scholar] [CrossRef]
- Wächtershäuser, G. Origin of Life in an Iron-Sulfur World. In The Molecular Origins of Life: Assembling Pieces of the Puzzle; A. Brack: Cambridge, UK, 1998; pp. 206–216. [Google Scholar]
- Russell, M. Why and How Life Is Driven into Being at Ancient Submarine Alkaline Springs. In Proceedings of the 41st COSPAR Scientific Assembly, Istanbul, Turkey, 30 July–7 August 2016; Volume 41. [Google Scholar]
- Clark, B.C.; Kolb, V.M. Macrobiont: Cradle for the Origin of Life and Creation of a Biosphere. Life 2020, 10, 278. [Google Scholar] [CrossRef]
- Lingam, M.; Loeb, A. Life in the Cosmos: From Biosignatures to Technosignatures; Harvard University Press: Cambridge, MA, USA, 2021; ISBN 9780674987579. [Google Scholar]
- Damer, B.; Deamer, D. The Hot Spring Hypothesis for an Origin of Life. Astrobiology 2020, 20, 429–452. [Google Scholar] [CrossRef]
- Stüeken, E.E.; Anderson, R.E.; Bowman, J.S.; Brazelton, W.J.; Colangelo-Lillis, J.; Goldman, A.D.; Som, S.M.; Baross, J.A. Did Life Originate from a Global Chemical Reactor? Geobiology 2013, 11, 101–126. [Google Scholar] [CrossRef]
- Spiess, F.N.; Macdonald, K.C.; Atwater, T.; Ballard, R.; Carranza, A.; Cordoba, D.; Cox, C.; Garcia, V.M.D.; Francheteau, J.; Guerrero, J.; et al. East Pacific Rise: Hot Springs and Geophysical Experiments. Science 1980, 207, 1421–1433. [Google Scholar] [CrossRef]
- Russell, M.J. The Alkaline Solution to the Emergence of Life: Energy, Entropy and Early Evolution. Acta Biotheor. 2007, 55, 133–179. [Google Scholar] [CrossRef]
- Russell, M.J.; Nitschke, W. Methane: Fuel or Exhaust at the Emergence of Life? Astrobiology 2017, 17, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Daniel, R.M.; Hall, A.J.; Sherringham, J.A. A Hydrothermally Precipitated Catalytic Iron Sulphide Membrane as a First Step toward Life. J. Mol. Evol. 1994, 39, 231–243. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Turner, D. In Vitro Growth of Iron Sulphide Chimneys: Possible Culture Chambers for Origin-of-Life Experiments. Terra Nova 1989, 1, 238–241. [Google Scholar] [CrossRef]
- Nakamura, R.; Takashima, T.; Kato, S.; Takai, K.; Yamamoto, M.; Hashimoto, K. Electrical Current Generation across a Black Smoker Chimney. Angew. Chem. Int. Ed. 2010, 49, 7692–7694. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nakamura, R.; Kasaya, T.; Kumagai, H.; Suzuki, K.; Takai, K. Spontaneous and Widespread Electricity Generation in Natural Deep-Sea Hydrothermal Fields. Angew. Chem. Int. Ed. 2017, 56, 5725–5728. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nakamura, R.; Oguri, K.; Kawagucci, S.; Suzuki, K.; Hashimoto, K.; Takai, K. Generation of Electricity and Illumination by an Environmental Fuel Cell in Deep-Sea Hydrothermal Vents. Angew. Chem. Int. Ed. 2013, 52, 10758–10761. [Google Scholar] [CrossRef] [PubMed]
- Karato, S.-I.; Wang, D. Electrical Conductivity of Minerals and Rocks. In Physics and Chemistry of the Deep Earth; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 145–182. ISBN 9781118529492. [Google Scholar]
- Georgieva, M.N.; Little, C.T.S.; Maslennikov, V.V.; Glover, A.G.; Ayupova, N.R.; Herrington, R.J. The History of Life at Hydrothermal Vents. Earth Sci. Rev. 2021, 217, 103602. [Google Scholar] [CrossRef]
- Wong, M.L.; Charnay, B.D.; Gao, P.; Yung, Y.L.; Russell, M.J. Nitrogen Oxides in Early Earth’s Atmosphere as Electron Acceptors for Life’s Emergence. Astrobiology 2017, 17, 975–983. [Google Scholar] [CrossRef]
- Ranjan, S.; Todd, Z.R.; Rimmer, P.B.; Sasselov, D.D.; Babbin, A.R. Nitrogen Oxide Concentrations in Natural Waters on Early Earth. Geochem. Geophys. Geosyst. 2019, 20, 2021–2039. [Google Scholar] [CrossRef]
- Bratsch, S.G. Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Martin, W. Serpentinization as a Source of Energy at the Origin of Life. Geobiology 2010, 8, 355–371. [Google Scholar] [CrossRef]
- Garcia, A.K.; Schopf, J.W.; Yokobori, S.; Akanuma, S.; Yamagishi, A. Reconstructed Ancestral Enzymes Suggest Long-Term Cooling of Earth’s Photic Zone since the Archean. Proc. Natl. Acad. Sci. USA 2017, 114, 4619–4624. [Google Scholar] [CrossRef] [PubMed]
- James, R.H.; Green, D.R.H.; Stock, M.J.; Alker, B.J.; Banerjee, N.R.; Cole, C.; German, C.R.; Huvenne, V.A.I.; Powell, A.M.; Connelly, D.P. Composition of Hydrothermal Fluids and Mineralogy of Associated Chimney Material on the East Scotia Ridge Back-Arc Spreading Centre. Geochim. Cosmochim. Acta 2014, 139, 47–71. [Google Scholar] [CrossRef]
- Matear, R.J.; Hirst, A.C. Long-Term Changes in Dissolved Oxygen Concentrations in the Ocean Caused by Protracted Global Warming. Glob. Biogeochem. Cycles 2003, 17, 452–457. [Google Scholar] [CrossRef]
- Hopwood, M.J.; Rapp, I.; Schlosser, C.; Achterberg, E.P. Hydrogen Peroxide in Deep Waters from the Mediterranean Sea, South Atlantic and South Pacific Oceans. Sci. Rep. 2017, 7, 43436. [Google Scholar] [CrossRef]
- Woodward, E.M.S.; Rees, A.P. Nutrient Distributions in an Anticyclonic Eddy in the Northeast Atlantic Ocean, with Reference to Nanomolar Ammonium Concentrations. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 775–793. [Google Scholar] [CrossRef]
- Lilley, M.D.; Butterfield, D.A.; Olson, E.J.; Lupton, J.E.; Macko, S.A.; McDuff, R.E. Anomalous CH4 and NH4+ Concentrations at an Unsedimented Mid-Ocean-Ridge Hydrothermal System. Nature 1993, 364, 45–47. [Google Scholar] [CrossRef]
- Pillot, G.; Amin Ali, O.; Davidson, S.; Shintu, L.; Godfroy, A.; Combet-Blanc, Y.; Bonin, P.; Liebgott, P.-P. Identification of Enriched Hyperthermophilic Microbial Communities from a Deep-Sea Hydrothermal Vent Chimney under Electrolithoautotrophic Culture Conditions. Sci. Rep. 2021, 11, 14782. [Google Scholar] [CrossRef]
- Pillot, G.; Amin Ali, O.; Davidson, S.; Shintu, L.; Combet-Blanc, Y.; Godfroy, A.; Bonin, P.; Liebgott, P.-P. Evolution of Thermophilic Microbial Communities from a Deep-Sea Hydrothermal Chimney under Electrolithoautotrophic Conditions with Nitrate. Microorganisms 2021, 9, 2475. [Google Scholar] [CrossRef] [PubMed]
- Popall, R.M.; Heussner, A.; Kerzenmacher, S.; Liebgott, P.-P.; Pillot, G. Screening for Hyperthermophilic Electrotrophs for the Microbial Electrosynthesis of Organic Compounds. Microorganisms 2022, 10, 2249. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takaki, Y.; Kashima, H.; Tsuda, M.; Tanizaki, A.; Nakamura, R.; Takai, K. In Situ Electrosynthetic Bacterial Growth Using Electricity Generated by a Deep-Sea Hydrothermal Vent. ISME J. 2022, 17, 12–20. [Google Scholar] [CrossRef]
- Lovley, D.R. Electrotrophy: Other Microbial Species, Iron, and Electrodes as Electron Donors for Microbial Respirations. Bioresour. Technol. 2022, 345, 126553. [Google Scholar] [CrossRef] [PubMed]
- Reiner, J.E.; Jung, T.; Lapp, C.J.; Siedler, M.; Bunk, B.; Overmann, J.; Gescher, J. 2018 Kyrpidia Spormannii Sp. Nov., a Thermophilic, Hydrogen-Oxidizing, Facultative Autotroph, Isolated from Hydrothermal Systems at São Miguel Island, and Emended Description of the Genus Kyrpidia. Int. J. Syst. Evol. Microbiol. 2018, 68, 3735–3740. [Google Scholar] [CrossRef]
- Carbajosa, S.; Malki, M.; Caillard, R.; Lopez, M.F.; Palomares, F.J.; Martín-Gago, J.A.; Rodríguez, N.; Amils, R.; Fernández, V.M.; De Lacey, A.L. Electrochemical Growth of Acidithiobacillus Ferrooxidans on a Graphite Electrode for Obtaining a Biocathode for Direct Electrocatalytic Reduction of Oxygen. Biosens. Bioelectron. 2010, 26, 877–880. [Google Scholar] [CrossRef]
- Summers, Z.M.; Gralnick, J.A.; Bond, D.R. Cultivation of an Obligate Fe(II)-Oxidizing Lithoautotrophic Bacterium Using Electrodes. mBio 2013, 4, e00420-12. [Google Scholar] [CrossRef]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A New Model for Electron Flow during Anaerobic Digestion: Direct Interspecies Electron Transfer to Methanosaeta for the Reduction of Carbon Dioxide to Methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Yee, M.O.; Snoeyenbos-West, O.L.; Thamdrup, B.; Ottosen, L.D.M.; Rotaru, A.-E. Extracellular Electron Uptake by Two Methanosarcina Species. Front. Energy Res. 2019, 7, 29. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Zhang, Y.; Lovley, D.R. Sparking Anaerobic Digestion: Promoting Direct Interspecies Electron Transfer to Enhance Methane Production. iScience 2020, 23, 101794. [Google Scholar] [CrossRef]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of Organic Compounds from Carbon Dioxide Is Catalyzed by a Diversity of Acetogenic Microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Kitadai, N.; Maruyama, S. Origins of Building Blocks of Life: A Review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Moran, J. Nonenzymatic Metabolic Reactions and Life’s Origins. Chem. Rev. 2020, 120, 7708–7744. [Google Scholar] [CrossRef] [PubMed]
- Ooka, H.; McGlynn, S.E.; Nakamura, R. Electrochemistry at Deep-Sea Hydrothermal Vents: Utilization of the Thermodynamic Driving Force towards the Autotrophic Origin of Life. ChemElectroChem 2019, 6, 1316–1323. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Yamamoto, M.; Takai, K.; Ishii, T.; Hashimoto, K.; Nakamura, R. Electrochemical CO2 Reduction by Ni-Containing Iron Sulfides: How Is CO2 Electrochemically Reduced at Bisulfide-Bearing Deep-Sea Hydrothermal Precipitates? Electrochim. Acta 2014, 141, 311–318. [Google Scholar] [CrossRef]
- Kitadai, N.; Nakamura, R.; Yamamoto, M.; Takai, K.; Li, Y.; Yamaguchi, A.; Gilbert, A.; Ueno, Y.; Yoshida, N.; Oono, Y. Geoelectrochemical CO Production: Implications for the Autotrophic Origin of Life. Sci. Adv. 2018, 4, eaao7265. [Google Scholar] [CrossRef] [PubMed]
- Budin, I.; Bruckner, R.J.; Szostak, J.W. Formation of Protocell-like Vesicles in a Thermal Diffusion Column. J. Am. Chem. Soc. 2009, 131, 9628–9629. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, A.I.; Simoneit, B.R. Lipid Formation by Aqueous Fischer-Tropsch-Type Synthesis over a Temperature Range of 100 to 400 °C. Orig. Life Evol. Biosph. 2001, 31, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.F.; Rammu, H.; Zheludev, I.N.; Hartley, A.M.; Maréchal, A.; Lane, N. Promotion of Protocell Self-Assembly from Mixed Amphiphiles at the Origin of Life. Nat. Ecol. Evol. 2019, 3, 1705–1714. [Google Scholar] [CrossRef]
- Koga, Y.; Morii, H. Biosynthesis of Ether-Type Polar Lipids in Archaea and Evolutionary Considerations. Microbiol. Mol. Biol. Rev. 2007, 71, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Weber, A.L. Bioenergetics and Life’s Origins. Cold Spring Harb. Perspect. Biol. 2010, 2, a004929. [Google Scholar] [CrossRef] [PubMed]
- McCollom, T.M.; Ritter, G.; Simoneit, B.R.T. Lipid Synthesis Under Hydrothermal Conditions by Fischer- Tropsch-Type Reactions. Orig. Life Evol. Biosph. 1999, 29, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Preiner, M.; Xavier, J.C.; Sousa, F.L.; Zimorski, V.; Neubeck, A.; Lang, S.Q.; Greenwell, H.C.; Kleinermanns, K.; Tüysüz, H.; McCollom, T.M.; et al. Serpentinization: Connecting Geochemistry, Ancient Metabolism and Industrial Hydrogenation. Life 2018, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.; Russell, M.J. Green Rust: The Simple Organizing ‘Seed’ of All Life? Life 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Ducluzeau, A.-L.; van Lis, R.; Duval, S.; Schoepp-Cothenet, B.; Russell, M.J.; Nitschke, W. Was Nitric Oxide the First Deep Electron Sink? Trends Biochem. Sci. 2009, 34, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Barge, L.M.; Bhartia, R.; Bocanegra, D.; Bracher, P.J.; Branscomb, E.; Kidd, R.; McGlynn, S.; Meier, D.H.; Nitschke, W.; et al. The Drive to Life on Wet and Icy Worlds. Astrobiology 2014, 14, 308–343. [Google Scholar] [CrossRef] [PubMed]
- Singireddy, S.; Gordon, A.D.; Smirnov, A.; Vance, M.A.; Schoonen, M.A.A.; Szilagyi, R.K.; Strongin, D.R. Reduction of Nitrite and Nitrate to Ammonium on Pyrite. Orig. Life Evol. Biosph. 2012, 42, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Schoepp-Cothenet, B.; van Lis, R.; Philippot, P.; Magalon, A.; Russell, M.J.; Nitschke, W. The Ineluctable Requirement for the Trans-Iron Elements Molybdenum and/or Tungsten in the Origin of Life. Sci. Rep. 2012, 2, 263. [Google Scholar] [CrossRef]
- Baymann, F.; Lebrun, E.; Brugna, M.; Schoepp-Cothenet, B.; Giudici-Orticoni, M.-T.; Nitschke, W. The Redox Protein Construction Kit: Pre-Last Universal Common Ancestor Evolution of Energy-Conserving Enzymes. Philos. Trans. Biol. Sci. 2003, 358, 267–274. [Google Scholar] [CrossRef]
- Marques, A.F.A.; Barriga, F.; Chavagnac, V.; Fouquet, Y. Mineralogy, Geochemistry, and Nd Isotope Composition of the Rainbow Hydrothermal Field, Mid-Atlantic Ridge. Min. Depos. 2006, 41, 52. [Google Scholar] [CrossRef]
- Lane, N. Proton Gradients at the Origin of Life. BioEssays 2017, 39, 1600217. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, W.; Russell, M.J. Beating the Acetyl Coenzyme A-Pathway to the Origin of Life. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120258. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.J.; Muchowska, K.B.; Chatelain, P.; Moran, J. Native Iron Reduces CO2 to Intermediates and End-Products of the Acetyl-CoA Pathway. Nat. Ecol. Evol. 2018, 2, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Kitadai, N.; Nakamura, R.; Yamamoto, M.; Takai, K.; Yoshida, N.; Oono, Y. Metals Likely Promoted Protometabolism in Early Ocean Alkaline Hydrothermal Systems. Sci. Adv. 2019, 5, eaav7848. [Google Scholar] [CrossRef]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common Origins of RNA, Protein and Lipid Precursors in a Cyanosulfidic Protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Chevallot-Beroux, E.; Lethuillier-Karl, L.; Li, G.; Moran, J. Metals Promote Sequences of the Reverse Krebs Cycle. Nat. Ecol. Evol. 2017, 1, 1716–1721. [Google Scholar] [CrossRef]
- Kopetzki, D.; Antonietti, M. Hydrothermal Formose Reaction. New J. Chem. 2011, 35, 1787–1794. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, W.; Lan, X.; Wang, Y.; Li, T.; Han, S.; Yu, Y.; Zhang, B. Electrochemical Upgrading of Formic Acid to Formamide via Coupling Nitrite Co-Reduction. J. Am. Chem. Soc. 2022, 144, 16006–16011. [Google Scholar] [CrossRef]
- Fukushima, T.; Yamauchi, M. Electrosynthesis of Amino Acids from Biomass-Derivable Acids on Titanium Dioxide. Chem. Commun. 2019, 55, 14721–14724. [Google Scholar] [CrossRef]
- Kim, C.; Eom, T.; Jee, M.S.; Jung, H.; Kim, H.; Min, B.K.; Hwang, Y.J. Insight into Electrochemical CO2 Reduction on Surface-Molecule-Mediated Ag Nanoparticles. ACS Catal. 2017, 7, 779–785. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New Insights into the Electrochemical Reduction of Carbon Dioxide on Metallic Copper Surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Martin, C.; Huser, H.; Servat, K.; Kokoh, K.B. Selective Electroreduction of Pyruvic Acid on Lead Electrode in Acid Medium. Electrochim. Acta 2005, 50, 2431–2435. [Google Scholar] [CrossRef]
- Lopez, A.; Fiore, M. Investigating Prebiotic Protocells for a Comprehensive Understanding of the Origins of Life: A Prebiotic Systems Chemistry Perspective. Life 2019, 9, 49. [Google Scholar] [CrossRef]
- Gomes, C.S.F.; Rautureau, M. Minerals and the Origin of Life. In Minerals Latu Sensu and Human Health: Benefits, Toxicity and Pathologies; Gomes, C., Rautureau, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 377–403. ISBN 9783030657062. [Google Scholar]
- Alhedabi, T.; Cattey, H.; Roussel, C.; Blondeau-Patissier, V.; Gharbi, T.; Herlem, G. Experimental and Theoretical Studies on Electropolymerization of Polar Amino Acids on Platinum Electrode. Mater. Chem. Phys. 2017, 185, 183–194. [Google Scholar] [CrossRef]
- Herlem, G.; Alhedabi, T.; Picaud, F. From Anodic Oxidation of Aliphatic α-Amino Acids to Polypeptides by Quantum Electrochemistry Approach: Beyond Miller–Urey Experiments. J. Am. Chem. Soc. 2019, 141, 14230–14238. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hartman, H. Clays and the Origin of Life: The Experiments. Life 2022, 12, 259. [Google Scholar] [CrossRef]
- Lackschewitz, K.S.; Botz, R.; Garbe-Schönberg, D.; Scholten, J.; Stoffers, P. Mineralogy and Geochemistry of Clay Samples from Active Hydrothermal Vents off the North Coast of Iceland. Mar. Geol. 2006, 225, 177–190. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Ishibashi, J.; Shimada, K.; Inoue, H.; Uehara, S.; Tsukimura, K. Clay Minerals in an Active Hydrothermal Field at Iheya-North-Knoll, Okinawa Trough. Resour. Geol. 2015, 65, 346–360. [Google Scholar] [CrossRef]
- Hao, J.; Mokhtari, M.; Pedreira-Segade, U.; Michot, L.J.; Daniel, I. Transition Metals Enhance the Adsorption of Nucleotides onto Clays: Implications for the Origin of Life. ACS Earth Space Chem. 2019, 3, 109–119. [Google Scholar] [CrossRef]
- Hudson, R.; de Graaf, R.; Strandoo Rodin, M.; Ohno, A.; Lane, N.; McGlynn, S.E.; Yamada, Y.M.A.; Nakamura, R.; Barge, L.M.; Braun, D.; et al. CO2 Reduction Driven by a PH Gradient. Proc. Natl. Acad. Sci. USA 2020, 117, 22873–22879. [Google Scholar] [CrossRef]
- Sojo, V.; Ohno, A.; McGlynn, S.E.; Yamada, Y.M.A.; Nakamura, R. Microfluidic Reactors for Carbon Fixation under Ambient-Pressure Alkaline-Hydrothermal-Vent Conditions. Life 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Kiran, R.; Patil, S.A. Microbial Electroactive Biofilms. In Introduction to Biofilm Engineering; ACS Symposium Series; American Chemical Society: New York, NY, USA, 2019; Volume 1323, pp. 159–186. ISBN 9780841234734. [Google Scholar]
- Kelley, D.S.; Karson, J.A.; Früh-Green, G.L.; Yoerger, D.R.; Shank, T.M.; Butterfield, D.A.; Hayes, J.M.; Schrenk, M.O.; Olson, E.J.; Proskurowski, G.; et al. A Serpentinite-Hosted Ecosystem: The Lost City Hydrothermal Field. Science 2005, 307, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Straub, K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef]
- Li, K.-M.; Wilkinson, C.; Kellosalo, J.; Tsai, J.-Y.; Kajander, T.; Jeuken, L.J.C.; Sun, Y.-J.; Goldman, A. Membrane Pyrophosphatases from Thermotoga Maritima and Vigna Radiata Suggest a Conserved Coupling Mechanism. Nat. Commun. 2016, 7, 13596. [Google Scholar] [CrossRef]
- Schoonen, M.; Smirnov, A.; Cohn, C. A Perspective on the Role of Minerals in Prebiotic Synthesis. AMBIO J. Hum. Environ. 2004, 33, 539–551. [Google Scholar] [CrossRef]
- Kitadai, N. Dissolved Divalent Metal and PH Effects on Amino Acid Polymerization: A Thermodynamic Evaluation. Orig. Life Evol. Biosph. 2017, 47, 13–37. [Google Scholar] [CrossRef]
- Bernhardt, H.S.; Tate, W.P. Primordial Soup or Vinaigrette: Did the RNA World Evolve at Acidic PH? Biol. Direct 2012, 7, 4. [Google Scholar] [CrossRef]
- Barge, L.M.; Doloboff, I.J.; Russell, M.J.; VanderVelde, D.; White, L.M.; Stucky, G.D.; Baum, M.M.; Zeytounian, J.; Kidd, R.; Kanik, I. Pyrophosphate Synthesis in Iron Mineral Films and Membranes Simulating Prebiotic Submarine Hydrothermal Precipitates. Geochim. Cosmochim. Acta 2014, 128, 1–12. [Google Scholar] [CrossRef]
- Prabahar, K.J.; Ferris, J.P. Adenine Derivatives as Phosphate-Activating Groups for the Regioselective Formation of 3′,5′-Linked Oligoadenylates on Montmorillonite: Possible Phosphate-Activating Groups for the Prebiotic Synthesis of RNA. J. Am. Chem. Soc. 1997, 119, 4330–4337. [Google Scholar] [CrossRef]
- Wang, Q.; Barge, L.M.; Steinbock, O. Microfluidic Production of Pyrophosphate Catalyzed by Mineral Membranes with Steep PH Gradients. Chemistry 2019, 25, 4732–4739. [Google Scholar] [CrossRef]


| Critical Aspects | Iron–Sulfur World | Alkaline Hydrothermal Vent | Electrotrophy |
|---|---|---|---|
| Driving process |
|
|
|
| Energy source |
|
|
|
| Electron sink |
|
|
|
| Carbon fixation |
|
|
|
| Localization |
|
|
|
| Proton gradient |
|
|
|
| Availability of Phosphorous |
|
|
|
| Availability of Nitrogen |
|
|
|
| Heavy metals |
|
|
|
| Temperature |
|
|
|
| Amino acids formation |
|
|
|
| Polymerization |
|
|
|
| Translocation of the energetic system |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillot, G.; Santiago, Ó.; Kerzenmacher, S.; Liebgott, P.-P. Spark of Life: Role of Electrotrophy in the Emergence of Life. Life 2023, 13, 356. https://doi.org/10.3390/life13020356
Pillot G, Santiago Ó, Kerzenmacher S, Liebgott P-P. Spark of Life: Role of Electrotrophy in the Emergence of Life. Life. 2023; 13(2):356. https://doi.org/10.3390/life13020356
Chicago/Turabian StylePillot, Guillaume, Óscar Santiago, Sven Kerzenmacher, and Pierre-Pol Liebgott. 2023. "Spark of Life: Role of Electrotrophy in the Emergence of Life" Life 13, no. 2: 356. https://doi.org/10.3390/life13020356
APA StylePillot, G., Santiago, Ó., Kerzenmacher, S., & Liebgott, P.-P. (2023). Spark of Life: Role of Electrotrophy in the Emergence of Life. Life, 13(2), 356. https://doi.org/10.3390/life13020356






