Cardiovascular Risk Assessment in Rheumatoid Arthritis: Accelerated Atherosclerosis, New Biomarkers, and the Effects of Biological Therapy
Abstract
1. Introduction
2. Cardiovascular Risk Factors in RA and Their Relationship with Atherosclerosis
2.1. Arterial Hypertension
2.2. Dyslipidemia
2.3. Obesity and Insulin Resistance
| Adipokine | Functions | Source | Effects in RA Patients | Reference |
|---|---|---|---|---|
| Adiponectin | Anti-inflammatory effect Anti-atherogenic effect | Adipocytes | Pro-inflammatory effect Correlated with disease activity, disease progression, and inflammatory markers | [44,45] |
| Leptin | Pro-inflammatory effect Appetite and weight regulator | Adipocytes | Pro-inflammatory effect Correlated with disease activity and progression, as well as with IL-6 levels | [44,46,47] |
| Visfatin | Pro-inflammatory effect Promotion of B-cell differentiation | Adipose tissue, liver, bone marrow, muscle | Pro-inflammatory effect Correlated with inflammatory markers and disease activity Expression of visfatin seems to be linked to decreased cardiometabolic risk | [44,48,49] |
| Resistin | Pro-inflammatory effect Promotion of immune cell recruitment and immune cell activation | Macrophages, adipocytes | Pro-inflammatory effect Systemic levels may depend on RA disease duration or severity Synovial levels seem to be correlated with inflammatory markers and disease activity | [44,50] |
| Omentin | Anti-inflammatory effect Anti-atherogenic effect | Stromal vascular cells, adipocytes | Systemic levels were associated with inflammatory markers, while tissue concentrations were neutral | [44,51] |
| Progranulin | Anti-inflammatory effect (by competitive binding to tumor necrosis factor (TNF)) | Adipocytes, macrophages, chondrocytes | Pro-inflammatory marker Correlated with disease activity and progression Is a key player in the preservation of cartilage integrity | [44,46,52] |
2.4. Homocysteine
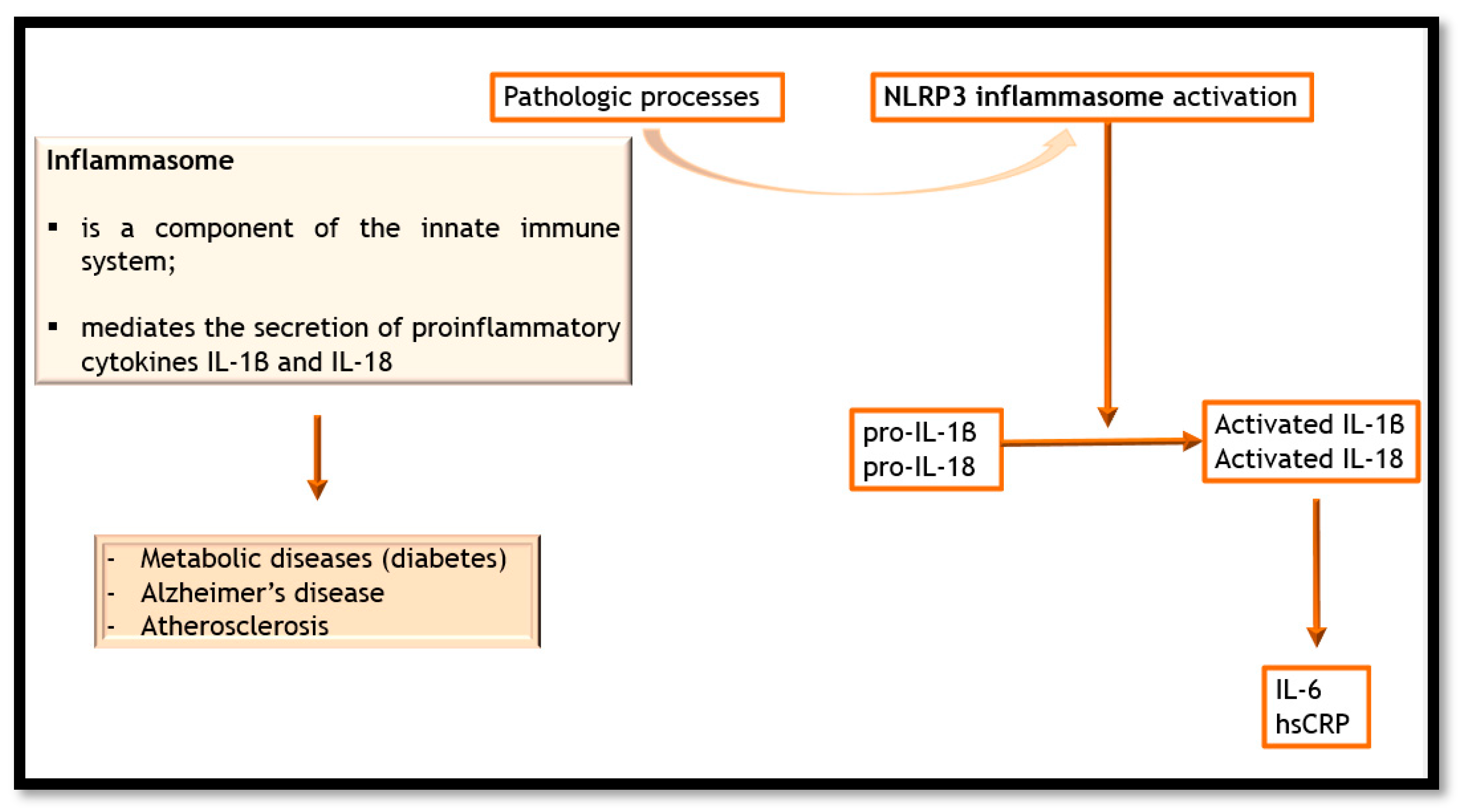
3. Inflammation and Atherosclerosis
4. Assessing Cardiovascular Risk in RA
4.1. Biomarkers Predictive of Cardiovascular Risk in RA
4.1.1. Lipid Profile
4.1.2. Homocysteinemia and ADMA
4.1.3. MicroRNAs
4.1.4. Anti-β2-Glycoprotein-1 (anti-β2GPI) IgA Antibodies
4.2. Predictive Imaging Markers of Cardiovascular Risk in RA
4.2.1. cIMT
4.2.2. CAC Scores
5. The Effects of Biological Therapy on Cardiovascular Risk Factors in RA
5.1. Anti-IL-6
5.2. Anti-TNF-α
5.3. Anti-CD20
5.4. Anti-CD80/86
6. Conclusions
- ☐
- RA patients are complex patients requiring a multidisciplinary approach, especially because the interaction between traditional cardiovascular risk factors and disease-specific inflammation increases cardiovascular risk.
- ☐
- RA patient management involves the following:
- ■
- Caution when prescribing medication that contributes to increased cardiovascular risk (e.g., COX-2 inhibitors, glucocorticoids, leflunomide);
- ■
- Management of cardiovascular risk factors (i.e., antihypertensive and hypolipidemic treatment should be administered according to current guidelines);
- ■
- Induction of disease remission and optimal control of systemic inflammation;
- ■
- Quantifying cardiovascular risk and detecting early atherosclerosis;
- ■
- Early implementation of targeted bDMARDs in selected patients.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes f death in 195 countries and territories. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Kerola, A.M.; Rollefstad, S.; Semb, A.G. Atherosclerotic cardiovascular disease in rheumatoid arthritis: Impact of inflammation and antirheumatic treatment. Eur. Cardiol. 2021, 16, e18. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Boavida, J.M.; Capodanno, D.; Crawford, C.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Karger, A.B.; Steffen, B.T.; Nomura, S.O.; Guan, W.; Garg, P.K.; Szklo, M.; Budoff, M.J.; Tsai, M.Y. Associations between homocysteine and vascular calcification incidence, prevalence, and progression in the MESA Cohort. J. Am. Heart Assoc. 2020, 9, e013934. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, Regional and National Burden of RheumaToid Arthritis 1990–2017: A Systematic analysis of The Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Carmona, L.; Wolfe, F.; Vos, T.; Williams, B.; Gabriel, S.; Lassere, M.; Johns, N.; et al. The global burden of rheumatoid arthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1316–1322. [Google Scholar] [CrossRef]
- Van der Woude, D.; van der Helm-van Mil, A.H.M. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 174–187. [Google Scholar] [CrossRef]
- Hansildaar, R.; Vedder, D.; Baniaamam, M.; Tausche, A.K.; Gerritsn, M.; Nurmohamed, M.T. Cardiovascular risk in inflammatory arthritis: Rheumatoid arthritis and gout. Lancet Rheumatol. 2020, 3, E58–E70. [Google Scholar] [CrossRef]
- Crowson, C.S.; Rollefstad, S.; Ikdahl, E.; Kitas, G.D.; van Riel, P.L.C.M.; Gabriel, S.E.; Matteson, E.L.; Kvien, T.K.; Douglas, K.; Sandoo, A.; et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 48–54. [Google Scholar] [CrossRef]
- Schieir, O.; Toseviski, C.; Glazier, R.H.; Hogg-Johnson, S.; Badley, E.M. Incident myocardial infarction associated with major types of arthritis in the general population: A systematic review and meta-analysis. Ann. Rheum. Dis. 2017, 76, 1396–1404. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.; Thomas, J.; Sadatsafavi, M.; Lehman, A.; Lacaille, D. Risk of incident Cardiovascular Events in Patients with rheumatoid Arthritis: A Meta-analysis of Observational Studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef]
- Hannawi, S.; Haluska, B.; Marwick, T.H.; Thomas, R. Atherosclerotic disease is increased in recent-onset rheumatoid arthritis: A critical role for inflammation. Arthritis Res. Ther. 2007, 9, R116. [Google Scholar] [CrossRef]
- Houge, I.S.; Hoff, M.; Thomas, R.; Videm, V. Mortality is increased in patients with rheumatoid arthritis or diabetes compared to the general population—The Nord-Trondelag health study. Sci. Rep. 2020, 10, 3593. [Google Scholar] [CrossRef]
- Van der Hoek, J.; Boshuizen, H.; Roorda, L.; Tijhuis, G.; Nurmohamed, M.; van den Bos, G.; Dekker, J. Mortality in patients with rheumatoid arthritis: A 15-year prospective cohort study. Rheumatol. Int. 2016, 37, 487–493. [Google Scholar] [CrossRef]
- Myasoedova, E.; Gabriel, S.E.; Matteson, E.L.; Davis, J.M., 3rd; Therneau, T.M.; Crowson, C.S. Decreased cardiovascular mortality in patients with incident rheumatoid arthtritis (RA) in recent years: Dawn of a new era in cardiovascular disease in RA? J. Rheumatol. 2017, 44, 732–739. [Google Scholar] [CrossRef]
- Hitchon, C.A.; Boire, G.; Haraoui, B.; Keystone, E.; Pope, J.; Jamal, S.; Tin, D.; Throne, C.; Bykerk, V.P. Self-reported comorbidity is common in early inflammatory arthritis and associated with poorer function and worse arthritis disease outcomes: Results from the Canadian Early Arthritis Cohort. Rheumatology 2016, 55, 1751–1762. [Google Scholar] [CrossRef]
- Chen, C.I.; Wang, L.; Wei, W.; Yuce, H.; Phillips, K. Burden of rheumatoid arthritis among US Medicare population: Co-morbidities, health-care resource utilization and costs. Rheumatol. Adv. Pract. 2018, 2, rky005. [Google Scholar] [CrossRef]
- Ramos, A.L.; Redeker, I.; Hoffmann, F.; Callhoff, J.; Zink, A.; Albrecht, K. Comorbidities in patients with rheumatoid arthritis and the association with patient-reported outcomes: Results of claims data linked to questionnaire survey. J. Rheumatol. 2019, 46, 564–571. [Google Scholar] [CrossRef]
- Panoulas, V.F.; Douglas, K.M.J.; Smith, J.P.; Stavropoulos-Kalinoglou, A.; Metsios, G.S.; Nightingale, P.; Kitas, G.D. Transforming growth factor-β1 869T/C, but not interleukin-6-174G/C, polymorphism associates with hypertension in rheumatoid arthritis. Rheumatology 2009, 48, 113–118. [Google Scholar] [CrossRef]
- Jagpal, A.; Navarro-Millan, I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: A narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol. 2018, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Hadwen, B.; Stranges, S.; Barra, L. Risk factors for hypertension in rheumatoid arthritis patients: A systematic review. Autoimmune Dis. 2021, 20, 102786. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, S.M.A.; Hannawi, H.; Al Salmi, I. Cardiovascular risk in rheumatoid arthritis: Literature review. Oman Med. J. 2021, 36, e262. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Sauer, B.; Teng, C.C.; Michael, G.; Cannon, G.W.; Said, I.; Cannella, A.; Bryant, R.; Michaud, K.; Caplan, L.; et al. Initiation of disease-modifying therapies in rheumatoid arthtritis is associated with changes in blood pressure. J. Clin. Rheumatol. 2018, 24, 203–209. [Google Scholar] [CrossRef]
- Seong-Kyu, K.; Sang Gyu, K.; Jung-Yoon, C. Association between biological disease modifying anti-rheumatic drugs and incident hypertension in patients with rheumatoid arthritis: Results from prospective nationwide KOBIO Registry. Medicine 2020, 99, e19415. [Google Scholar] [CrossRef]
- Patrick, D.M.; Van Beusecum, J.P.; Kirabo, A. The role of inflammation in hypertension: Novel concepts. Curr. Opin. Physiol. 2021, 19, 92–98. [Google Scholar] [CrossRef]
- Wenzel, U.O.; Ehmke, H.; Bode, M. Immune mechanisms in arterial hypertension. Recent advances. Cell Tissue Res. 2021, 385, 393–404. [Google Scholar] [CrossRef]
- Baghdadi, L.R.; Woodman, R.J.; Shanahan, E.M.; Mangoni, A.A. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0117952. [Google Scholar] [CrossRef]
- Yu, Z.; Kim, S.C.; Vanni, K.; Huang, J.; Desai, R.; Murphy, S.N.; Solomon, D.H.; Liao, K.P. Association between inflammation and systolic blood pressure in RA compared to patients without RA. Arthritis Res. Ther. 2018, 20, 107. [Google Scholar] [CrossRef]
- Kypreos, K.E.; Bitzur, R.; Karavia, E.A.; Xepapadaki, E.; Panayiotakopoulos, G.; Constantinou, C. Pharmacological management of dyslipidemia in atherosclerosis: Limitations, challenges and therapeutic opportunities. Angiology 2018, 70, 1–13. [Google Scholar] [CrossRef]
- London, M.G.; Muriden, K.D.; Hewitt, J.V. Serum cholesterol in rheumatic diseases. Br. Med. J. 1963, 1, 1380–1383. [Google Scholar] [CrossRef]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef]
- Liao, K.P.; Liu, J.; Lu, B.; Solomon, D.H.; Kim, S.C. Associations between lipid levels and major adverse cardiovascular events in rheumatoid arthritis compared to non-rheumatoid arthritis patients. Arthritis Rheumatol. 2015, 67, 2004–2010. [Google Scholar] [CrossRef]
- Rodriguez-Carrio, J.; Alperi-Lopez, M.; Lopez, P.; Lopez-Mejias, R.; Alonso-Castro, S.; Abal, F.; Ballina-Garcia, F.J.; Gonzalez-Gay, M.A.; Suarez, A. High triglycerides and low HDL-c lipid profile in rheumatoid arthritis: A potential link among inflammation, oxidative status and dysfunctional HDL. J. Clin. Lipidol. 2017, 11, 1043–1054. [Google Scholar] [CrossRef]
- Venetsanopoulou, A.I.; Pelechas, E.; Voulgari, P.V.; Drosos, A.A. The lipid paradox in rheumatoid arthritis: The dark horse of the augmented cardiovascular risk. Rheumatol. Int. 2020, 40, 1181–1191. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Lee, Y.Y.; Grijalva, V.; Amjadi, S.; FitzGerald, J.; Ranganath, V.K.; Taylor, M.; McMahon, M.; Paulus, H.E.; Reddy, S.T. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2012, 71, 1157–1162. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Henning, R.J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: A review of the pathophysiology and treatment of obesity. Am. J. Cardiovasc. Dis. 2021, 11, 504–529. [Google Scholar]
- Lu, B.; Hiraki, L.T.; Sparks, J.A.; Malspeis, S.; Chen, C.-Y.; Awosogba, J.A.; Arkema, E.V.; Costenbader, K.H.; Karlson, E.W. Being overweight or obese and risk of developing rheumatoid arthritis among women: A prospective cohort study. Ann. Rheum. Dis. 2014, 73, 1914–1922. [Google Scholar] [CrossRef]
- Brandao de Resende Guimaraes, M.F.; Rodrigues, C.E.M.; Gomes, K.W.P.; Machado, C.J.; Brenol, C.V.; Krampe, S.F.; Bueno de Andrade, N.P.; Kakehasi, A.M. High prevalence of obesity in rheumatoid arthritis patients: Association with disease activity, hypertension, dyslipidemia and diabetes, a multi-center study. Adv. Rheumatol. 2019, 59, 1–9. [Google Scholar] [CrossRef]
- Wolfe, F.; Michaud, K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res. 2012, 64, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; McInnes, I.B. Debunking the obesity-mortality paradox in RA. Nat. Rev. Rheumatol. 2015, 11, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.F.; Billing, E.; Michaud, K.; Ibrahim, S.; Caplan, L.; Cannon, G.W.; Stokes, A.; Majithia, V.; Mikuls, T.R. Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol. 2015, 67, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Hassali, R.; Ohl, S.; Lange, U.; Frommer, K.W.; Muller-Ladner, U. Adipokines and autoimmunity in inflammatory arthritis. Cells 2021, 10, 216. [Google Scholar] [CrossRef]
- Lei, Y.; Li, X.; Gao, Z.; Liu, Y.; Zhang, B.; Xia, L.; Lu, J.; Shen, H. Association Between Adiponectin and Clinical Manifestations in Rheumatoid Arthritis. J. Interferon Cytokine Res. 2020, 40, 501–508. [Google Scholar] [CrossRef]
- Carrion, M.; Frommer, K.W.; Perez-Garcia, S.; Muller-Ladner, U.; Gomariz, R.P.; Neumann, E. The Adipokine Network in Rheumatic Joint Diseases. Int. J. Mol. Sci. 2019, 20, 4091. [Google Scholar] [CrossRef]
- Batun-Garrido, J.A.J.; Salas-Magana, M.; Juarez-Rojop, I.E. Association between leptin and IL-6 concentrations with cardiovascular risk in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 631–637. [Google Scholar] [CrossRef]
- Fatel, E.C.S.; Rosa, F.T.; Simao, A.N.C.; Dichi, I. Adipokines in rheumatoid arthritis. Adv. Rheumatol. 2018, 58, 25. [Google Scholar] [CrossRef]
- Robinson, C.; Tsang, L.; Solomon, A.; Woodiwiss, A.J.; Gunter, S.; Mer, M.; Hsu, H.C.; Gomes, M.; Norton, G.R.; Millen, A.M.E.; et al. Nesfatin-1 and visfatin expression is associated with reduced atherosclerotic disease risk in patients with rheumatoid arthritis. Peptides 2018, 102, 31–37. [Google Scholar] [CrossRef]
- Senolt, L.; Housa, D.; Vernerova, Z.; Jirasek, T.; Svobodova, R.; Veigl, D.; Anderlova, K.; Muller-Ladner, U.; Pavelka, K.; Haluzik, M. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann. Rheum. Dis. 2007, 66, 458–463. [Google Scholar] [CrossRef]
- Frommer, K.W.; Vasile, M.; Muller-Ladner, U.; Neumann, E. The Adipokine Omentin in Late-stage Rheumatoid Arthritis and Endstage Osteoarthritis. J. Rheumatol. 2017, 44, 539–541. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Shi, J.; Zhang, L.; Li, J.; Chen, S.; Wu, C.; Shen, B. Serum progranulin irrelated with Breg cell levels, but elevated in RA patients, reflecting high disease activity. Rheumatol. Int. 2016, 36, 359–364. [Google Scholar] [CrossRef]
- Zhang, S.; Bai, Y.Y.; Luo, L.M.; Xiao, W.K.; Wu, H.M.; Ye, P. Association between serum homocysteine and arterial stiffness in elderly: A community-based study. J. Geriatr. Cardiol. 2014, 11, 32–38. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Kim, B.J.; Lim, B.S.; Kang, J.H. Plasma homocysteine and coronary artery calcification in Korean men. Eur. J. Prev. Cardiol. 2015, 22, 478–485. [Google Scholar] [CrossRef]
- Roubenoff, R.; Dellaripa, P.; Nadeau, M.R.; Abad, L.W.; Muldoon, B.A.; Selhub, J.; Rosenberg, I.H. Abnormal homocysteine metabolism in rheumatoid arthritis. Arthritis Rheum. 1997, 40, 718–722. [Google Scholar] [CrossRef]
- Tekaya, R.; Rouached, L.; Ahmed, H.B.; Tekaya, A.B.; Bouden, S.; Saidane, O.; Bouzid, K.; Mahnoud, I.; Abdelmoula, L. Variation of homocysteine levels in rheumatoid arthritis patients: Relationship to inflammation, cardiovascular risk factors, and mehotrexate. Z. Rheumatol. 2021, 82, 38–43. [Google Scholar] [CrossRef]
- Katsushima, M.; Minamino, H.; Shirakashi, M.; Onishi, A.; Fujita, Y.; Yamamoto, W.; Onizawa, H.; Tsuji, H.; Watanabe, R.; Murakami, K.; et al. High plasma homocysteine level is associated with increased prevalence of non-remission state in rheumatoid arthritis: Findings from the KURAMA cohort. Mod. Rheumatol. 2022, roac106. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Bisogno, S.; Galeazzi, M.; Marcolongo, R.; Pasini, F.L. Reduction in plasma homocysteine level in patients with rheumatoid arthritis given pulsed glucocorticoid treatment. Ann. Rheum. Dis. 2003, 63, 694–695. [Google Scholar] [CrossRef]
- Haagsma, C.J.; Blom, H.J.; van Riel, P.L.C.M.; van’t Hof, M.A.; Giesendorf, B.A.J.; van Oppenraaij-Emmerzaal, D.; van de Putte, L.B.A. Influence of sulphasalazine, methotrexate, and the combination of both on plasma homocysteine concentrations in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1999, 58, 79–84. [Google Scholar] [CrossRef]
- Libby, P. History of discovery: Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Gao, Y.; Galis, Z.S. Exploring the role of endothelial cell resilience in cardiovascular health and disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 179–185. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 2019, 40, 20–33. [Google Scholar] [CrossRef]
- Furmanik, M.; van Gorp, R.; Whitehead, M.; Ahmad, S.; Bordoloi, J.; Kapustin, A.; Schurgers, L.J.; Shanahan, C.M. Endoplasmic reticulum stress mediates vascular smooth muscle cell calcification via increased release of Grp78 (glucose-regulated protein, 78kDa)-loaded extracellular vesicles. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 898–914. [Google Scholar] [CrossRef]
- Dobnikar, L.; Taylor, A.L.; Chappell, J.; Oldach, P.; Harman, J.L.; Oerton, E.; Dzierzak, E.; Bennett, M.R.; Spivakov, M.; Jorgensen, H.F. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat. Commun. 2018, 9, 4567. [Google Scholar] [CrossRef]
- Kaur, H.; Carvalho, J.; Looso, M.; Singh, P.; Chennupati, R.; Preussner, J.; Gunther, S.; Albarran-Juarez, J.; Tischner, D.; Classen, S.; et al. Single-cell profiling reveals heterogeneity and functional patterning of GPCR expression in the vascular system. Nat. Commun. 2017, 8, 15700. [Google Scholar] [CrossRef]
- Hong, X.; Margariti, A.; Le Bras, A.; Jacquet, L.; Kong, W.; Hu, Y.; Xu, Q. Transdifferentiated human vascular smooth muscle cells are a new potential cell source for endothelial regeneration. Sci. Rep. 2017, 7, 5590. [Google Scholar] [CrossRef]
- Pan, H.; Xue, C.; Auerbach, B.J.; Fan, J.; Bashore, A.C.; Cui, J.; Yang, D.Y.; Trignano, S.B.; Liu, W.; Shi, J. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 2020, 142, 2060–2075. [Google Scholar] [CrossRef]
- Back, M.; Yurdagul, A.; Tabas, I.; Oorni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–405. [Google Scholar] [CrossRef]
- Ley, K. Role of the adaptive immune system in atherosclerosis. Biochem. Soc. Trans. 2020, 48, 2273–2281. [Google Scholar] [CrossRef]
- Winkels, H.; Wolf, D. Heterogeneity of T cells in atherosclerosis defined by single-cell RNA-sequencing and citometry by time of flight. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 549–563. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFayden, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; Mac Fayden, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tollefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damas, J.K.; et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Huse, C.; Anstensrud, A.K.; Michelsen, A.E.; Ueland, T.; Broch, K.; Woxholt, S.; Yang, K.; Sharma, K.; Tollefsen, I.M.; Bendz, B.; et al. Interleukin-6 inhibition in ST-elevation myocardial infarction: Immune cell profile in the randomised ASSAIL-MI trial. Lancet 2022, 80, 104013. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Post, W.S.; Blumenthal, R.S.; Polak, J.; Petri, M.; Gelber, A.C.; Szklo, M.; Bathon, J.M. Longitudinal predictors of progression of carotid atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2011, 63, 3216–3225. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Gonzalez-Juanatey, C.; Lopez-Diaz, M.J.; Pineiro, A.; Garcia-Porrua, C.; Miranda-Filloy, J.A.; Ollier, W.E.R.; Martin, J.; Llorca, J. HLA-DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2007, 57, 125–132. [Google Scholar] [CrossRef]
- Lopez-Mejias, R.; Genre, F.; Remuzgo-Martinez, S.; Robustillo-Villarino, M.; Garcia-Bermudez, M.; Llorca, J.; Corrales, A.; Gonzalez-Juanatey, C.; Ubilla, B.; Miranda-Filloy, J.A.; et al. Protective role of the interleukin 33 rs3939286 gene polymorphism in the development of subclinical atherosclerosis in rheumatoid arthritis patients. PLoS ONE 2015, 10, e0143153. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bermudez, M.; Lopez-Mejias, R.; Genre, F.; Castaneda, S.; Llorca, J.; Gonzalez-Juanatey, C.; Corrales, A.; Ubilla, B.; Miranda-Filloy, J.A.; Pina, T.; et al. Interferon regulatory factor 5 genetic variants are associated with cardiovascular disease in patients with rheumatoid arthritis. Arthritis Res. Ther. 2014, 16, R146. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Su, D.; Li, Z.; Li, X.; Chen, Y.; Zhang, Y.; Ding, D.; Deng, X.; Xia, M.; Qiu, J.; Ling, W. Association between serum interleukin-6 concentration and mortality in patients with coronary artery disease. Mediat. Inflamm. 2013, 2013, 726178. [Google Scholar] [CrossRef]
- Navarro-Millan, I.; Yang, S.; DuVall, S.L.; Baddley, J.; Cannon, G.W.; Delzell, E.S.; Zhang, J.; Safford, M.M.; Patkar, N.M.; Mikuls, T.R.; et al. Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: Data from the National Veterans Health Administration. Ann. Rheum. Dis. 2015, 75, 341–347. [Google Scholar] [CrossRef]
- Spinelli, F.R.; Pecani, A.; Conti, F.; Mancini, R.; Alessandri, C.; Valesini, G. Post-translational modifications in rheumatoid arthritis and atherosclerosis: Focus on citrullination and carbamylation. J. Int. Med. Res. 2016, 44 (Suppl. 1), 81–84. [Google Scholar] [CrossRef]
- Carbone, F.; Bonaventura, A.; Liberale, L.; Paolino, S.; Torre, F.; Dallegri, F.; Montecucco, F.; Cutolo, M. Atherosclerosis in rheumatoid arthritis: Promoters and opponents. Clin. Rev. Allergy Immunol. 2020, 58, 1–14. [Google Scholar] [CrossRef]
- Majka, D.S.; Vu, T.H.T.; Pope, R.M.; Teodorescu, M.; Karlson, E.W.; Liu, K.; Chang, R.W. Associations of rheumatoid factors with subclinical and clinical atherosclerosis in african american women: The multiethnic study of atherosclerosis. Arthritis Care Res. 2017, 69, 166–174. [Google Scholar] [CrossRef]
- Shi, J.; van de Stadt, L.A.; Nivine Levarht, E.W.; Huizinga, T.W.J.; Toes, R.E.M.; Trouw, L.A.; van Schaardenburg, D. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum. 2013, 65, 911–915. [Google Scholar] [CrossRef]
- Holzer, M.; Gauster, M.; Pfeifer, T.; Wadsack, C.; Fauler, G.; Stiegler, P.; Koefeler, H.; Beubler, E.; Schuligoi, R.; Heinemann, A.; et al. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid. Redox Signal. 2011, 14, 2337–2346. [Google Scholar] [CrossRef]
- Speer, T.; Owala, F.O.; Holy, E.W.; Zewinger, S.; Frenzel, F.L.; Stahli, B.E.; Razavi, M.; Triem, S.; Cvija, H.; Rohrer, L.; et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur. Heart J. 2014, 35, 3021–3032. [Google Scholar] [CrossRef]
- Spinelli, F.R.; Pecani, A.; Ciciarello, F.; Colasanti, T.; Di Franco, M.; Miranda, F.; Conti, F.; Valesini, G.; Alessandri, C. Associations between antibodies to carbamylated protein and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2017, 18, 214. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Wilson Tang, W.H.; Hazen, S.L. Protein carbamylated and cardiovascular disease. Kidney Int. 2015, 88, 474–478. [Google Scholar] [CrossRef]
- Giles, J.T.; Wasko, M.C.M.; Chung, C.; Szklo, M.; Blumenthal, R.S.; Kao, A.; Bokhari, S.; Zartoshti, A.; Stein, C.M.; Bathon, J.M. Exploring the lipid paradox theory in rheumatoid arthritis: Associations of low circulating low-density lipoprotein concentration with subclinical coronary atherosclerosis. Arthritis Rheumatol. 2019, 71, 1426–1436. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Sun, Y.; Chien, K.L.; Hsu, H.C.; Su, T.C.; Chen, M.F.; Lee, Y.T. Use of serum homocysteine to predict stroke, coronary heart disease and death in ethnic Chinese. 12-years prospective cohort study. Circ. J. 2009, 73, 1423–1430. [Google Scholar] [CrossRef]
- Martin-Herrero, F.; Martin-Moreiras, J.; Pabon, P.; Sanchez, P.L.; Morinigo-Munoz, J.L.; Jimenez-Candil, J.; Cruz-Gonzalez, I.; Alberca, I.; Gonzalez-Porras, J.R.; Martin-Luengo, C. Homocysteine and outcome in young patients with acute coronary syndromes. Int. J. Cardiol. 2007, 118, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, L.; Liu, Y.S.; Zheng, M.Q.; Ma, F.F.; Qi, Y.C.; Liu, G. Homocysteine as a potential predictive factor for high major adverse cardiovascular events risk in female patients with premature acute coronary syndrome. Medicine 2019, 98, e18019. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Freitag, D.F.; Laukkanen, J.A.; Chowdhury, S.; Gobin, R.; Mayr, M.; Di Angelantonio, E.; Chowdhury, R. Asymmetric dimethylarginine and cardiovascular risk: Systematic review and meta-analysis of 22 prospective studies. J. Am. Heart Assoc. 2015, 5, e001833. [Google Scholar] [CrossRef] [PubMed]
- Balkarli, A.; Tekinturk, S.; Kaptanoglu, B.; Cobankara, V. Relationship between plasma levels of homocysteine and proinflammatory cytokines in patients with rheumatoid arthritis. J. Clin. Exp. Investig. 2016, 7, 163–167. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, L.; Du, L.; Zhang, T.; Xin, W.; Lan, X.; Du, G. Association of circulating levels of asymmetric dimethylarginine (ADMA) with carotid intima-media thickness: Evidence from 6168 participants. Ageing Res. Rev. 2013, 12, 699–707. [Google Scholar] [CrossRef]
- Moroni, L.; Selmi, C.; Angelini, C.; Meroni, P.L. Evaluation of endothelial function by flow-mediated dilation: A comprehensive review in rheumatic disease. Arch. Immunol. Ther. Exp. 2017, 65, 463–475. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Hodson, J.; Sandoo, A.; Smith, J.; Kitas, G.D. Endothelial injury in rheumatoid arthritis: A crosstalk between dimethylarginines and systemic inflammation. Arthritis Res. Ther. 2017, 19, 32. [Google Scholar] [CrossRef]
- Senturk, T.; Yılmaz, N.; Sargın, G.; Koseoglu, K.; Yenisey, C. Relationship between asymmetric dimethylarginine and endothelial dysfunction in patients with rheumatoid arthritis. Eur. J. Rheumatol. 2016, 3, 106–108. [Google Scholar] [CrossRef]
- Feinberg, M.W.; Moore, K.J. MicroRNA regulation of atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef]
- Staszel, T.; Zapala, B.; Polus, A.; Sadakierska-Chudy, A.; Kiec-Wilk, B.; Stepien, E.; Wybranska, I.; Chojnacka, M.; Dembinska-Kiec, A. Role of microRNAs in endothelial cell pathophysiology. Pol. Arch. Int. Med. 2011, 121, 361–366. [Google Scholar] [CrossRef]
- Mir, R.; Elfaki, I.; Khallar, N.; Waza, A.A.; Jha, C.; Mir, M.M.; Nisa, S.; Mohammad, B.; Mir, T.A.; Maqbool, M.; et al. Role of selected miRNAs as diagnostic and prognostic biomarkers in cardiovascular diseases, including coronary artery disease, myocardial infarction and atherosclerosis. J. Cardiovasc. Dev. Dis. 2021, 8, 22. [Google Scholar] [CrossRef]
- Sharma, A.R.; Sharma, G.; Bhattacharya, M.; Lee, S.S.; Chakraborty, C. Circulating miRNA in atherosclerosis: A clinical biomarker and early diagnostic tool. Curr. Mol. Med. 2022, 22, 250–262. [Google Scholar] [CrossRef]
- Tabaei, S.; Tabaee, S.S. Implications for microRNA involvement in the prognostic and treatment of atherosclerosis. Mol. Cell. Biochem. 2021, 476, 1327–1336. [Google Scholar] [CrossRef]
- Tavasolian, F.; Abdollahi, E.; Rezaei, R.; Momtazi-Borojeni, A.A.; Henrotin, Y.; Sahebkar, A. Altered expression of microRNAs in rheumatoid arthritis. J. Cell. Biochem. 2018, 119, 478–487. [Google Scholar] [CrossRef]
- Evangelatos, G.; Fragoulis, G.E.; Koulouri, V.; Lambrou, G.I. MicroRNAs in Rheumatoid Arthritis: From Pathogenesis to Clinical Impact. Autoimmun. Rev. 2019, 18, 102391. [Google Scholar] [CrossRef]
- Renman, E.; Brink, M.; Arlestig, L.; Rantapaa-Dahlqvist, S.; Lejon, K. Dysregulated microRNA expression in rheumatoid arthritis families-a comparison between rheumatoid arthritis patients, their first-degree relatives, and healthy controls. Clin. Rheumatol. 2021, 40, 2387–2394. [Google Scholar] [CrossRef]
- Bae, S.C.; Lee, Y.H. MiR-146a levels in rheumatoid arthritis and their correlation with disease activity: A meta-analysis. Int. J. Rheum. Dis. 2018, 21, 1335–1342. [Google Scholar] [CrossRef]
- Ciesla, M.; Kolarz, B.; Majdan, M.; Dryglewska, M. The Value of MIR-20B, MIR-22, MIR-26A, MIR-125B and MIR-221 in Rheumatoid Arthritis. Ann. Rheum. Dis. 2021, 80, 309–310. [Google Scholar] [CrossRef]
- Smigielska-Czepiel, K.; Van Den Berg, A.; Jellema, P.; Van Der Lei, R.J.; Bijzet, J.; Kluiver, J.; Boots, A.M.H.; Brouwer, E.; Kroesen, B.J. Comprehensive analysis of miRNA expression in T-cell subsets of rheumatoid arthritis patients reveals defined signatures of naive and memory Tregs. Genes Immun. 2014, 15, 115–125. [Google Scholar] [CrossRef]
- Taverner, D.; Llop, D.; Rosales, R.; Ferre, R.; Masana, L.; Vallve, J.C.; Paredes, S. Plasma expression of microRNA-425-5p and microRNA-451a as biomarkers of cardiovascular disease in rheumatoid arthritis patients. Sci. Rep. 2021, 11, 15670. [Google Scholar] [CrossRef]
- Paredes, S.; Taverner, D.; Ferre, R.; Alegret, J.M.; Masana, L.; Vallve, J.C. MicroRNA differential expression shared between rheumatoid arthritis and acute myocardial infarction: An exploratory study. Clin. Exp. Rheumatol. 2019, 37, 886–887. [Google Scholar] [PubMed]
- Ormseth, M.J.; Solus, J.F.; Sheng, Q.; Chen, S.C.; Ye, F.; Wu, Q.; Oeser, A.M.; Allen, R.; Raggi, P.; Vickers, K.C.; et al. Plasma miRNAs improve the prediction of coronary atherosclerosis in patients with rheumatoid arthritis. Clin. Rheumatol. 2021, 40, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, E.; Kobayashi, K.; Inoue, K.; Lopez, L.R.; Shoenfeld, Y. Oxidized LDL/β2-glycoprotein I complexes: New aspects in atherosclerosis. Lupus 2005, 14, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Matsuura, E.; Liu, Q.; Furukawa, J.; Kaihara, K.; Inagaki, J.; Atsumi, T.; Sakairi, N.; Yasuda, T.; Voelker, D.R.; et al. A specific ligand for beta(2)-glycoprotein I mediates autoantibody-dependent uptake of oxidized low density lipoprotein by macrophages. J. Lipid Res. 2001, 42, 697–709. [Google Scholar] [CrossRef]
- Lopez, L.R.; Hurley, B.L.; Simpson, D.F.; Matsuura, E. Oxidized low-density lipoprotein/ β2-glycoprotein I complexes and autoantibodies in patients with type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2005, 1051, 97–103. [Google Scholar] [CrossRef]
- Kasahara, J.; Kobayashi, K.; Maeshima, Y.; Yamasaki, Y.; Yasuda, T.; Matsuura, E.; Makino, H. Clinical significance of serum oxidized low-density lipoprotein/ β2-glycoprotein I complexes in patients with chronic renal diseases. Nephron Clin. Pract. 2004, 98, 15–24. [Google Scholar] [CrossRef]
- Staub, H.L.; Franck, M.; Ranzolin, A.; Norman, G.L.; Iverson, G.M.; von Muhler, C.A. IgA antibodies to beta2-glycoprotein I and atherosclerosis. Autoimmun. Rev. 2006, 6, 104–106. [Google Scholar] [CrossRef]
- Pahor, A.; Hojs, R.; Holc, I.; Ambrozic, A.; Cucnik, S.; Kveder, T.; Rozman, B. Antiphospholipid antibodies as a possible risk factor for atherosclerosis in patients with rheumatoid arthritis. Immunobiology 2006, 211, 689–694. [Google Scholar] [CrossRef]
- Pereira, I.; Laurindo, I.; Burlingame, R.; Anjos, L.; Viana, V.; Leon, E.; Vendramini, M.; Borba, E. Auto-antibodies do not influence development of atherosclerosis plaques in rheumatoid arthritis. Jt. Bone Spine 2008, 75, 416–421. [Google Scholar] [CrossRef]
- Holc, I.; Hojs, R.; Cikes, N.; Ambrozic, A.; Cucnik, S.; Kveder, T.; Rozman, B.; Pahor, A. Antiphospholipid antibodies and atherosclerosis: Insights from rheumatoid arthritis—A five-years follow-up study. Immunobiology 2011, 216, 1331–1337. [Google Scholar] [CrossRef]
- Karpouzas, G.A.; Ormseth, S.R.; Hernandez, E.; Bui, V.L.; Budoff, M.J. Beta-2-glycoprotein-I IgA antibodies predict coronary plaque progression in rheumatoid arthritis. Semin. Arthritis Rheum. 2011, 51, 20–27. [Google Scholar] [CrossRef]
- Hannawi, S.; Hannawi, H.; Alokaily, F.; Salmi, I. Variables associated with Subclinical Atherosclerosis Among rheumatoid Arthritis Patients of Gulf Cooperative Council Countries. Saudi Med. J. 2020, 41, 128–137. [Google Scholar] [CrossRef]
- Hannawi, S.; Hannawi, H.; Al Salmi, I. Cardiovascular Disease and Subclinical Atherosclerosis in rheumatoid Arthritis. Hypertens. Res. 2020, 43, 982–984. [Google Scholar] [CrossRef]
- Sedrakyan, S.; Fatima, T.; Khatun, M.; Awan, M.; Okam, N.; Jahan, N. Evaluation of The Risk of Getting Peripheral Artery Disease in Rheumatoid Arthritis and The Selection of Appropriate Diagnostic Methods. Cureus 2020, 12, e9782. [Google Scholar] [CrossRef]
- Tehan, P.; Stewart, S.; Chuter, V.; Carroll, M.; Rutherfurd, K.; Brenton-Rule, A. Relationship Between Lower Limb Vascular Characteristics, Peripheral Arterial Disease and Gait in Rheumatoid Arthritis. Int. J. Rheum. Dis. 2019, 22, 2017–2024. [Google Scholar] [CrossRef]
- Gonzalez-Juanatey, C.; Llorca, J.; Martin, J.; Gonzalez-Gay, M.A. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 2009, 38, 366–371. [Google Scholar] [CrossRef]
- Evans, M.R.; Escalante, A.; Battafarano, D.F.; Freeman, G.L.; O’Leary, D.H.; del Rincon, I. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011, 63, 1211–1220. [Google Scholar] [CrossRef]
- Ajeganova, S.; de Faire, U.; Jogestrand, T.; Frostegard, J.; Hafstrom, I. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis—An inception cohort study. Rheumatology 2012, 39, 1146–1154. [Google Scholar] [CrossRef]
- Okazaki, S.; Sakaguchi, M.; Miwa, K.; Furukado, S.; Yamagami, H.; Yagita, Y.; Mochizuki, H.; Kitagawa. K. Association of interleukin-6 with the progression of carotid atherosclerosis. A 9-year follow-up study. Stroke 2014, 45, 2924–2929. [Google Scholar] [CrossRef]
- Kaseem, E.; Ghonimy, R.; Adel, M.; El-Sharnoby, G. Non-traditional risk factors of carotid atherosclerosis in rheumatoid arthritis. Egypt Rheumatol. 2011, 33, 113–119. [Google Scholar] [CrossRef]
- Corrales, A.; Gonzalez-Juanatey, C.; Peiro, M.E.; Blanco, R.; Llorca, J.; Gonzalez-Gay, M.A. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann. Rheum. Dis. 2014, 73, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Lupoli, R.; Di Manno, A.; Tasso, M.; Peluso, R.; Di Minno, M.N.D. Subclinical atherosclerosis in patients with rheumatoid arthritis. Thromb. Haemost. 2015, 113, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Wah-Suarez, M.I.; Galarza-Delgado, D.A.; Azpiri-Lopez, J.R.; Colunga-Pedraza, I.J.; Abundis-Marquez, E.E.; Davila-Jimenez, J.A.; Guillen-Gutierrez, C.Y.; Elizondo-Riojas, G. Carotid ultrasound findings in rheumatoid arthritis and control subjects: A case-control study. Int. J. Rheum. Dis. 2019, 22, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Mazario, R.; Fragio Gil, J.J.; Martinez Calabuig, P.; Grau Garcia, E.; Canada Martinez, A.J.; Roman Ivorra, J.A. Cardiovascular risk assessment with carotid ultrasound in rheumatoid arthritis. Med. Clin. 2022, 159, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Vegas-Revenga, N.; Rueda-Gotor, J.; Portilla, V.; Atienza-Mateo, B.; Blanco, R.; Castaneda, S.; Ferraz-Amaro, I.; Llorca, J. Gonzalez-Gay, M.A. Carotid plaques as predictors of cardiovascular events in patients with rheumatoid arthritis. Results from a 5-year-prospective follow-up study. Semin. Arthritis Rheum. 2020, 50, 1333–1338. [Google Scholar] [CrossRef]
- Gerasimova, E.; Popkova, T.; Gerasimova, D.; Nasonov, E. Clinical and diagnostic significance of intima-media thickness levels of soluble cell adhesion molecules and CD40 ligand in rheumatoid arthritis patients with low cardiovascular risk. Ann. Rheum. Dis. 2022, 81, 341. [Google Scholar] [CrossRef]
- Chung, C.P.; Oeser, A.; Raggi, P.; Gebretsadik, T.; Shintani, A.K.; Sokka, T.; Pincus, T.; Avalos, I.; Stein, C.M. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005, 52, 3045–3053. [Google Scholar] [CrossRef]
- Karpouzas, G.A.; Malpeso, J.; Choi, T.Y.; Li, D.; Munoz, S.; Budoff, M.I. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann. Rheum. Dis. 2014, 73, 1797–1804. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.H.; Kim, H.R.; Min, H.K. Cardiovascular disease risk calculators to reflect the subclinical atherosclerosis of coronary artery in rheumatoid arthritis: A pilot study. BMC Rheumatol. 2021, 5, 39. [Google Scholar] [CrossRef]
- Findeisen, K.E.; Sewell, J.; Ostor, A.J.K. Biological therapies for rheumatoid arthritis: An overview for the clinician. Biologics 2021, 15, 343–352. [Google Scholar] [CrossRef]
- Smole, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewe, R.B.M.; Bijlsma, J.W.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Buch, M.H.; Bingham, S.J.; Bryer, D.; Emery, P. Long-term infliximab treatment in rheumatoid arthritis: Subsequent outcome of initial responders. Rheumatology 2007, 46, 1153–1156. [Google Scholar] [CrossRef]
- Raffeiner, B.; Botsios, C.; Sfriso, P.; Ometto, F.; Bernardi, L.; Todesco, S.; Punzi, L. Efficacy of low dose etanercept in maintaining clinical and radiological remission in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 102. [Google Scholar]
- Boyadzieva, V.V.; Stoilov, N.; Stoilov, R.M.; Tachkov, K.; Kamusheva, M.; Mitov, K.; Petrova, G.I. Quality of life and cost study of rheumatoid arthritis therapy with biological medicines. Front. Pharmacol. 2018, 9, 794. [Google Scholar] [CrossRef]
- Smolen, J.S.; Goncalves, J.; Quinn, M.; Benedetti, F.; Lee, J.Y. Era of biosimilars in rheumatology: Reshaping the healthcare environment. RMD Open 2019, 5, e000900. [Google Scholar] [CrossRef]
- Feagan, B.G.; Marabani, M.; Wu, J.J.; Faccin, F.; Spronk, C.; Castaneda-Hernandez, G. The challenges of switching therapies in an evolving multiple biosimilars landscape: A narrative review of current evidence. Adv. Ther. 2020, 37, 4491–4518. [Google Scholar] [CrossRef]
- Schett, G. Physiological effects of modulating the interleukin-6 axis. Rheumatology 2018, 57, 43–50. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Saremi, A.; Anderson, R.J.; Luo, P.; Moritz, T.E.; Schwenke, D.C.; Allison, M.; Reaven, P.D. Association between IL-6 and the extent of coronary atherosclerosis in the veterans affairs diabetes trial (VADT). Atherosclerosis 2009, 203, 610–614. [Google Scholar] [CrossRef]
- McInnes, I.B.; Thompson, L.; Giles, J.T.; Bathon, J.M.; Salmon, J.E.; Beaulieu, A.D.; Codding, C.E.; Carlson, T.H.; Delles, C.; Lee, J.S.; et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis. 2015, 74, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Cacciapaglia, F.; Anelli, M.G.; Rinaldi, A.; Fornaro, M.; Lopalco, G.; Scioscia, C.; Lapadula, G.; Iannone, F. Lipids and atherogenic indices fluctuation in rheumatoid arthritis patients on long-term tocilizumab treatment. Mediat. Inflamm. 2018, 2018, 2453265. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, S.Y.; Kawakami, A.; Yamasaki, S.; Imazato, T.; Iwamoto, N.; Fujikawa, K.; Aramaki, T.; Tamai, M.; Nalamura, H.; Ida, H.; et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol. Int. 2011, 31, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; McInnes, I.B.; Kavanaugh, A.; Tuckwell, K.; Klearman, M.; Pulley, J.; Sattar, N. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Clin. Trial 2016, 75, 1806–1812. [Google Scholar] [CrossRef]
- Greco, D.; Gualtierotti, R.; Agosti, P.; Adorni, M.P.; Ingegnoli, F.; Rota, M.; Bernini, F.; Meroni, P.L.; Ronda, N. Anti-atherogenic modification of serum lipoprotein function in patients with rheumatoid arthritis after tocilizumab treatment, a pilot study. J. Clin. Med. 2020, 9, 2157. [Google Scholar] [CrossRef]
- Garcia-Gomez, C.; Martin-Martinez, M.A.; Castaneda, S.; Sanchez-Alonso, F.; Uriarte-Ecenarro, M.; Gonzalez-Juanatey, C.; Romera-Baures, M.; Santos-Rey, J.; Pinto-Tasende, J.A.; Quesada-Masachs, E. Lipoprotein(a) concentrations in rheumatoid arthritis on biologic therapy: Results from the cardiovascular in rheumatology study project. J. Clin. Lipidol. 2017, 11, 749–756. [Google Scholar] [CrossRef]
- Ruiz-Limon, P.; Ortega, R.; de la Rosa, I.A.; Abalos-Aguilera, M.C.; Perez-Sanchez, C.; Jimenez-Gomez, Y.; Peralbo-Santaella, E.; Font, P.; Ruiz-Vilches, D.; Ferrin, G. Tocilizumab improves the proatherothrombotic profile of rheumatoid arthritis patients modulating endothelial dysfunction, NETosis, and inflammation. Transl. Res. 2017, 183, 87–103. [Google Scholar] [CrossRef]
- Kume, K.; Amano, K.; Yamada, S.; Hatta, K.; Ohta, H.; Kuwaba, N. Tocilizumab monotherapy reduces arterial stiffness as effectively as etanercept or adalimumab monotherapy in rheumatoid arthritis: An open-label randomized controlled trial. J. Rheumatol. 2011, 38, 2169–2171. [Google Scholar] [CrossRef]
- Provan, S.A.; Berg, I.J.; Berner Hammer, H.; Mathiessen, A.; Kvien, T.K.; Semb, A.G. The impact of newer biological disease modifying anti-rheumatic drugs on cardiovascular risk factors: A 12-month longitudinal study in rheumatoid arthritis patients treated with rituximab, abatacept and tocilizumab. PLoS ONE 2015, 10, e0130709. [Google Scholar] [CrossRef]
- Jones, G.; Sebba, A.; Lowenstein, M.B.; Calvo, A.; Gomez-Reino, J.J.; Siri, D.A.; Tomsic, M.; Alecock, E.; Woodworth, T.; Genovese, M.C. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION study. Ann. Rheum. Dis. 2010, 69, 88–96. [Google Scholar] [CrossRef]
- Tournadre, A.; Pereira, B.; Dutheil, F.; Giraud, C.; Courteix, D.; Sapin, V.; Frayssac, T.; Mathieu, S.; Malochet-Guinamand, S.; Soubrier, M. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J. Cachexia Sarcopenia Muscle 2017, 8, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, F.; Yun, H.; Chen, L.; Muntner, P.; Levitan, E.B.; Safford, M.M.; Kent, S.T.; Osterman, M.T.; Lewis, J.D.; et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Singht, S.; Fumery, M.; Singh, A.G.; Singh, N.; Prokop, L.J.; Dulai, P.S.; Sandborn, W.J.; Curtis, J.R. Comparative risk of cardiovascular events with biologic and synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: A systematic review and meta-analysis. Arthritis Care Res. 2020, 72, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Rondon, J.; Parrino, J.; Lin, Y.; Pena-Rossi, C.; van Hoogstraten, H.; Graham, N.M.H.; Liu, N.; Paccaly, A.; Wu, R.; et al. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology 2019, 58, 849–858. [Google Scholar] [CrossRef]
- Burmester, G.R.; Lin, Y.; Patel, R.; van Adelsberg, J.; Mangan, E.K.; Graham, N.M.H.; van Hoogstraten, H.; Bauer, D.; Vargas, J.I.; Lee, E.B. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): A randomised, double-blind, parallel-group phase III trial. Ann. Rheum. Dis. 2017, 76, 840–847. [Google Scholar] [CrossRef]
- Fleischmann, R.; Genovese, M.C.; Lin, Y.; St John, G.; van der Heijde, D.; Wang, S.; Gomez-Reino, J.J.; Maldonado-Cocco, J.A.; Stanislav, M.; Kivitz, A.J.; et al. Long-term safety of sarilumab in rheumatoid arthritis: An integrated analysis with up to 7 years’ follow-up. Rheumatology 2020, 59, 292–302. [Google Scholar] [CrossRef]
- Jang, D.; Lee, A.H.; Shin, H.Y.; Song, Y.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021, 22, 2791. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Pfeffer, M.; Sacks, F.; Braunwald, E. Elevation of tumor necrosis factor-alpha and increased risk of reccurrent coronary events after myocardial infarction. Circulation 2000, 101, 2149–2153. [Google Scholar] [CrossRef]
- Solomon, D.H.; Curtis, J.R.; Saag, K.G.; Lii, J.; Chen, L.; Harrold, L.R.; Herrinton, L.J.; Graham, D.J.; Kowal, M.K.; Kuriya, B.; et al. Cardiovascular risk in rheumatoid arthritis: Comparing TNF-α blockade with nonbiologic DMARDs. Am. J. Med. 2013, 126, 9–17. [Google Scholar] [CrossRef]
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The effects of tumor necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 480–489. [Google Scholar] [CrossRef]
- Low, A.S.L.; Symmons, D.P.M.; Lunt, M.; Mercer, L.K.; Gale, C.P.; Watson, K.D.; Dixon, W.G.; Hyrich, K.L. Relationship between exposure to tumor necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 654–660. [Google Scholar] [CrossRef]
- Lee, J.L.; Sinnthurai, P.; Buchbinder, R.; Hill, C.; Lassere, M.; March, L. Biologics and cardiovascular events in inflammatory arthritis: A prospective national cohort study. Atrhritis Res. Ther. 2018, 20, 171. [Google Scholar] [CrossRef]
- Karpouzas, G.A.; Orsmeth, S.R.; Hernandez, E.; Budoff, M.J. Biologics may prevent cardiovascular events in rheumatoid arthritis by inhibiting coronary plaque formation and stabilizing high-risk lesions. Arthritis Rheumatol. 2020, 72, 1467–1475. [Google Scholar] [CrossRef]
- Ljung, L.; Rantapaa-Dahlqvist, S.; Jacobsson, L.T.H.; Askling, J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 2087–2094. [Google Scholar] [CrossRef]
- Bergstrom, U.; Jovinge, S.; Persson, J.; Jacobsson, L.T.H.; Turesson, C. Effects of treatment with adalimumab on blood lipid levels and atherosclerosis in patients with rheumatoid arthritis. Curr. Ther. Res. Clin. Exp. 2018, 89, 1–6. [Google Scholar] [CrossRef]
- Gabay, C.; Emery, P.; van Vollenhoven, R.; Dikranian, A.; Alten, R.; Pavelka, K.; Klearman, M.; Musselman, D.; Agarwal, S.; Green, J.; et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): A randomised, double-blind, controlled phase 4 trial. Lancet 2013, 381, 1541–1550. [Google Scholar] [CrossRef]
- Gonzalez-Juanatey, C.; Vasquez-Rodriguez, T.R.; Miranda-Filloy, J.A.; Gomez-Acebo, I.; Testa, A.; Garcia-Porrua, C.; Sanchez-Andrade, A.; Llorca, J.; Gonzalez-Gay, M.A. Anti-TNF-alpha-adalimumab therapy is associated with persistent improvement of endothelial function without progression of carotid intima-media wall thickness in patients with rheumatoid arthritis refractory to conventional therapy. Mediat. Inflamm. 2012, 2012, 674265. [Google Scholar] [CrossRef]
- Ronda, N.; Greco, D.; Adorni, M.P.; Zimetti, F.; Favari, E.; Hjeltnes, G.; Mikkelsen, L.; Borghi, M.O.; Favalli, E.G.; Gatti, R.; et al. Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol. 2015, 67, 1155–1164. [Google Scholar] [CrossRef]
- Biseell, L.A.; Hensor, E.M.A.; Kozera, L.; Mackie, S.L.; Burska, A.N.; Nam, J.L.; Keen, H.; Villeneuve, E.; Donica, H.; Buch, M.H. Improvement in insulin resistance is greater when infliximab is added to methotrexate during intensinve treatment of early rheumatoid arthritis-results from the IDEA study. Rheumatology 2016, 55, 2181–2190. [Google Scholar] [CrossRef]
- Popa, C.; van Tits, L.J.H.; Barreta, P.; Lemmers, H.L.M.; van den Hoogen, F.H.J.; van Riel, P.L.C.; Radstake, T.R.D.J.; Netea, M.G.; Roest, M.; Stalenhoef, A.F.H. Anti-inflammatory therapy with tumor necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann. Rheum. Dis. 2009, 68, 868–872. [Google Scholar] [CrossRef]
- Kirkham, B.W.; Wasko, M.C.; Hsia, E.C.; Fleischmann, R.M.; Genovese, M.C.; Matteson, E.L.; Liu, H.; Rahman, M.U. Effects of golimumab, an anti-tumor necrosis factor-α human monoclonal antibody, on lipids and markers of inflammation. Ann. Rheum. Dis. 2014, 73, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Daien, C.I.; Fesler, P.; du Cailar, G.; Daien, V.; Mura, T.; Dupuy, A.M.; Cristol, J.P.; Ribstein, J.; Combe, B.; Morel, J. Etanercept normalises left ventricular mass in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jimenez, N.A.; Garcia-Gonzalez, C.E.; Ayala-Lopez, K.P.; Trujillo-Hernandez, B.; Aguilar-Chavez, E.A.; Rocha-Munoz, A.D.; Vasquez-Jimenez, J.C.; Olivas-Flores, E.; Salazar-Paramao, M.; Corona-Sanchez, E.G.; et al. Modifications in lipid levels are independent of serum TNF-α in rheumatoid arthritis: Results of an observational 24-weeks cohort study comparing patients receiving etanercept plus methotrexate or methotrexate as monotherapy. Biomed Res. Int. 2014, 2014, 510305. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Leporini, C.; Bene, F.; D’Angelo, S.; Mauro, D.; Russo, E.; De Sarro, G.; Olivieri, I.; Pitzalis, C.; Lewis, M.; et al. Anti-TNF-alpha agents and endothelial function in rheumatoid arthritis: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 5346. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, F.; Charakida, M.; Topham, E.; McLoughlin, E.; Patel, N.; Sutill, E.; Kay, C.W.M.; D’Aiuto, F.; Landmesser, U.; Taylor, P.C.; et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart 2017, 103, 766–773. [Google Scholar] [CrossRef]
- Spinelli, F.R.; Metere, A.; Barbati, C.; Pierdominici, M.; Iannuccelli, C.; Lucchino, B.; Ciciarello, F.; Agati, L.; Valesini, G.; Di Franco, M. Effect of therapeutic inhibition of TNF on circulating endothelial progenitor cells in patients with rheumatoid arthritis. Mediat. Inflamm. 2013, 2013, 537539. [Google Scholar] [CrossRef]
- Kume, K.; Amano, K.; Yamada, S.; Hatta, K.; Amano, K.; Ohta, H.; Kuwaba, N. Tocilizumab improves arterial stiffness as well as other biologics with methtrexate-resistant active rheumatoid arthritis—An opel label, randomized cohort multi center study. J. Radiol. 2015, 4, 186. [Google Scholar] [CrossRef]
- Tam, L.S.; Shang, Q.; Li, E.K.; Wang, S.; Li, R.J.; Lee, K.L.; Leung, Y.Y.; Ying, K.Y.; Ym, C.W.; Kun, E.W.; et al. Infliximab is associated with improvement in arterial stiffness in patients with early rheumatoid arthritis—A randomized trial. J. Rheumatol. 2012, 39, 2267–2275. [Google Scholar] [CrossRef]
- Angel, K.; Provan, S.A.; Fagerhol, M.K.; Mowinckel, P.; Kvien, T.; Atar, D. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: A controlled study. Am. J. Hypertens. 2012, 25, 644–650. [Google Scholar] [CrossRef]
- Tam, L.S.; Kitas, G.D.; Gonzalez-Gay, M.A. Can suppression of inflammation by anti-TNF prevent progression of subclinical atherosclerosis in inflammatory arthritis? Rheumatology 2014, 53, 1108–1119. [Google Scholar] [CrossRef]
- Zhao, Q.; Hong, D.; Zhang, Y.; Sang, Y.; Yang, Z.; Zhang, X. Association between anti-TNF therapy for rheumatoid arthritis and hypertension: A meta-analysis of randomized controlled trials. Medicine 2015, 94, 731. [Google Scholar] [CrossRef]
- Giles, J.T.; Sattar, N.; Gabriel, S.; Ridker, P.M.; Gay, S.; Warne, C.; Musselman, D.; Brockwell, L.; Shittu, E.; Klearman, M.; et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: A randomised controlled trial. Arthritis Rheum. 2019, 72, 31–40. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Buch, D.H.; Dougados, M.; Bhatt, D.L.; Giles, J.T.; Ytterberg, S.R.; Koch, G.G.; Vranic, I.; Wu, J.; Wang, C.; et al. Risk of major adverse cardiovascular events with tofacitinib versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: A post hoc analysis from ORAL Surveillance. Ann. Rheum. Dis. 2022, 81, 119–129. [Google Scholar] [CrossRef]
- Hoisnard, L.; Vegas, L.P.; Dray-Spira, R.; Weill, A.; Zureik, M.; Sbidian, E. Risk of major adverse cardiovascular and venous thromboembolism events in patients with rheumatoid arthritis exposed to JAK inhibitors versus adalimumab: A nationwide cohort study. Ann. Rheum. Dis. 2022, 74. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Herbin, O.; Bouaziz, J.D.; Binder, C.J.; Uyttenhove, C.; Laurans, L.; Taleb, S.; Van Vre, E.; Esposito, B.; Vilar, J.; et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010, 207, 1579–1587. [Google Scholar] [CrossRef]
- Tsiantoulas, D.; Diehl, C.J.; Witztum, J.L.; Binder, C.J. B cells and humoral immunity in atherosclerosis. Circ. Res. 2014, 114, 1743–1756. [Google Scholar] [CrossRef]
- Correa, J.E.B.; Cortez, M.A.F.; Uribe, J.A.; Camacho, L.S.R. Comparison of plasma cytokine levels before and after treatment with rituximab in patients with rheumatoid arthritis and systemic lupus erythematosus-associated polyautoimmunity. Univ. Med. 2018, 59, 21–36. [Google Scholar] [CrossRef]
- Srikakulapu, P.; McNamara, C.A. B cells and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, 1060–1067. [Google Scholar] [CrossRef]
- Tsiantoulas, D.; Sage, A.P.; Mallat, Z.; Binder, C.J. Targeting B cells in atherosclerosis: Closing the gap from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 296–302. [Google Scholar] [CrossRef]
- Raterman, H.G.; Levels, H.; Voskuyl, A.E.; Lems, W.F.; Dijkmans, B.A.; Nurmohamed, M.T. HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann. Rheum. Dis. 2013, 72, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Novikova, D.S.; Popkova, T.V.; Lukina, G.V.; Luchikhina, E.L.; Karateev, D.E.; Volkov, A.V.; Novikov, A.A.; Aleksandrova, E.N.; Nasonov, E.L. The effects of rituximab on lipids, arterial stiffness and carotid intima-media thickness in rheumatoid arthritis. J. Korean Med. Sci. 2016, 31, 202–207. [Google Scholar] [CrossRef]
- Mathieu, S.; Pereira, B.; Dubost, J.J.; Lusson, J.R.; Soubrier, M. No significant change in arterial stiffness in RA after 6 months and 1 year of rituximab treatment. Rheumatology 2012, 51, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Saviola, G.; Manfredi, M.; Sarzi-Puttini, P.; Atzeni, F. Factors correlated with improvement of endothelial dysfunction during rituximab therapy in patients with rheumatoid arthritis. Biologics 2013, 7, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Hsue, P.Y.; Scherzer, R.; Grunfeld, C.; Imboden, J.; Wu, Y.; Del Puerto, G.; Nitta, E.; Shigenaga, J.; Schnell Heringer, A.; Ganz, P.; et al. Depletion of B-cell rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J. Am. Heart Assoc. 2014, 3, e001267. [Google Scholar] [CrossRef] [PubMed]
- Van Vollenhove, R.F.; Emery, P.; Bingham, C.O.; Keystone, E.C.; Fleischmann, R.M.; Furst, D.E.; Tyson, N.; Collinson, N.; Lehane, P.B. Long-term safety of rituximab in rheumatoid arthritis: 9.5 years follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann. Rheum. Dis. 2013, 72, 1496–1502. [Google Scholar] [CrossRef]
- Ewing, M.M.; Karper, J.C.; Abdul, S.; de Jong, R.C.M.; Peters, H.A.B.; de Vries, M.R.; Redeker, A.; Kuiper, J.; Toes, R.E.M.; Arens, R.; et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int. J. Cardiol. 2013, 168, 1965–1974. [Google Scholar] [CrossRef]
- Pappas, D.A.; John, A.; Curtis, J.A.; Reed, G.W.; Greenberg, J.D.; Shewade, A.; Solomon, D.H.; Kremer, J.M.; Soomers, T. Effect of biological agents on lipid and cardiovascular risk in rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 49. [Google Scholar] [CrossRef]
- Saito, K.; Hanami, K.; Hirata, S.; Kubo, S.; Nawata, M.; Yamaoka, K.; Nakayamada, S.; Nakano, K.; Tanaka, Y. Comparison of lipid profile including high molecular weight adiponectin (HMW-AN) after treatment with three different biologics in the patients with bio-naïve rheumatoid arthritis. Ann. Rheum. Dis. 2014, 72, 459. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Gugiu, G.B.; Ge, H.; Shahbazian, A.; Lee, Y.Y.; Wang, X.; Furst, D.E.; Ranganath, V.K.; Maldonado, M.; Lee, T.; et al. Remodeling of the HDL proteome with treatment response to abatacept or adalimumab in the AMPLE trial of patients with rheumatoid arthritis. Artheriosclerosis 2018, 275, 107–114. [Google Scholar] [CrossRef]
- Mathieu, S.; Couderc, M.; Glace, B.; Pereira, B.; Tournadre, A.; Dubost, J.J.; Soubrier, M. Effects of 6 months of abatacept treatment on aortic stiffness in patients with rheumatoid arthritis. Biologics 2013, 7, 259–264. [Google Scholar] [CrossRef]
- Ursini, F.; Russo, E.; Hribal, M.L.; Mauro, D.; Savarino, F.; Bruno, C.; Tripolino, C.; Rubino, M.; Naty, S.; Grembiale, R.D. Abatacept improves whole body insulin sensitivity in rheumatoid arthritis: An observational study. Medicine 2015, 94, e888. [Google Scholar] [CrossRef]
- Jin, Y.; Kang, E.H.; Brill, G.; Desai, R.J.; Kim, S.C. Cardiovascular risk after initiation of abatacept versus TNF inhibitors in rheumatoid arthritis patients with and without baseline cv disease. J. Rheumatol. 2018, 45, 1240–1248. [Google Scholar] [CrossRef]
- Kang, E.H.; Jin, Y.; Brill, G.; Lewey, J.; Patorno, E.; Desai, R.J.; Kim, S.C. Comparative cardiovascular risk of abatacept tumor necrosis factor inhibitors in patients with rheumatoid arthritis with and without diabetes mellitus: A multidatabase cohort study. J. Am. Heart Assoc. 2018, 24, e001393. [Google Scholar] [CrossRef]
- Meune, C.; Touze, E.; Trinquart, L.; Allanore, Y. High risk of clinical cardiovascular events in rheumatoid arthritis: Levels of associations of myocardial infarction and stroke through a systematic review and meta-analysis. Arch. Cardiovasc. Dis. 2010, 103, 253–261. [Google Scholar] [CrossRef]
| Classes of bDMARDs | bDMARDs | Biosimilar | Mechanism | Effects |
|---|---|---|---|---|
| Anti-IL-6 | Tocilizumab | - | Monoclonal antibodies act as IL-6 receptor antagonists, to which they bind and prevent this cytokine from being fixed at this level | Clinical and biological improvement, slowing or stopping disease progression; preventing joint destruction Increased efficiency as a therapy in RA patients [150,152,155,158] |
| Sarilumab | - | |||
| Anti-TNF-α | Infliximab | √ | Chimeric IgG1k monoclonal antibody | Neutralization of biological effects of TNF-α, such as stimulation of synthesis and release of pro-inflammatory cytokines, prostaglandins, and platelet-activating factors; endothelial dysfunction; development and progression of atheromatous plaques; cardiac remodeling [150,152] |
| Adalimumab | √ | Human IgG1 monoclonal antibody | ||
| Golimumab | - | Fully human monoclonal antibody | ||
| Certolizumab pegol | - | PEGylated monoclonal antibody formed with a humanized Fab fragment | ||
| Etanercept | √ | Soluble TNF-α receptor | ||
| Anti-CD20 (anti-LB) | Rituximab | √ | Chimeric monoclonal anti-CD20 antibody; the antigen CD20 is expressed on the surface of LB | Induces B2 cell depletion Clinical improvement, slowing or stopping disease progression; preventing joint destruction Increased efficacy in combination with MTX [150,152,155] |
| Anti-CD80/86 (anti-LT) | Abatacept | - | Soluble receptor consisting of a fusion molecule that blocks the binding of CD80 and CD86 receptors on the antigen-presenting cell (APC), thereby inhibiting T-cell activation | Clinical improvement, slowing disease progression; preventing joint destruction Therapeutic effects and safety profile similar to adalimumab [150,152,155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, D.; Rezus, E.; Badescu, M.C.; Dima, N.; Seritean Isac, P.N.; Dragoi, I.-T.; Rezus, C. Cardiovascular Risk Assessment in Rheumatoid Arthritis: Accelerated Atherosclerosis, New Biomarkers, and the Effects of Biological Therapy. Life 2023, 13, 319. https://doi.org/10.3390/life13020319
Popescu D, Rezus E, Badescu MC, Dima N, Seritean Isac PN, Dragoi I-T, Rezus C. Cardiovascular Risk Assessment in Rheumatoid Arthritis: Accelerated Atherosclerosis, New Biomarkers, and the Effects of Biological Therapy. Life. 2023; 13(2):319. https://doi.org/10.3390/life13020319
Chicago/Turabian StylePopescu, Diana, Elena Rezus, Minerva Codruta Badescu, Nicoleta Dima, Petronela Nicoleta Seritean Isac, Ioan-Teodor Dragoi, and Ciprian Rezus. 2023. "Cardiovascular Risk Assessment in Rheumatoid Arthritis: Accelerated Atherosclerosis, New Biomarkers, and the Effects of Biological Therapy" Life 13, no. 2: 319. https://doi.org/10.3390/life13020319
APA StylePopescu, D., Rezus, E., Badescu, M. C., Dima, N., Seritean Isac, P. N., Dragoi, I.-T., & Rezus, C. (2023). Cardiovascular Risk Assessment in Rheumatoid Arthritis: Accelerated Atherosclerosis, New Biomarkers, and the Effects of Biological Therapy. Life, 13(2), 319. https://doi.org/10.3390/life13020319








