The Association between the Level of Advanced Glycation End Products and Objective Skin Quality Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Subjects
2.3. Objective Skin Assessment
2.4. AGEs Measurement
2.5. Statistical Analyses and Sample Size Calculation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.F.L.; Schumacher, B. Principles of the Molecular and Cellular Mechanisms of Aging. J. Investig. Dermatol. 2021, 141, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Krieg, T.M. The Aging Skin: From Basic Mechanisms to Clinical Applications. J. Investig. Dermatol. 2021, 141, 949–950. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin Ageing: Pathophysiology and Current Market Treatment Approaches. Curr. Aging Sci. 2020, 13, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.H.; Wang, Z.Q.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. The skin as a mirror of the aging process in the human organism—State of the art and results of the aging research in the German National Genome Research Network 2 (NGFN-2). Exp. Gerontol. 2007, 42, 879–886. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 18, 3818196. [Google Scholar] [CrossRef]
- Sharma, C.; Kaur, A.; Thind, S.S.; Singh, B.; Raina, S. Advanced glycation End-products (AGEs): An emerging concern for processed food industries. J. Food Sci. Technol. 2015, 52, 7561–7576. [Google Scholar] [CrossRef] [PubMed]
- van der Lugt, T.; Weseler, A.R.; Gebbink, W.A.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Dietary Advanced Glycation Endproducts Induce an Inflammatory Response in Human Macrophages in Vitro. Nutrients 2018, 10, 1868. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16R–18R. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Fotheringham, A.K.; Gallo, L.A.; Borg, D.J.; Forbes, J.M. Advanced Glycation End Products (AGEs) and Chronic Kidney Disease: Does the Modern Diet AGE the Kidney? Nutrients 2022, 14, 2675. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Paula, D.P.; de Araujo, B.P.; da Fonseca, M.J.M.; Diniz, M.; Daliry, A.; Griep, R.H. Advanced glycation end product: A potential biomarker for risk stratification of non-alcoholic fatty liver disease in ELSA-Brasil study. World J. Gastroenterol. 2021, 27, 4913–4928. [Google Scholar] [CrossRef]
- Peppa, M.; Raptis, S.A. Advanced glycation end products and cardiovascular disease. Curr. Diabetes Rev. 2008, 4, 92–100. [Google Scholar] [CrossRef]
- Luketin, M.; Mizdrak, M.; Boric-Skaro, D.; Martinovic, D.; Tokic, D.; Vilovic, M.; Supe-Domic, D.; Ticinovic Kurir, T.; Bozic, J. Plasma Catestatin Levels and Advanced Glycation End Products in Patients on Hemodialysis. Biomolecules 2021, 11, 456. [Google Scholar] [CrossRef]
- Grahovac, M.; Kumric, M.; Vilovic, M.; Martinovic, D.; Kreso, A.; Ticinovic Kurir, T.; Vrdoljak, J.; Prizmic, K.; Božić, J. Adherence to Mediterranean diet and advanced glycation endproducts in patients with diabetes. World J. Diabetes 2021, 12, 1942–1956. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.Q.; Li, L.; Guo, M.M.; He, Y.F.; Dong, Y.M.; Meng, H.; Yi, F. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Park, S.; Kim, J. The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. J. Exerc. Nutr. Biochem. 2017, 21, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Jeanmaire, C.; Danoux, L.; Pauly, G. Glycation during human dermal intrinsic and actinic ageing: An in vivo and in vitro model study. Br. J. Dermatol. 2001, 145, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Prisayanh, P.; Haque, Z.; Woodley, D.T. Collagen cross-linking in sun-exposed and unexposed sites of aged human skin. J. Investig. Dermatol. 1991, 97, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Avery, N.C.; Bailey, A.J. The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol. Biol. 2006, 54, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, Z.; Alikhani, M.; Boyd, C.M.; Nagao, K.; Trackman, P.C.; Graves, D.T. Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J. Biol. Chem. 2005, 280, 12087–12095. [Google Scholar] [CrossRef]
- Rosado, C.; Pinto, P.; Rodrigues, L.M. Comparative assessment of the performance of two generations of Tewameter: TM210 and TM300. Int. J. Cosmet. Sci. 2005, 27, 237–241. [Google Scholar] [CrossRef]
- Snatchfold, J.; Targett, D. Exploratory study to evaluate two clinical methods for assessing moisturizing effect on skin barrier repair. Skin Res. Technol. 2019, 25, 251–257. [Google Scholar] [CrossRef]
- Martinovic, D.; Lupi-Ferandin, S.; Tokic, D.; Usljebrka, M.; Rados, A.; Pojatina, A.; Kadic, S.; Puizina, E.; Mihovilovic, A.; Kumric, M.; et al. Objective Skin Quality Assessment after Reconstructive Procedures for Facial Skin Defects. J. Clin. Med. 2022, 11, 4471. [Google Scholar] [CrossRef]
- Waqas, K.; Chen, J.; Rivadeneira, F.; Uitterlinden, A.G.; Voortman, T.; Zillikens, M.C. Skin Autofluorescence, a Noninvasive Biomarker of Advanced Glycation End-products, Is Associated With Frailty: The Rotterdam Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2032–2039. [Google Scholar] [CrossRef]
- Crisan, M.; Taulescu, M.; Crisan, D.; Cosgarea, R.; Parvu, A.; Cãtoi, C.; Drugan, T. Expression of Advanced Glycation End-Products on Sun-Exposed and Non-Exposed Cutaneous Sites during the Ageing Process in Humans. PLoS ONE 2013, 8, e75003. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Ouyang, M.; Qu, Y.; Wang, M.; Huang, X.; Lan, J.; Lai, W.; Xu, Q. Advanced Glycation End Products Promote Melanogenesis by Activating NLRP3 Inflammasome in Human Dermal Fibroblasts. J. Investig. Dermatol. 2022, 142, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Murawsky, M.; LaCount, T.; Kasting, G.B.; Li, S.K. Transepidermal water loss and skin conductance as barrier integrity tests. Toxicol. In Vitro 2018, 51, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Jansen van Rensburg, S.; Franken, A.; Du Plessis, J.L. Measurement of transepidermal water loss, stratum corneum hydration and skin surface pH in occupational settings: A review. Ski. Res. Technol. 2019, 25, 595–605. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300. [Google Scholar] [CrossRef]
- Yokota, M.; Masaki, H.; Okano, Y.; Tokudome, Y. Effect of glycation focusing on the process of epidermal lipid synthesis in a reconstructed skin model and membrane fluidity of stratum corneum lipids. Dermatoendocrinology 2017, 9, e1338992. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, J.H.; Jung, M.; Chung, C.H.; Hasham, R.; Park, C.S.; Choi, E.H. A long-standing hyperglycaemic condition impairs skin barrier by accelerating skin ageing process. Exp. Dermatol. 2011, 20, 969–974. [Google Scholar] [CrossRef]
- Lee, K.H.; Ng, Y.P.; Cheah, P.S.; Lim, C.K.; Toh, M.S. Molecular characterization of glycation-associated skin ageing: An alternative skin model to study in vitro antiglycation activity of topical cosmeceutical and pharmaceutical formulations. Br. J. Dermatol. 2017, 176, 159–167. [Google Scholar] [CrossRef]
- Kottner, J.; Lichterfeld, A.; Blume-Peytavi, U. Transepidermal water loss in young and aged healthy humans: A systematic review and meta-analysis. Arch. Dermatol. Res. 2013, 305, 315–323. [Google Scholar] [CrossRef]
- Liu, Z.; Fluhr, J.W.; Song, S.P.; Sun, Z.; Wang, H.; Shi, Y.J.; Elias, P.M.; Man, M.Q. Sun-induced changes in stratum corneum function are gender and dose dependent in a Chinese population. Ski. Pharmacol. Physiol. 2010, 23, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Cua, A.B.; Wilhelm, K.P.; Maibach, H.I. Frictional properties of human skin: Relation to age, sex and anatomical region, stratum corneum hydration and transepidermal water loss. Br. J. Dermatol. 1990, 123, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.C.; Strässle, V.; Lenz, A.; Spencer, N.D.; Derler, S. Influence of epidermal hydration on the friction of human skin against textiles. J. R. Soc. Interface 2008, 5, 1317–1328. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Song, S.P.; Luo, W.; Elias, P.M.; Man, M.Q. Characterization of skin friction coefficient, and relationship to stratum corneum hydration in a normal Chinese population. Ski. Pharmacol. Physiol. 2011, 24, 81–86. [Google Scholar] [CrossRef]
- Lodén, M.; Olsson, H.; Axéll, T.; Linde, Y.W. Friction, capacitance and transepidermal water loss (TEWL) in dry atopic and normal skin. Br. J. Dermatol. 1992, 126, 137–141. [Google Scholar] [CrossRef]

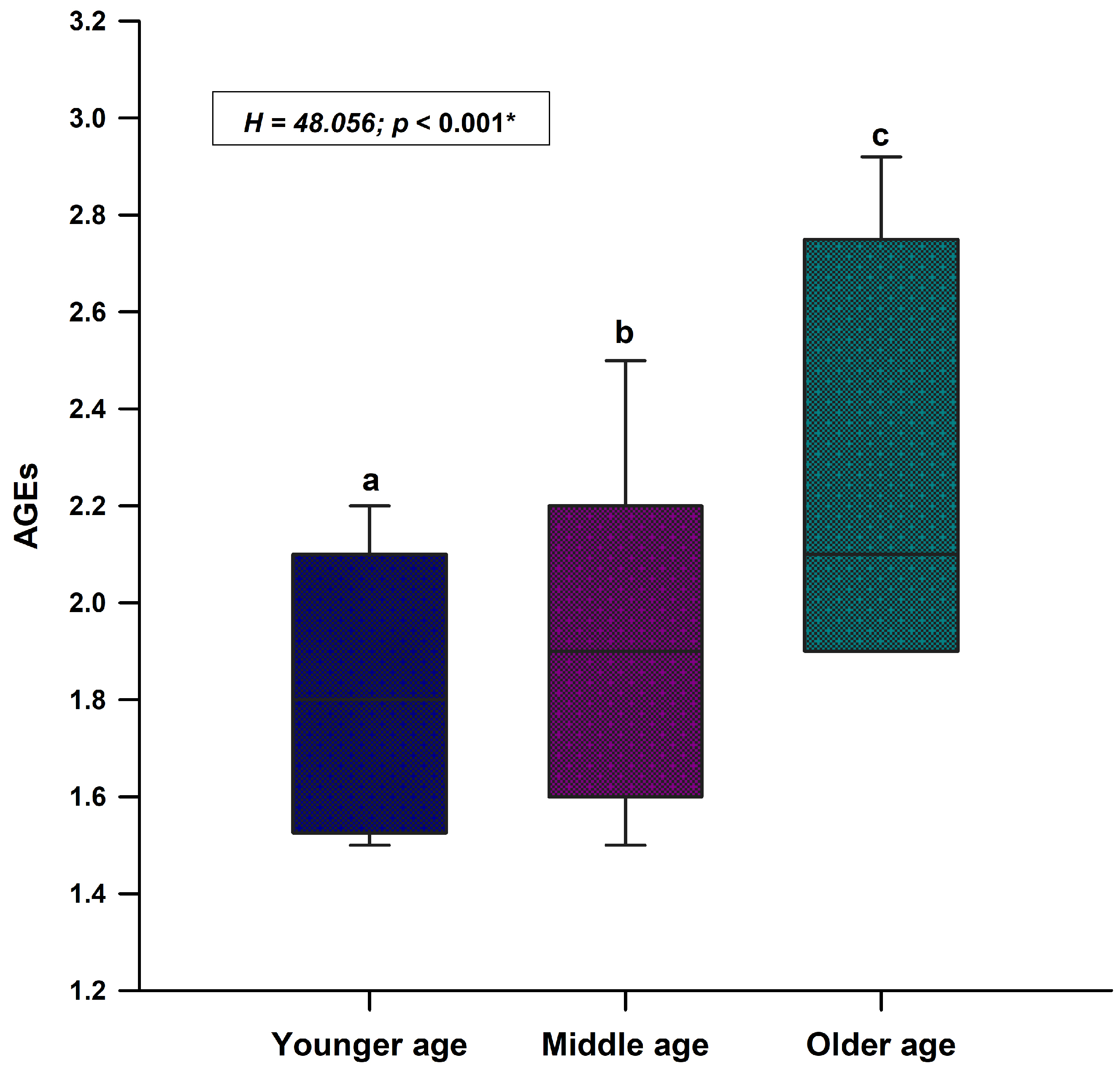
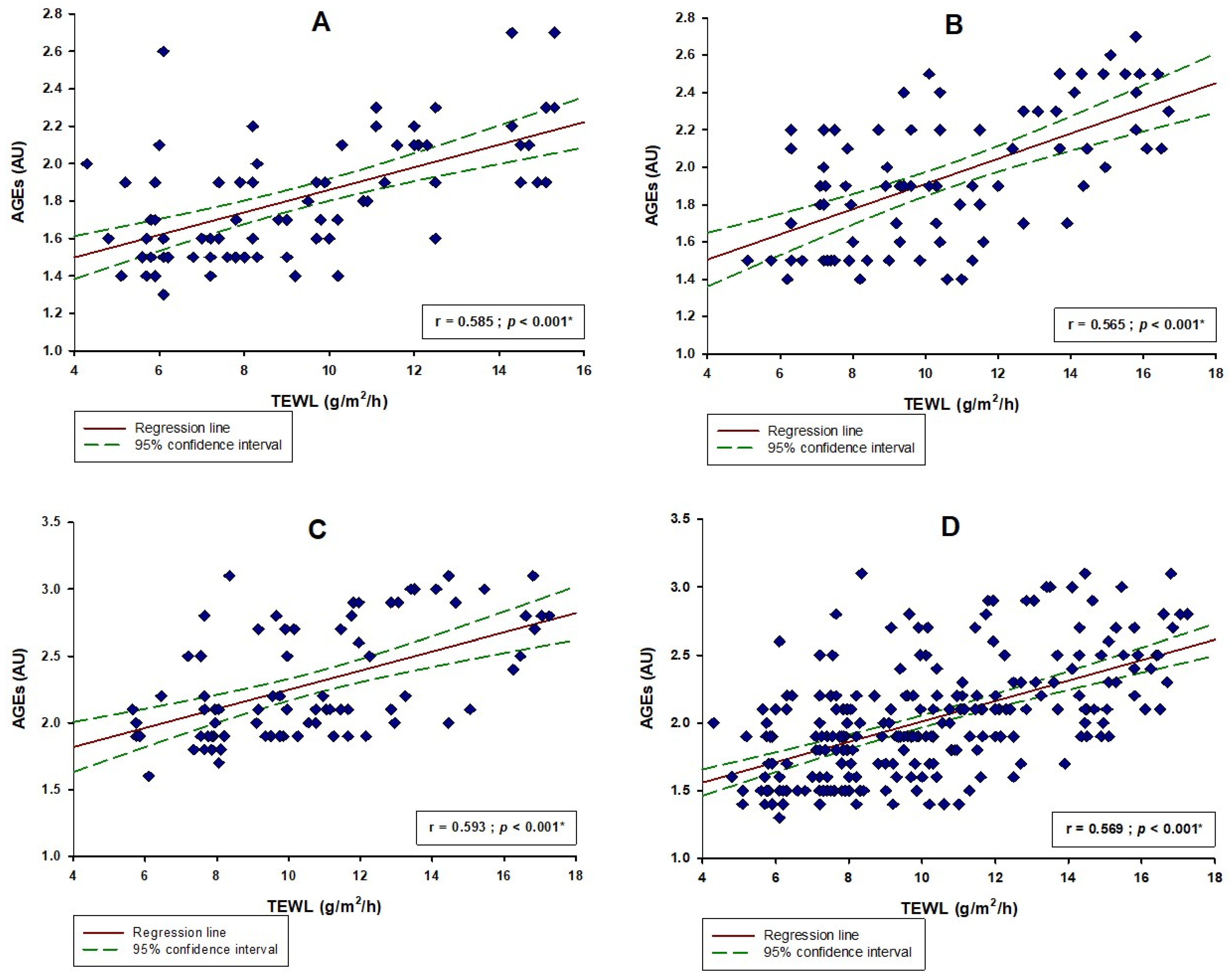
| Parameter | Study Sample (N = 237) | Younger Age (<33 Years) N = 80 | Middle Age (33–56 Years) N = 80 | Older Age (>56 Years) N = 77 | p † |
|---|---|---|---|---|---|
| Male gender (N, %) | 142 (59.9) | 48 (60) | 46 (57.5) | 48 (62.3) | 0.825 |
| Age (years) | 41.0 (22.0–62.0) | 21.0 (19.0–22.0) | 41.5 (37.0–48.0) | 67.0 (62.0–74.0) | <0.001 abc |
| Body height (cm) | 178.0 (170.0–185.0) | 177.0 (170.0–185.0) | 174.0 (167.0–181.5) | 181.0 (175.0–186.0) | 0.009c |
| Body mass (kg) | 78.2 ± 8.9 | 77.2 ± 15.2 | 76.7 ± 15.5 | 88.4 ± 16.6 | <0.001 bc |
| BMI (kg/m2) | 24.5 (22.2–26.4) | 24.5 (22.3–26.3) | 24.7 (23.0–27.4) | 27.4 (25.4–29.0) | <0.001 bc |
| Melanin (AU) | 99.6 (69.1–133.8) | 94.2 (66.0–127.7) | 90.8 (65.4–124.4) | 112.0 (78.2–145.1) | 0.010 bc |
| Erythema (AU) | 256.2 ± 75.4 | 244.1 ± 75.4 | 250.4 ± 78.1 | 274.6 ± 69.9 | 0.028 b |
| Hydration (AU) | 48.8 (41.0–60.3) | 50.6 (44.2–62.6) | 50.9 (41.8–63.4) | 45.2 (37.1–53.9) | <0.001 bc |
| Friction (AU) | 249.0 (145.0–378.5) | 273.4 (189.2–462.2) | 297.8 (181.1–465.2) | 190.1 (125.4–301.8) | <0.001 bc |
| TEWL (g/m2/h) | 9.7 (7.6–12.4) | 9.3 (6.9–12.2) | 9.7 (7.5–12.7) | 9.9 (7.9–12.8) | 0.165 |
| Parameter | Study Sample N = 237 r (p) | Younger Age (< 33 Years) N = 80 r (p) | Middle Age (33–56 Years) N = 80 r (p) | Older Age (>56 Years) N = 77 r (p) |
|---|---|---|---|---|
| Melanin (AU) | 0.488 (<0.001) ‡ | 0.482 (<0.001) ‡ | 0.499 (<0.001) ‡ | 0.491 (<0.001) ‡ |
| Erythema (AU) | 0.237 (<0.001) † | 0.162 (0.150) † | 0.180 (0.102) † | 0.193 (0.092) † |
| Hydration (AU) | −0.501 (<0.001) ‡ | −0.492 (<0.001) ‡ | −0.478 (<0.001) ‡ | −0.522 (<0.001) ‡ |
| Friction (AU) | −0.307 (<0.001) ‡ | −0.191 (0.089) ‡ | −0.192 (0.087) ‡ | −0.224 (0.051) ‡ |
| Parameter | β † | SE ‡ | t | p |
|---|---|---|---|---|
| Age | 0.006 | 0.001 | 5.879 | <0.001 |
| BMI | −0.012 | 0.006 | −1.811 | 0.071 |
| Melanin | 0.002 | 0.0005 | 4.834 | <0.001 |
| Erythema | 0.001 | 0.0002 | 2.802 | 0.005 |
| Hydration | −0.007 | 0.002 | −3.901 | <0.001 |
| Friction | −0.001 | 0.0001 | −2.394 | 0.017 |
| TEWL | 0.027 | 0.006 | 4.084 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinovic, D.; Tokic, D.; Usljebrka, M.; Lupi-Ferandin, S.; Cigic, L.; Vanjaka Rogosic, L.; Ercegovic, S.; Kontic, M.; Kumrić, M.; Rusic, D.; et al. The Association between the Level of Advanced Glycation End Products and Objective Skin Quality Parameters. Life 2023, 13, 256. https://doi.org/10.3390/life13020256
Martinovic D, Tokic D, Usljebrka M, Lupi-Ferandin S, Cigic L, Vanjaka Rogosic L, Ercegovic S, Kontic M, Kumrić M, Rusic D, et al. The Association between the Level of Advanced Glycation End Products and Objective Skin Quality Parameters. Life. 2023; 13(2):256. https://doi.org/10.3390/life13020256
Chicago/Turabian StyleMartinovic, Dinko, Daria Tokic, Mislav Usljebrka, Slaven Lupi-Ferandin, Livia Cigic, Lucija Vanjaka Rogosic, Sasa Ercegovic, Mirko Kontic, Marko Kumrić, Doris Rusic, and et al. 2023. "The Association between the Level of Advanced Glycation End Products and Objective Skin Quality Parameters" Life 13, no. 2: 256. https://doi.org/10.3390/life13020256
APA StyleMartinovic, D., Tokic, D., Usljebrka, M., Lupi-Ferandin, S., Cigic, L., Vanjaka Rogosic, L., Ercegovic, S., Kontic, M., Kumrić, M., Rusic, D., Vilovic, M., Leskur, M., & Bozic, J. (2023). The Association between the Level of Advanced Glycation End Products and Objective Skin Quality Parameters. Life, 13(2), 256. https://doi.org/10.3390/life13020256












