Precision and Safety of Ultrasound-Guided versus Palpation-Guided Needle Placement on the Patellar Tendon: A Cadaveric Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
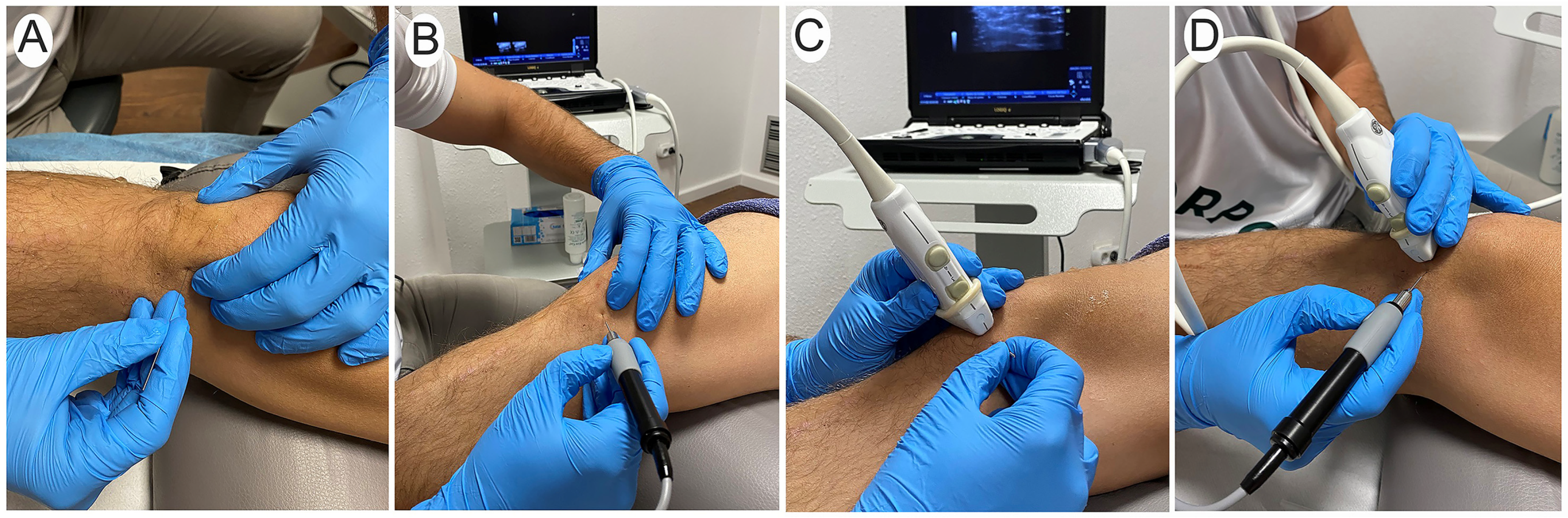
2.2. Procedure
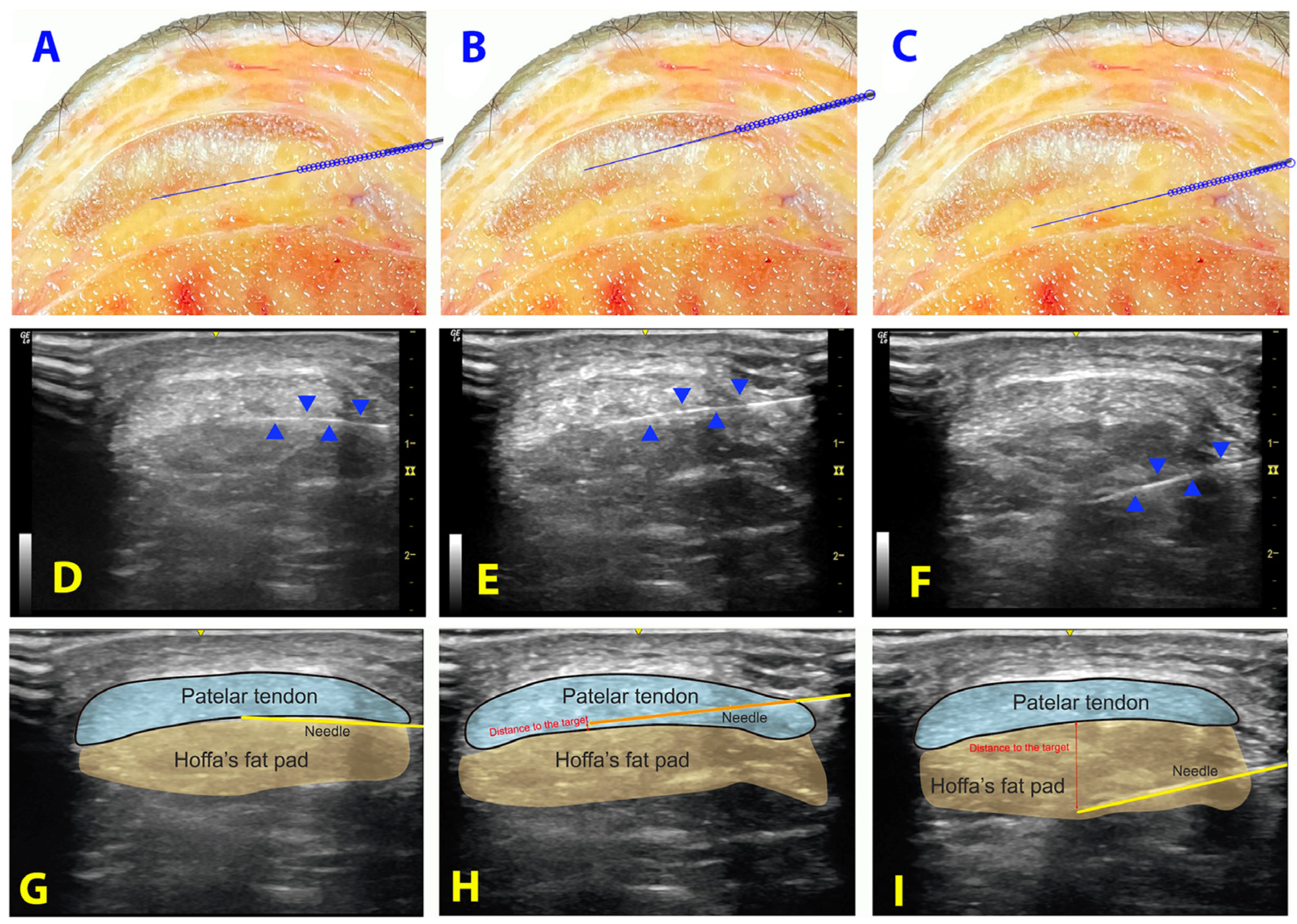
2.3. Measurements
- Distance: Distance of the needle’s tip to the target (in millimeters).
- Target contact and surface of contact: Contact of the needle with the interface between patellar tendon and Hoffa’s fat pad (yes/no) and the distance of contact (in millimeters).
- Punctured structure: If a different structure was punctured (patellar tendon or cortical bone). If the patellar tendon and not the interface was punctured, the distance from the needle to the interface between patellar tendon and Hoffa’s fat pad was recorded (in millimeters).
- Time: The time needed for a single needle insertion (in seconds).
- Passes: The number of needle insertion (each time the participant/therapist advanced the needle after a change of direction was considered one pass) [28].
- Needle length outside: The length of the needle that was located outside the body (in millimeters).
- Accuracy: The inserted attempt was considered accurate if the tip of the needle was properly placed less than 3 mm from the target or the contact with the interface was properly achieved.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassel, M.; Baur, H.; Hirschmüller, A.; Carlsohn, A.; Fröhlich, K.; Mayer, F. Prevalence of Achilles and Patellar Tendinopathy and Their Association to Intratendinous Changes in Adolescent Athletes. Scand. J. Med. Sci. Sports 2015, 25, e310–e318. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Voos, J.E.; Nguyen, J.T.; Callahan, L.; Hannafin, J.A. Injury Profile in Elite Female Basketball Athletes at the Women’s National Basketball Association Combine. Am. J. Sports Med. 2013, 41, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Lian, Ø.B.; Engebretsen, L.; Bahr, R. Prevalence of Jumper’s Knee among Elite Athletes from Different Sports: A Cross-Sectional Study. Am. J. Sports Med. 2005, 33, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L. A Cross Sectional Study of 100 Athletes with Juniper’s Knee Managed Conservatively and Surgically. Br. J. Sports Med. 1997, 31, 332–336. [Google Scholar] [CrossRef]
- Fu, S.C.; Rolf, C.; Cheuk, Y.C.; Lui, P.P.Y.; Chan, K.M. Deciphering the Pathogenesis of Tendinopathy: A Three-Stages Process. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2010, 2, 30. [Google Scholar] [CrossRef]
- Van Der Worp, H.; Van Ark, M.; Roerink, S.; Pepping, G.J.; Van Den Akker-Scheek, I.; Zwerver, J. Risk Factors for Patellar Tendinopathy: A Systematic Review of the Literature. Br. J. Sports Med. 2011, 45, 446–452. [Google Scholar] [CrossRef]
- Griffiths, G.P.; Selesnick, F.H. Operative Treatment and Arthroscopic Findings in Chronic Patellar Tendinitis. Arthroscopy 1998, 14, 836–839. [Google Scholar] [CrossRef]
- Kannus, P.; Józsa, L.; Natri, A.; Järvinen, M. Effects of Training, Immobilization and Remobilization on Tendons. Scand. J. Med. Sci. Sports 1997, 7, 67–71. [Google Scholar] [CrossRef]
- Johnson, D.P.; Wakeley, C.J.; Watt, I. Magnetic Resonance Imaging of Patellar Tendonitis. J. Bone Jt. Surg. Ser. B 1996, 78, 452–457. [Google Scholar] [CrossRef]
- Draghi, F.; Ferrozzi, G.; Urciuoli, L.; Bortolotto, C.; Bianchi, S. Hoffa’s Fat Pad Abnormalities, Knee Pain and Magnetic Resonance Imaging in Daily Practice. Insights Imaging 2016, 7, 373–383. [Google Scholar] [CrossRef]
- Burton, I. Combined Extracorporeal Shockwave Therapy and Exercise for the Treatment of Tendinopathy: A Narrative Review. Sports Med. Health Sci. 2022, 4, 8–17. [Google Scholar] [CrossRef]
- Cardoso, T.B.; Pizzari, T.; Kinsella, R.; Hope, D.; Cook, J.L. Current Trends in Tendinopathy Management. Best. Pr. Res. Clin. Rheumatol. 2019, 33, 122–140. [Google Scholar] [CrossRef] [PubMed]
- Marigi, E.M.; Buckley, P.; Razi, F.; Abbas, M.J.; Jildeh, T.R.; Camp, C.L.; Krych, A.J.; Okoroha, K.R. Patellar Tendinopathy: Critical Analysis Review of Current Nonoperative Treatments. JBJS Rev. 2022, 10, e21. [Google Scholar] [CrossRef] [PubMed]
- Abat, F.; Sánchez-Sánchez, J.L.; Martín-Nogueras, A.M.; Calvo-Arenillas, J.I.; Yajeya, J.; Méndez-Sánchez, R.; Monllau, J.C.; Gelber, P.E. Randomized Controlled Trial Comparing the Effectiveness of the Ultrasound-Guided Galvanic Electrolysis Technique (USGET) versus Conventional Electro-Physiotherapeutic Treatment on Patellar Tendinopathy. J. Exp. Orthop. 2016, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fernández, A.C.; Barragán-Carballar, C.; Villafañe, J.H.; Martín-Pérez, S.; Alonso-Pérez, J.L.; Díaz-Meco, R.; Jiménez, D.G.; Sánchez Romero, E.A. A New Ultrasound-Guided Percutaneous Electrolysis and Exercise Treatment in Patellar Tendinopathy: Three Case Reports. Front. Biosci. Landmark 2021, 26, 1166–1175. [Google Scholar]
- Muñoz-Fernández, A.C.; Barragán-Carballar, C.; Villafañe, J.H.; Martin-Pérez, S.; Alonso-Pérez, J.L.; Díaz-Meco, R.; García-Jiménez, D.; Sánchez-Romero, E.A. Correction: Muñoz-Fernández et al. A New Ultrasound-Guided Percutaneous Electrolysis and Exercise Treatment in Patellar Tendinopathy: Three Case Reports. Frontiers in Bioscience-Landmark. 2021; 26: 1166–1175. Front. Biosci. 2022, 27, 109. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Chiguano, G.F.; Navarro-Santana, M.J.; Cleland, J.A.; Arias-Buría, J.L.; Fernández-De-Las-Peñas, C.; Ortega-Santiago, R.; Plaza-Manzano, G. Effectiveness of Ultrasound-Guided Percutaneous Electrolysis for Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Pain. Med. 2021, 22, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Abat, F.; Valles, S.L.; Gelber, P.E.; Polidori, F.; Jorda, A.; García-Herreros, S.; Monllau, J.C.; Sanchez-Ibáñez, J.M. An Experimental Study of Muscular Injury Repair in a Mouse Model of Notexin-Induced Lesion with EPI® Technique. BMC Sports Sci. Med. Rehabil. 2015, 7, 7. [Google Scholar] [CrossRef]
- Margalef, R.; Valera-Garrido, F.; Minaya-Muñoz, F.; Bosque, M.; Ortiz, N.; Santafe, M.M. Percutaneous Needle Electrolysis Reverses Neurographic Signs of Nerve Entrapment by Induced Fibrosis in Mice. Evid. Based Complement. Altern. Med. 2020, 2020, 6615563. [Google Scholar] [CrossRef]
- Sánchez-sánchez, J.L.; Calderón-díez, L.; Herrero-turrión, J.; Méndez-sánchez, R.; Arias-buría, J.L.; Fernández-de-las-peñas, C. Changes in Gene Expression Associated with Collagen Regeneration and Remodeling of Extracellular Matrix after Percutaneous Electrolysis on Collagenase-Induced Achilles Tendinopathy in an Experimental Animal Model: A Pilot Study. J. Clin. Med. 2020, 9, 3316. [Google Scholar] [CrossRef]
- Neal, J.M.; Brull, R.; Chan, V.W.S.; Grant, S.A.; Horn, J.-L.; Liu, S.S.; McCartney, C.J.L.; Narouze, S.N.; Perlas, A.; Salinas, F.V.; et al. The ASRA Evidence-Based Medicine Assessment of Ultrasound-Guided Regional Anesthesia and Pain Medicine: Executive Summary. Reg. Anesth. Pain. Med. 2010, 35 (Suppl. S1), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.A.; Brismée, J.M.; Riley, S.P.; Wang-Price, S.; Denninger, T.; Vugrin, M. Lack of Standardization in Dry Needling Dosage and Adverse Event Documentation Limits Outcome and Safety Reports: A Scoping Review of Randomized Clinical Trials. J. Man. Manip. Ther. 2023, 31, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, M.S.; Aziz, M.F.; Fu, R.F.; Horn, J.L. Ultrasound Guidance Compared with Electrical Neurostimulation for Peripheral Nerve Block: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Br. J. Anaesth. 2009, 102, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, H.J.; Ouanes, J.P.P.; Lesley, M.R.; Ko, P.S.; Murphy, J.D.; Sumida, S.M.; Isaac, G.R.; Kumar, K.; Wu, C.L. Analgesic Efficacy of Ultrasound-Guided Regional Anesthesia: A Meta-Analysis. J. Clin. Anesth. 2011, 23, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.J.; McGrattan, K.; Aas-Eng, K.; Smith, A.F. Ultrasound Guidance for Peripheral Nerve Blockade. Cochrane Database Syst. Rev. 2009, 4, CD006459. [Google Scholar] [CrossRef]
- Klop, A.C.; Vester, M.E.M.; Colman, K.L.; Ruijter, J.M.; Van Rijn, R.R.; Oostra, R.J. The Effect of Repeated Freeze-Thaw Cycles on Human Muscle Tissue Visualized by Postmortem Computed Tomography (PMCT). Clin. Anat. 2017, 30, 799–804. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, J.; Sun, K.; Zhang, X.; Tian, S. Effects of Repetitive Multiple Freeze-Thaw Cycles on the Biomechanical Properties of Human Flexor Digitorum Superficialis and Flexor Pollicis Longus Tendons. Clin. Biomech. 2011, 26, 419–423. [Google Scholar] [CrossRef]
- Johnson, A.N.; Peiffer, J.S.; Halmann, N.; Delaney, L.; Owen, C.A.; Hersh, J. Ultrasound-Guided Needle Technique Accuracy Prospective Comparison of Passive Magnetic Tracking versus Unassisted Echogenic Needle Localization. Reg. Anesth. Pain. Med. 2017, 42, 223–232. [Google Scholar] [CrossRef]
- Barrington, M.J.; Wong, D.M.; Slater, B.; Ivanusic, J.J.; Ovens, M. Ultrasound-Guided Regional Anesthesia: How Much Practice Do Novices Require before Achieving Competency in Ultrasound Needle Visualization Using a Cadaver Model. Reg. Anesth. Pain. Med. 2012, 37, 334–339. [Google Scholar] [CrossRef]
- McVicar, J.; Niazi, A.U.; Murgatroyd, H.; Chin, K.J.; Chan, V.W. Novice Performance of Ultrasound-Guided Needling Skills: Effect of a Needle Guidance System. Reg. Anesth. Pain. Med. 2015, 40, 150–153. [Google Scholar] [CrossRef]
- Diep, D.; Chen, K.J.Q.; Kumbhare, D. Ultrasound-Guided Interventional Procedures for Myofascial Trigger Points: A Systematic Review. Reg. Anesth. Pain. Med. 2021, 46, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Folli, A.; Schneebeli, A.; Ballerini, S.; Mena, F.; Soldini, E.; Fernández-De-las-peñas, C.; Barbero, M. Enhancing Trigger Point Dry Needling Safety by Ultrasound Skin-to-Rib Measurement: An Inter-Rater Reliability Study. J. Clin. Med. 2020, 9, 1958. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morales, C.; Bravo-Aguilar, M.; Abuín-Porras, V.; Almazán-Polo, J.; Calvo-Lobo, C.; Martínez-Jiménez, E.M.; López-López, D.; Navarro-Flores, E. Current Advances and Novel Research on Minimal Invasive Techniques for Musculoskeletal Disorders. Dis.-A-Mon. 2021, 67, 101210. [Google Scholar] [CrossRef] [PubMed]
- Koscielniak-Nielsen, Z.J. Ultrasound-Guided Peripheral Nerve Blocks: What Are the Benefits? Acta Anaesthesiol Scand. Acta Anaesthesiol. Scand. 2008, 52, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, P.; Bieryło, A.; Duniec, L.; Kosson, D.; Łazowski, T. The Substantial Impact of Ultrasound-Guided Regional Anaesthesia on the Clinical Practice of Peripheral Nerve Blocks. Anaesthesiol. Intensive Ther. 2013, 45, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Boctor, E.M.; Choti, M.A.; Burdette, E.C.; Webster, R.J. Three-Dimensional Ultrasound-Guided Robotic Needle Placement: An Experimental Evaluation. Int. J. Med. Robot. Comput. Assist. Surg. 2008, 4, 180–191. [Google Scholar] [CrossRef]
- Stolka, P.J.; Foroughi, P.; Rendina, M.; Weiss, C.R.; Hager, G.D.; Boctor, E.M. Needle Guidance Using Handheld Stereo Vision and Projection for Ultrasound-Based Interventions. Med. Image Comput. Comput. Assist. Interv. 2014, 17, 684–691. [Google Scholar]
- Hocking, G.; Hebard, S.; Mitchell, C.H. A Review of the Benefits and Pitfalls of Phantoms in Ultrasound-Guided Regional Anesthesia. Reg. Anesth. Pain. Med. 2011, 36, 162–170. [Google Scholar] [CrossRef]
- Srinivasan, K.K.; Iohom, G.; Loughnane, F.; Lee, P.J. Conventional Landmark-Guided Midline versus Preprocedure Ultrasound-Guided Paramedian Techniques in Spinal Anesthesia. Anesth. Analg. 2015, 121, 1089–1096. [Google Scholar] [CrossRef]
- Louis, L.J. Musculoskeletal Ultrasound Intervention: Principles and Advances. Radiol. Clin. N. Am. 2008, 46, 515–533. [Google Scholar] [CrossRef]
- Chin, K.J.; Perlas, A.; Chan, V.W.S.; Brull, R. Needle Visualization in Ultrasound-Guided Regional Anesthesia: Challenges and Solutions. Reg. Anesth. Pain. Med. 2008, 33, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.A.; Johnson, D.; Bodenham, A.R. Visualisation of Needle Position Using Ultrasonography. Anaesthesia 2006, 61, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Tsui, B.C.H.; Finucane, B.; Schafhalter-Zoppoth, I.; Gray, A.T. Practical Recommendations for Improving Needle-Tip Visibility under Ultrasound Guidance? Reg. Anesth. Pain. Med. 2005, 30, 596–597. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, S.; McCreesh, K.; Culloty, F.; Purtill, H.; O’Sullivan, K. Can Ultrasound Imaging Predict the Development of Achilles and Patellar Tendinopathy? A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2016, 50, 1516–1523. [Google Scholar] [CrossRef]
- Jorda, A.; Campos-Campos, J.; Aldasoro, C.; Colmena, C.; Aldasoro, M.; Alvarez, K.; Valles, S.L. Protective Action of Ultrasound-Guided Electrolysis Technique on the Muscle Damage Induced by Notexin in Rats. PLoS ONE 2022, 17, e0276634. [Google Scholar] [CrossRef]
- Backhaus, T.; von Cranach, M.; Brich, J. Ultrasound-Guided Lumbar Puncture with a Needle-Guidance System: A Prospective and Controlled Study to Evaluate the Learnability and Feasibility of a Newly Developed Approach. PLoS ONE 2018, 13, e0195317. [Google Scholar] [CrossRef]
- White, M.L.; Jones, R.; Zinkan, L.; Tofil, N.M. Transfer of Simulated Lumbar Puncture Training to the Clinical Setting. Pediatr. Emerg. Care 2012, 28, 1009–1012. [Google Scholar] [CrossRef]
- Xu, D.; Abbas, S.; Chan, V.W.S. Ultrasound Phantom for Hands-on Practice. Reg. Anesth. Pain. Med. 2005, 30, 593–594. [Google Scholar] [CrossRef]
| Mean (SD) | |
|---|---|
| Male/Female (n) | 4/1 |
| Experience with invasive techniques (years) | 10.0 ± 5.4 |
| Experience with ultrasound (years) | 8.8 ± 3.5 |
| Total needle procedures (n) | 100 |
| Palpation-guided/Ultrasound-guided (n) | 50/50 |
| With/without handpiece (n) | 50/50 |
| Palpation-Guided | Ultrasound-Guided | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p | |
| Distance to the target (mm) | 2.5 (1.9) | 0.25 (0.65) | <0.001 |
| Target contact (no/yes) | 24/26 | 0/50 | <0.001 |
| Surface of contact with the target (mm) | 4.7 (7.5) | 15.5 (6.65) | <0.001 |
| Patellar tendon punctured (no/yes) | 24/26 | 42/8 | <0.001 |
| Distance of patellar tendon punctured (mm) | 2.95 (6.25) | 2.3 (12.8) | 0.739 |
| Success/Failure (n) | 40/10 | 50/0 | 0.001 |
| Time required (s) | 23.75 (15.4) | 54.8 (26.8) | <0.001 |
| Passes (total number) | 1.5 (0.95) | 2.55 (1.9) | 0.001 |
| Needle length outside (mm) | 9.25 (5.95) | 6.0 (4.25) | 0.003 |
| With Handpiece | Needle | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p | |
| Distance to the target (mm) | 1.4 (1.8) | 1.3 (1.8) | 0.875 |
| Target contact (no/yes) | 11/39 | 13/37 | 0.812 |
| Surface of contact with the target (mm) | 9.9 (8.6) | 10.3 (9.3) | 0.789 |
| Patellar tendon punctured (no/yes) | 29/21 | 37/13 | 0.142 |
| Distance of patellar tendon punctured (mm) | 3.9 (13.4) | 1.4 (4.6) | 0.216 |
| Success/Failure (n) | 45/5 | 45/5 | 1.000 |
| Time required (s) | 39.6 (30.0) | 39.0 (23.4) | 0.901 |
| Passes (total number) | 1.9 (1.5) | 2.1 (1.7) | 0.534 |
| Needle length outside (mm) | 6.85 (5.1) | 8.5 (5.65) | 0.134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Buría, J.L.; Borrella-Andrés, S.; Rodríguez-Sanz, J.; López-de-Celis, C.; Malo-Urriés, M.; Fernández-de-las-Peñas, C.; Gallego-Sendarrubias, G.M.; González-Rueda, V.; Pérez-Bellmunt, A.; Albarova-Corral, I. Precision and Safety of Ultrasound-Guided versus Palpation-Guided Needle Placement on the Patellar Tendon: A Cadaveric Study. Life 2023, 13, 2060. https://doi.org/10.3390/life13102060
Arias-Buría JL, Borrella-Andrés S, Rodríguez-Sanz J, López-de-Celis C, Malo-Urriés M, Fernández-de-las-Peñas C, Gallego-Sendarrubias GM, González-Rueda V, Pérez-Bellmunt A, Albarova-Corral I. Precision and Safety of Ultrasound-Guided versus Palpation-Guided Needle Placement on the Patellar Tendon: A Cadaveric Study. Life. 2023; 13(10):2060. https://doi.org/10.3390/life13102060
Chicago/Turabian StyleArias-Buría, José L., Sergio Borrella-Andrés, Jacobo Rodríguez-Sanz, Carlos López-de-Celis, Miguel Malo-Urriés, César Fernández-de-las-Peñas, Gracia M. Gallego-Sendarrubias, Vanessa González-Rueda, Albert Pérez-Bellmunt, and Isabel Albarova-Corral. 2023. "Precision and Safety of Ultrasound-Guided versus Palpation-Guided Needle Placement on the Patellar Tendon: A Cadaveric Study" Life 13, no. 10: 2060. https://doi.org/10.3390/life13102060
APA StyleArias-Buría, J. L., Borrella-Andrés, S., Rodríguez-Sanz, J., López-de-Celis, C., Malo-Urriés, M., Fernández-de-las-Peñas, C., Gallego-Sendarrubias, G. M., González-Rueda, V., Pérez-Bellmunt, A., & Albarova-Corral, I. (2023). Precision and Safety of Ultrasound-Guided versus Palpation-Guided Needle Placement on the Patellar Tendon: A Cadaveric Study. Life, 13(10), 2060. https://doi.org/10.3390/life13102060










