Bioactive Compounds of Dietary Origin and Their Influence on Colorectal Cancer as Chemoprevention
Abstract
1. Introduction
2. Generalities of Bioactive Compounds in CRC
2.1. Polyphenols
2.2. Flavonoids
2.3. Carotenoids
3. Regulation of Molecular Pathways in Colorectal Carcinogenesis by Bioactive Compounds
4. Chemosensitive and Chemopreventive Properties by Bioactive Compounds in CRC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| ABCB1 | ATP binding cassette gene B1 |
| AKT | Serine/threonine kinase |
| ALDH1A1 | Aldehyde dehydrogenase 1A1 |
| AP-1 | Activator protein-1 |
| APC | Adenomatous polyposis coli |
| BAX | Bcl-2 Associated X-protein |
| BCL-2 | B-cell lymphoma 2 |
| Bim | Bcl-2-interacting mediator |
| BRAF | V-Raf Murine Sarcoma Viral Oncogene Homolog B oncogene |
| COX 1 | Cyclooxygenase 1 |
| COX 2 | Cyclooxygenase 2 |
| CRC | Colorectal Cancer |
| CSC | Cancer Stem Cells |
| DNA | Deoxiribonucleic acid |
| DNMT3A | DNA methyltransferase 3 alpha |
| DR5 | Death Receptor 5 |
| EGCG | Epigallocatechin-3-gallate |
| EMT | Epithelial Mesenchymal Transition |
| ERK | Extracellular Signal-Regulated Kinase |
| FOLFIRI | Folinic acid, Fluorouracil and Irinotecan |
| FOLFOX | Folinic acid, Fluorouracil and Oxaliplatin |
| GSK-3 | Glycogen Synthase Kinase-3 |
| IBD | Inflammatory Bowel Disease |
| IFN-γ | Interferon gamma |
| iNOS | inducible Nitric Oxide Synthase |
| JAK | Janus Kinases |
| KITLG | c-KIT ligand gene |
| MAPK | Mitogen-Activated Protein Kinases |
| MCL-1 | Myeloid Leukemia 1 |
| MiRNA | Micro RNA |
| MMP-2 | Matrix Metalloproteinases 2 |
| MMP-9 | Matrix Metalloproteinases 9 |
| mRNA | Messenger RNA |
| mTORC | mechanistic Target Of Rapamycin Complex 1 |
| NF-kB | Nuclear Factor Kappa-light-chain-enhancer of Activated B cells |
| Nrf2 | Nuclear Erytroid Factor 2 related factor 2 |
| OCT 4 | Octamer-Binding Transcription Factor 4 |
| PARP | The Poly (ADP-ribose) Polymerase |
| PCN | Phenazine-1-carboxamide |
| p-FAK | Phospho- Focal Adhesion Kinase |
| PGC1α | Peroxisome Proliferator-activated receptor-gamma Coactivator α |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidylinositol 3-kinase |
| PPARβ | Perosyxomel Proliferator Activated Receptor β |
| PPARδ | Perosyxomel Proliferator Activated Receptor δ |
| PPARγ | Perosyxomel Proliferator Activated Receptor γ |
| PTEN | Phosphatase and Tensin homolog |
| ROS | Reactive Oxigen Species |
| SIRT1 | Silent Information Regulator 1 |
| SLC1A5 | Solute Carrier Family 1 Member 5 |
| STAT | Signal Transducer and Activator of Transcription |
| STR1 | Stromelysin-1 |
| SUMO | Small Ubiquitin-like Modifier |
| TGF-β | Transforming Growth Factor beta |
| TLR4 | Toll Type Receptor 4 |
| TNF-α | Tumor Necrosis Factor Alpha α |
| TSC1 | Tuberous Sclerosis Complex 1 |
| TSC2 | Tuberous Dclerosis Complex 2 |
| UVR | Ultraviolet Radiation |
| VEGF | Vascular Endothelial Growth Factor |
| WNT | Wingless-related integration site |
References
- Nosrati, N.; Bakovic, M.; Paliyath, G. Molecular mechanisms and pathways as targets for cancer prevention and progression with dietary compounds. Int. J. Mol. Sci. 2017, 18, 2050. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.; Cottet, V.; Dossus, L.; Fassier, P.; Ginhac, J.; Latino-Martel, P.; Romieu, I.; Schneider, S.; Srour, B.; Touillaud, M.; et al. Nutritional Factors during and after Cancer: Impacts on Survival and Quality of Life. Nutrients 2022, 14, 2958. [Google Scholar] [CrossRef] [PubMed]
- Bullock, A.F.; Greenley, S.L.; McKenzie, G.A.G.; Paton, L.W.; Johnson, M.J. Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: Systematic review, narrative synthesis and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 1519–1535. [Google Scholar] [CrossRef] [PubMed]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Food Sci. Nutr. 2014, 3, 17–179. [Google Scholar] [CrossRef]
- González, S. Dietary Bioactive Compounds and Human Health and Disease. Nutrients 2020, 12, 348. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Q.; Liu, Q.; Li, H.; Zhang, W.; Sun, C. Focus on immune checkpoint PD-1/PD-L1 pathway: New advances of polyphenol phytochemicals in tumor immunotherapy. Biomed. Pharmacother. 2022, 154, 113618. [Google Scholar] [CrossRef]
- Zheng, C.; Luo, W.; Liu, Y.; Chen, J.; Deng, H.; Zhou, Z.; Shen, J. Killing three birds with one stone: Multi-stage metabolic regulation mediated by clinically usable berberine liposome to overcome photodynamic immunotherapy resistance. Chem. Eng. J. 2023, 454, 140164. [Google Scholar] [CrossRef]
- Lim, S.O.; Li, C.W.; Xia, W.; Cha, J.H.; Chan, L.C.; Wu, Y.; Chang, S.-S.; Lin, W.-C.; Hsu, J.-M.; Hsu, Y.-H.; et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell 2016, 30, 925–939. [Google Scholar] [CrossRef]
- Kang, D.Y.; Sp, N.; Jo, E.S.; Rugamba, A.; Hong, D.Y.; Lee, H.G.; Yoo, J.-S.; Liu, Q.; Jang, K.-J.; Yang, Y.M. The Inhibitory Mechanisms of Tumor PD-L1 Expression by Natural Bioactive Gallic Acid in Non-Small-Cell Lung Cancer (NSCLC) Cells. Cancers 2020, 12, 727. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Jiang, X.; Zheng, C.; Luo, W.; Xiang, X.; Qi, X.; Shen, J. Metformin modified chitosan as a multi-functional adjuvant to enhance cisplatin-based tumor chemotherapy efficacy. Int. J. Biol. Macromol. 2023, 224, 797–809. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef]
- Kciuk, M.; Kołat, D.; Kałuzińska-Kołat, Ż.; Gawrysiak, M.; Drozda, R.; Celik, I.; Kontek, R. PD-1/PD-L1 and DNA Damage Response in Cancer. Cells 2023, 12, 530. [Google Scholar] [CrossRef] [PubMed]
- Toche Tuesta, A.; Curay Carhuamaca, V.L.; Diaz Barrientos, R.; Fernández Rebaza, G.A.; Bonilla Rivera, P.E. Estructura química de compuestos fenólicos del extracto etanólico de hojas de Clinopodium pulchellum (Kunth) Govaerts “panisara”. Rev. Peru Med. Integr. 2017, 2, 803–809. [Google Scholar] [CrossRef][Green Version]
- Chiu, H.F.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Gastroprotective Effects of Polyphenols against Various Gastro-Intestinal Disorders: A Mini-Review with Special Focus on Clinical Evidence. Molecules 2021, 26, 2090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol–Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.N.; Felipe da Costa, S.; Colquhoun, A. Eicosanoids and cancer. Clinics 2018, 73, e530s. [Google Scholar] [CrossRef] [PubMed]
- Gholamalizadeh, M.; Majidi, N.; Tajaddod, S.; Abdollahi, S.; Poorhosseini, S.M.; Ahmadzadeh, M.; Joubani, M.N.; Dahka, S.M.; Shafaei, H.; Hajiesmaeil, M.; et al. Interactions of Colorectal Cancer, Dietary Fats, and Polymorphisms of Arachidonate Lipoxygenase and Cyclooxygenase Genes: A Literature Review. Front. Oncol. 2022, 12, 865208. [Google Scholar] [CrossRef]
- Wasti, H.; Shawana, S. Comparision of BRAF V600E, COX–2 and p53 as Biomarkers for the Early Detection of Colorectal Cancer. J. Bahria Univ. Med. Dent. Coll. 2019, 9, 147–150. [Google Scholar] [CrossRef]
- Aldecoa, F.; Ávila, J. La vía canónica PI3K/AKT/mTOR y sus alteraciones en cáncer. Horiz. Médico. 2021, 21, e1547. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stefani, C.; Stănescu, I.I.; Stanescu, A.M.A.; Rusu, I.R.; Radulescu, R.; Rusu, G.C.; Greabu, M. Structure, activation and biological effects of AKT or protein kinase B. Rom. J. Med. Pract. 2019, 14, 246–250. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Wang, L.; Liu, P.; Zhao, S.; Li, H.; Jiang, Y.; Guo, Y.; Wang, X. Resveratrol inhibits paclitaxel-induced neuropathic pain by the activation of PI3K/Akt and SIRT1/PGC1&α; pathway. J. Pain Res. 2019, 12, 879–890. [Google Scholar]
- González-Sarrías, A.; Tomé-Carneiro, J.; Bellesia, A.; Tomás-Barberán, F.A.; Espín, J.C. The ellagic acid-derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5-fluorouracil chemotherapy on human colon cancer cells. Food Funct. 2015, 6, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Viggiano, T.R.; Buttar, N.S.; Song, L.M.W.K.; Schroeder, K.W.; Kraichely, R.E.; Kisiel, J.B.; Gostout, C.J.; Kalaiger, A.M. Randomized Phase II Trial of Polyphenon E versus Placebo in Patients at High Risk of Recurrent Colonic Neoplasia. Cancer Prev. Res. 2021, 14, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Herrero de la Parte, B.; Rodeño-Casado, M.; Iturrizaga Correcher, S.; Mar Medina, C.; García-Alonso, I. Curcumin Reduces Colorectal Cancer Cell Proliferation and Migration and Slows In Vivo Growth of Liver Metastases in Rats. Biomedicines 2021, 9, 1183. [Google Scholar] [CrossRef]
- Bahrami, A.; Jafari, S.; Rafiei, P.; Beigrezaei, S.; Sadeghi, A.; Hekmatdoost, A.; Rashidkhani, B.; Hejazi, E. Dietary intake of polyphenols and risk of colorectal cancer and adenoma—A case-control study from Iran. Complement. Ther. Med. 2019, 45, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Buneman, O.P.; et al. The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br. J. Pharmacol. 2015, 172, 5729–5743. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Kim, Y. Effects of β-carotene on Expression of Selected MicroRNAs, Histone Acetylation, and DNA Methylation in Colon Cancer Stem Cells. J. Cancer Prev. 2019, 24, 224–232. [Google Scholar] [CrossRef]
- Han, X.; Zhao, R.; Zhang, G.; Jiao, Y.; Wang, Y.; Wang, D.; Cai, H. Association of Retinol and Carotenoids Content in Diet and Serum With Risk for Colorectal Cancer: A Meta-Analysis. Front. Nutr. 2022, 9, 918777. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]
- Costea, T.; Hudiță, A.; Ciolac, O.A.; Gălățeanu, B.; Ginghină, O.; Costache, M.; Ganea, C.; Mocanu, M.M. Chemoprevention of Colorectal Cancer by Dietary Compounds. Int. J. Mol. Sci. 2018, 19, 3787. [Google Scholar] [CrossRef] [PubMed]
- Carini, F.; David, S.; Tomasello, G.; Mazzola, M.; Damiani, P.; Rappa, F.; Battaglia, L.; Geagea, A.G.; Jurjus, R.; Leone, A. Colorectal cancer: An update on the effects of lycopene on tumor progression and cell proliferation. J. Biol. Regul. Homeost. Agents 2017, 31, 769–774. [Google Scholar] [PubMed]
- Alhoshani, N.M.; Al-Zharani, M.; Almutairi, B.; Aljarba, N.H.; AL-Johani, N.S.; Alkeraishan, N.; AlKahtane, A.A.; Alarifi, S.; Ali, D.; Alkahtani, S. Antioxidant and anti-inflammatory activities of lycopene against 5-fluorouracil-induced cytotoxicity in Caco2 cells. Saudi Pharm. J. 2022, 30, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Alhoshani, N.M.; Al-Johani, N.S.; Alkeraishan, N.; Alarifi, S.; Alkahtani, S. Effect of lycopene as an adjuvant therapy with 5-florouracil in human colon cancer. Saudi J. Biol. Sci. 2022, 29, 103392. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef]
- Terasaki, M.; Uehara, O.; Ogasa, S.; Sano, T.; Kubota, A.; Kojima, H.; Miyashita, K.; Mutoh, M. Alteration of fecal microbiota by fucoxanthin results in prevention of colorectal cancer in AOM/DSS mice. Carcinogenesis 2021, 42, 210–219. [Google Scholar] [CrossRef]
- Terasaki, M.; Iida, T.; Kikuchi, F.; Tamura, K.; Endo, T.; Kuramitsu, Y.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Fucoxanthin potentiates anoikis in colon mucosa and prevents carcinogenesis in AOM/DSS model mice. J. Nutr. Biochem. 2019, 64, 198–205. [Google Scholar] [CrossRef]
- Lopes-Costa, E.; Abreu, M.; Gargiulo, D.; Rocha, E.; Ramos, A.A. Anticancer effects of seaweed compounds fucoxanthin and phloroglucinol, alone and in combination with 5-fluorouracil in colon cells. J. Toxicol. Environ. Health A 2017, 80, 776–787. [Google Scholar] [CrossRef]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Tanaka, T.; Miyamoto, S.; Mutoh, M. A marine bioofunctional lipid, fucoxanthinol, attenuates human colorectal cancer stemmlike cell tumorigenicity and sphere formation. J. Clin. Biochem. Nutr. 2017, 61, 25–32. [Google Scholar] [CrossRef]
- Bakshi, H.A.; Quinn, G.A.; Nasef, M.M.; Mishra, V.; Aljabali, A.A.A.; El-Tanani, M.; Serrano-Aroca, Á.; Webba Da Silva, M.; McCarron, P.A.; Tambuwala, M.M. Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-kB/VEGF Pathways. Cells 2022, 11, 1502. [Google Scholar] [CrossRef] [PubMed]
- Veisi, A.; Akbari, G.; Mard, S.A.; Badfar, G.; Zarezade, V.; Mirshekar, M.A. Role of crocin in several cancer cell lines: An updated review. Iran. J. Basic Med. Sci. 2020, 23, 3–12. [Google Scholar] [PubMed]
- Tao, W.; Ruan, J.; Wu, R.; Zhao, M.; Zhao, T.; Qi, M.; Yau, S.S.; Yao, G.; Zhang, H.; Hu, Y.; et al. A natural carotenoid crocin exerts antidepressant action by promoting adult hippocampal neurogenesis through Wnt/β-catenin signaling. J. Adv. Res. 2023, 43, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shi, N.; Afzali, A. Chemopreventive Effects of Strawberry and Black Raspberry on Colorectal Cancer in Inflammatory Bowel Disease. Nutrients 2019, 11, 1261. [Google Scholar] [CrossRef]
- La Vecchia, S.; Sebastián, C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev. Biol. 2020, 98, 63–70. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, M.; Bai, L.L.; Liao, W.; Zhou, K.; Zhang, M.; Wu, Q.; Wen, F.; Lei, W.; Zhang, P.; et al. Comprehensive analysis of EMT-related genes and lncRNAs in the prognosis, immunity, and drug treatment of colorectal cancer. J. Transl. Med. 2021, 19, 391. [Google Scholar] [CrossRef]
- Erdogan, F.; Radu, T.B.; Orlova, A.; Qadree, A.K.; de Araujo, E.D.; Israelian, J.; Valent, P.; Mustjoki, S.M.; Herling, M.; Moriggl, R.; et al. JAK-STAT core cancer pathway: An integrative cancer interactome analysis. J. Cell Mol. Med. 2022, 26, 2049–2062. [Google Scholar] [CrossRef]
- Ni, Y.; Low, J.T.; Silke, J.; O’Reilly, L.A. Digesting the Role of JAK-STAT and Cytokine Signaling in Oral and Gastric Cancers. Front. Immunol. 2022, 13, 835997. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Song, H.; Yin, H.; Jiang, T.; Xu, Y.; Liu, L.; Wang, H.; Gao, H.; Wang, R.; et al. The circular RNA circSPARC enhances the migration and proliferation of colorectal cancer by regulating the JAK/STAT pathway. Mol. Cancer 2021, 20, 81. [Google Scholar] [CrossRef]
- Kovács, T.; Mikó, E.; Ujlaki, G.; Sári, Z.; Bai, P. The Microbiome as a Component of the Tumor Microenvironment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 137–153. [Google Scholar]
- Hanus, M.; Parada-Venegas, D.; Landskron, G.; Wielandt, A.M.; Hurtado, C.; Alvarez, K.; Hermoso, M.A.; López-Köstner, F.; De la Fuente, M. Immune System, Microbiota, and Microbial Metabolites: The Unresolved Triad in Colorectal Cancer Microenvironment. Front. Immunol. 2021, 12, 612826. [Google Scholar] [CrossRef]
- Laplane, L.; Duluc, D.; Bikfalvi, A.; Larmonier, N.; Pradeu, T. Beyond the tumour microenvironment. Int. J. Cancer 2019, 145, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, Z.P.; Zhang, S.S.; Jing, Y.Y.; Bu, X.X.; Wang, C.Y.; Sun, K.; Jiang, G.-C.; Zhao, X.; Li, R.; et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J. Biol. Chem. 2011, 286, 25007–25015. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of Colorectal Carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.-R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells—Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Yang, Y.; Misra, B.B.; Liang, L.; Bi, D.; Weng, W.; Wu, W.; Cai, S.; Qin, H.; Goel, A.; Li, X.; et al. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics 2019, 9, 4101–4114. [Google Scholar] [CrossRef]
- Landskron, G.; De La Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor Necrosis Factor α and Regulatory T Cells in Oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γin tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Duchartre, Y.; Kim, Y.M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol./Hematol. 2016, 99, 141–149. [Google Scholar] [CrossRef]
- Wu, C.H.; Hsu, F.T.; Chao, T.L.; Lee, Y.H.; Kuo, Y.C. Revealing the suppressive role of protein kinase C delta and p38 mitogen-activated protein kinase (MAPK)/NF-κB axis associates with lenvatinib-inhibited progression in hepatocellular carcinoma in vitro and in vivo. Biomed. Pharmacother. 2022, 145, 112437. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tan, A.H.; Li, J. Curcumin Represses Colorectal Cancer Cell Proliferation by Triggering Ferroptosis via PI3K/Akt/mTOR Signaling. Nutr. Cancer 2023, 75, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, T.; Chen, C. The critical roles of miR-21 in anti-cancer effects of curcumin. Ann. Transl. Med. 2015, 4, 330. [Google Scholar]
- Xiang, T.; Fang, Y.; Wang, S.X. Quercetin suppresses HeLa cells by blocking PI3K/Akt pathway. J. Huazhong Univ. Sci. Technol. (Med. Sci.) 2014, 34, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Si, Y.; Wang, Z.; Wang, J.; Guo, Y.; Zhang, X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int. J. Mol. Med. 2016, 38, 619–626. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wu, K.; Huang, J.; Liu, Y.; Wang, X.; Meng, Z.J.; Yuan, S.-X.; Wang, D.-X.; Luo, J.-Y.; Zuo, G.-W.; et al. The PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. Int. J. Oncol. 2014, 45, 104–112. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; Jin, G.; Yao, K.; Zhao, Z.; Bie, L.; Guo, Y.; Li, N.; Deng, W.; Chen, X.; et al. Resveratrol suppresses colon cancer growth by targeting the AKT/STAT3 signaling pathway. Int. J. Mol. Med. 2019, 43, 630–640. [Google Scholar] [CrossRef]
- Wang, R.; Lu, X.; Yu, R. Lycopene Inhibits Epithelial–Mesenchymal Transition and Promotes Apoptosis in Oral Cancer via PI3K/AKT/m-TOR Signal Pathway. Drug Des. Dev. Ther. 2020, 14, 2461–2471. [Google Scholar] [CrossRef]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelialto-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2014, 36, 355–367. [Google Scholar] [CrossRef]
- Han, M.; Song, Y.; Zhang, X. Quercetin suppresses the migration and invasion in human colon cancer caco-2 cells through regulating toll-like receptor 4/nuclear factor-kappa B pathway. Pharmacogn. Mag. 2016, 12, S237. [Google Scholar]
- Qi, W.J.; Sheng, W.S.; Peng, C.; Xiaodong, M.; Yao, T.Z. Investigating into anti-cancer potential of lycopene: Molecular targets. Biomed. Pharmacother. 2021, 138, 111546. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Riahirad, H.; Pabarja, A.; Jafari, E.; Khandani, B.K. Effect of Cinnamic acid and FOLFOX in diminishing side population and downregulating cancer stem cell markers in colon cancer cell line HT-29. DARU. J. Pharm. Sci. 2018, 26, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.N. Curcumin: A therapeutic strategy in cancers by inhibiting the canonical WNT/β-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 323. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Brockmueller, A.; Shakibaei, M. Resveratrol suppresses cross-talk between colorectal cancer cells and stromal cells in multicellular tumor microenvironment: A bridge between in vitro and in vivo tumor microenvironment study. Molecules 2020, 25, 4292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.Q.; Zhang, Q.; Zhu, J.Y.; Li, Y.; Xie, C.F.; Li, X.-T.; Wu, J.-S.; Geng, S.-S.; Zhong, C.-Y.; et al. (-)-Epigallocatechin-3-gallate inhibits colorectal cancer stem cells by suppressing Wnt/β-catenin pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef]
- Afzal, M.; Safer, A.M.; Menon, M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting GSK-3β- and PP2A-independent β-catenin phosphorylation/degradation. BioFactors 2014, 40, 586–595. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90. [Google Scholar] [CrossRef]
- Raina, K.; Agarwal, C.; Agarwal, R. Effect of silibinin in human colorectal cancer cells: Targeting the activation of NF-κB signaling. Mol. Carcinog. 2013, 52, 195–206. [Google Scholar] [CrossRef]
- Özgöçmen, M.; Bayram, D.; Armağan, İ.; Türel, G.Y.; Sevimli, M.; Şenol, N. Is Quercetin Beneficial for Colon Cancer? A Cell Culture Study, Using the Apoptosis Pathways. Anticancer Agents Med. Chem. 2021, 22, 193–200. [Google Scholar] [CrossRef]
- Rajagopal, C.; Lankadasari, M.B.; Aranjani, J.M.; Harikumar, K.B. Targeting oncogenic transcription factors by polyphenols: A novel approach for cancer therapy. Pharmacol. Res. 2018, 130, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, H.; Zhang, X.; Zhang, S.; Zhu, S.; Wang, H. Resveratrol attenuates radiation enteritis through the SIRT1/FOXO3a and PI3K/AKT signaling pathways. Biochem. Biophys. Res. Commun. 2021, 554, 199–205. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; HLajis, N.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef]
- Lim, J.Y.; Wang, X.D. Mechanistic understanding of β-cryptoxanthin and lycopene in cancer prevention in animal models. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158652. [Google Scholar] [CrossRef] [PubMed]
- Urrego, D.; Tomczak, A.P.; Zahed, F.; Stühmer, W.; Pardo, L.A. Potassium channels in cell cycle and cell proliferation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130094. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; He, L.Y.; Chen, Y.; Wang, W.Y.; Zhao, X.H.; Wu, M.Y. Quercetin affects leptin and its receptor in human gastric cancer MGC-803 cells and JAK-STAT pathway. Chin. J. Cell Mol. Immunol. 2012, 28, 12–16. [Google Scholar]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martín-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Banala, R.R.; Mukherjee, S.; Uppula, P.; GPV, S.; AV, G.R.; Malarvilli, T. Novel biosynthesized gold nanoparticles as anti-cancer agents against breast cancer: Synthesis, biological evaluation, molecular modelling studies. Mater. Sci. Eng. C 2019, 99, 417–429. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-safi, I.; Conte, R.; Hano, C.; Amaghnouje, A.; Jawhari, F.Z.; Radouane, N.; Bencheikh, N.; Grafov, A.; Bousta, D. In Vivo and In Vitro Antidiabetic and Anti-Inflammatory Properties of Flax (Linum usitatissimum L.) Seed Polyphenols. Nutrients 2021, 13, 2759. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Cayssials, V.; Jenab, M.; Rothwell, J.A.; Fedirko, V.; Aleksandrova, K.; Tjønneland, A.; Kyrø, C.; Overvad, K.; Boutron-Ruault, M.C.; et al. Dietary intake of total polyphenol and polyphenol classes and the risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Eur. J. Epidemiol. 2018, 33, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hosokawa, M.; Kasajima, H.; Hatanaka, K.; Kudo, K.; Shimoyama, N.; Miyashita, K. Anticancer effects of fucoxanthin and fucoxanthinol on colorectal cancer cell lines and colorectal cancer tissues. Oncol. Lett. 2015, 10, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wu, H.; Fan, J.; Zhang, L.; Li, H.; Guo, H.; Yang, R.; Li, Z. The Mixture of Ferulic Acid and P-Coumaric Acid Suppresses Colorectal Cancer through lncRNA 495810/PKM2 Mediated Aerobic Glycolysis. Int. J. Mol. Sci. 2022, 23, 12106. [Google Scholar] [CrossRef]
- Goncalves, P.; Araujo, J.; Pinho, M.J.; Martel, F. In Vitro Studies on the Inhibition of Colon Cancer by Butyrate and Polyphenolic Compounds. Nutr. Cancer 2011, 63, 282–294. [Google Scholar] [CrossRef]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Bracci, L.; Fabbri, A.; Del Cornò, M.; Conti, L. Dietary Polyphenols: Promising Adjuvants for Colorectal Cancer Therapies. Cancers 2021, 13, 4499. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, S.; Xiang, B.; Li, L.; Lin, Y. Curcumin attenuates oxaliplatin-induced liver injury and oxidative stress by activating the Nrf2 pathway. Drug Des. Dev. Ther. 2020, 14, 73–85. [Google Scholar] [CrossRef]
- Ouyang, M.; Luo, Z.; Zhang, W.; Zhu, D.; Lu, Y.; Wu, J.; Yao, X. Protective effect of curcumin against irinotecan-induced intestinal mucosal injury via attenuation of NF-κB activation, oxidative stress and endoplasmic reticulum stress. Int. J. Oncol. 2019, 54, 1376–1386. [Google Scholar] [CrossRef]
- Sabet, N.S.; Atashbar, S.; Khanlou, E.M.; Kahrizi, F.; Salimi, A. Curcumin Attenuates Bevacizumab-Induced Toxicity via Suppressing Oxidative Stress and Preventing Mitochondrial Dysfunction in Heart Mitochondria. Naunyn-Schmiedeberg's Arch. Pharmacol. 2020, 393, 1447–1457. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Wang, K.; Han, W.; Wang, X.; Gao, M.; Wang, Z.; Sun, Y.; Yan, H.; Zhang, H.; et al. Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Eur. J. Pharmacol. 2020, 881, 173185. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Namkoong, K.; Shin, M.; Park, J.; Yang, E.; Ihm, J.; Thu, V.T.; Kim, H.K.; Han, J.; Ghaffar, S.; et al. Cardiovascular Protective Effects and Clinical Applications of Resveratrol. J. Med. Food 2017, 20, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective effect of resveratrol in a postinfarction heart failure model. Oxid. Med. Cell. Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Moranta, D.; Asensio, V.J.; Miralles, A.; Esteban, S. Effects of Resveratrol and Other Polyphenols on the Most Common Brain Age-Related Diseases. Curr. Med. Chem. 2017, 24, 4245–4266. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, W.; Lu, F.; Kong, W.; Zhou, X.; Miao, P.; Lei, C.; Wang, Y. Resveratrol attenuates neurological deficit and neuroinflammation following intracerebral hemorrhage. Exp. Ther. Med. 2018, 15, 4131–4138. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A review of resveratrol as a potent chemoprotective and synergistic agent in cancer chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Moutabian, H.; Majdaeen, M.; Ghahramani-Asl, R.; Yadollahi, M.; Gharepapagh, E.; Ataei, G.; Falahatpour, Z.; Bagheri, H.; Farhood, B. A systematic review of the therapeutic effects of resveratrol in combination with 5-fluorouracil during colorectal cancer treatment: With a special focus on the oxidant, apoptotic, and anti-inflammatory activities. Cancer Cell Int. 2022, 22, 142. [Google Scholar] [CrossRef]
- Santandreu, F.M.; Valle, A.; Oliver, J.; Roca, P. Resveratrol Potentiates the Cytotoxic Oxidative Stress Induced by Chemotherapy in Human Colon Cancer Cells. Cell. Physiol. Biochem. 2011, 28, 219–228. [Google Scholar] [CrossRef]
- Cocetta, V.; Quagliariello, V.; Fiorica, F.; Berretta, M.; Montopoli, M. Resveratrol as Chemosensitizer Agent: State of Art and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 2049. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.; Wang, Y. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell. Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef]
- Wang, J.; Huang, P.; Pan, X.; Xia, C.; Zhang, H.; Zhao, H.; Yuan, Z.; Liu, J.; Meng, C.; Liu, F. Resveratrol reverses TGF-β1-mediated invasion and metastasis of breast cancer cells via the SIRT3/AMPK/autophagy signal axis. Phytother. Res. 2023, 37, 211–230. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Xiao, K.; Jiang, J.; Guan, C.; Dong, C.; Wang, G.; Bai, L.; Sun, J.; Hu, C.; Bai, C. Curcumin Induces Autophagy via Activating the AMPK Signaling Pathway in Lung Adenocarcinoma Cells. J. Pharmacol. Sci. 2013, 123, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shen, G.; Khor, T.O.; Kim, J.H.; Kong, A.N.T. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2609–2620. [Google Scholar] [CrossRef] [PubMed]
- Holczer, M.; Besze, B.; Zámbó, V.; Csala, M.; Bánhegyi, G.; Kapuy, O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2018, 2018, 6721530. [Google Scholar] [CrossRef]
- Khan, K.; Quispe, C.; Javed, Z.; Iqbal, M.J.; Sadia, H.; Raza, S.; Irshad, A.; Salehi, B.; Reiner, Ž.; Sharifi-Rad, J. Resveratrol, curcumin, paclitaxel and miRNAs mediated regulation of PI3K/Akt/mTOR pathway: Go four better to treat bladder cancer. Cancer Cell Int. 2020, 20, 560. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Dutta, P.; Austin, D.; Wang, P.; Awad, A.; Vadgama, J.V. Combination of Resveratrol and 5-Flurouracil Enhanced Anti-Telomerase Activity and Apoptosis by Inhibiting STAT3 and Akt Signaling Pathways in Human Colorectal Cancer Cells. 2018. Available online: www.oncotarget.com (accessed on 28 June 2023).
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.U.; Nam, J.; Cha, M.D.; Kim, S.W. Inhibition of phosphodiesterase 4D decreases the malignant properties of DLD-1 colorectal cancer cells by repressing the AKT/mTOR/Myc signaling pathway. Oncol. Lett. 2019, 17, 3589–3598. [Google Scholar] [CrossRef]
- Celiberto, F.; Aloisio, A.; Girardi, B.; Pricci, M.; Iannone, A.; Russo, F.; Riezzo, G.; D’Attoma, B.; Ierardi, E.; Losurdo, G.; et al. Fibres and Colorectal Cancer: Clinical and Molecular Evidence. Int. J. Mol. Sci. 2023, 24, 13501. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernández, J.; Gutiérrez-del-Río, I.; Villar, C.J.; Lombó, F. New Insights toward Colorectal Cancer Chemotherapy Using Natural Bioactive Compounds. Front. Pharmacol. 2017, 8, 109. [Google Scholar] [CrossRef]
- Kumazaki, M.; Noguchi, S.; Yasui, Y.; Iwasaki, J.; Shinohara, H.; Yamada, N.; Akao, Y. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J. Nutr. Biochem. 2013, 24, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, W.; Dong, F.; Sun, H.; Wu, B.; Tan, J.; Zou, W.; Zhou, D. KITLG is a novel target of miR-34c that is associated with the inhibition of growth and invasion in colorectal cancer cells. J. Cell. Mol. Med. 2014, 18, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, B.M.; Weigert, A.; Scherzberg, M.C.; Ley, S.; Gilbert, B.; Brecht, K.; Brüne, B.; Steinhilber, D.; Stein, J.; Ulrich-Rückert, S. Resveratrol-induced potentiation of the antitumor effects of oxaliplatin is accompanied by an altered cytokine profile of human monocyte-derived macrophages. Apoptosis 2014, 19, 1136–1147. [Google Scholar] [CrossRef]
- Chang, T.K.; Yin, T.C.; Su, W.C.; Tsai, H.L.; Huang, C.W.; Chen, Y.C.; Li, C.-C.; Chen, P.-J.; Ma, C.-J.; Chuang, K.-H.; et al. A Pilot Study of Silymarin as Supplementation to Reduce Toxicities in Metastatic Colorectal Cancer Patients Treated with First-Line FOLFIRI Plus Bevacizumab. Oncol. Res. 2020, 28, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Koczurkiewicz-Adamczyk, P.; Klaś, K.; Gunia-Krzyżak, A.; Piska, K.; Andrysiak, K.; Stępniewski, J.; Lasota, S.; Wójcik-Pszczoła, K.; Dulak, J.; Madeja, Z.; et al. Cinnamic Acid Derivatives as Cardioprotective Agents against Oxidative and Structural Damage Induced by Doxorubicin. Int. J. Mol. Sci. 2021, 22, 6217. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, M.; Yang, T.; Qin, L.; Hu, Y.; Zhao, D.; Wu, L.; Liu, T. Cinnamic Acid Ameliorates Nonalcoholic Fatty Liver Disease by Suppressing Hepatic Lipogenesis and Promoting Fatty Acid Oxidation. Evid.-Based Complement. Altern. Med. 2021, 2021, 9561613. [Google Scholar] [CrossRef]
- Chae, H.K.; Kim, W.; Kim, S.K. Phytochemicals of Cinnamomi Cortex: Cinnamic Acid, but not Cinnamaldehyde, Attenuates Oxaliplatin-Induced Cold and Mechanical Hypersensitivity in Rats. Nutrients 2019, 11, 432. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, R.; Zhang, X.; Zhang, B.; Yao, Q. Curcumin may reverse 5-fluorouracil resistance on colonic cancer cells by regulating TET1-NKD-Wnt signal pathway to inhibit the EMT progress. Biomed. Pharmacother. 2020, 129, 110381. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
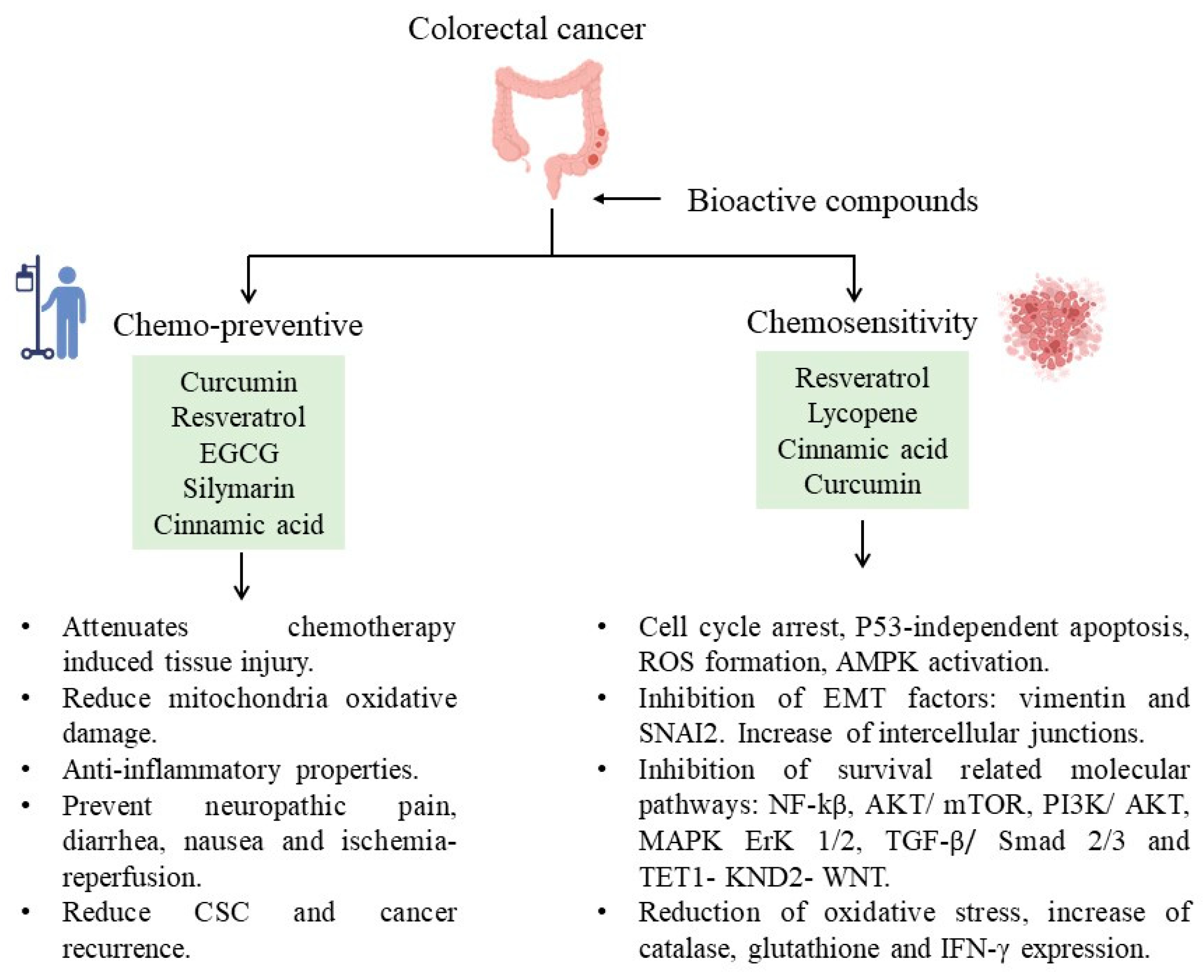
| Natural Products | Bioactive Compound | Food Sources |
|---|---|---|
| Flavonoids | Curcumin | Turmeric, Ginger, Curry |
| Resveratrol | Red wine, Red grapes, Peanuts | |
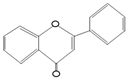 | Quercetin | Onions, Tea, Apples, Kale |
| Epigallocatechin Gallate | Green tea, White tea, Black tea | |
| Anthocyanins | Blackberries, Raspberries, Cherries | |
| Phenolic Acids | Caffeic Acid | Coffee beans, olives, potatoes, carrots, propolis |
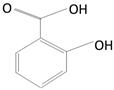 | Ellagic Acid | Pomegranates, blackberries, raspberries, strawberries |
| Gallic Acid | Grapes, strawberries, blueberries, mango, plums, hazelnut | |
| Carotenoids | α-carotenoid | Carrots, sweet potatoes, pumpkin, broccoli, spinach |
 | β-carotenoid | Carrots, sweet potatoes, pumpkin, spinach, kale |
| Lycopene | Tomatoes, watermelon, grapefruits | |
| Xanthophylls | β-cryptoxanthin | Citrus fruits, papaya, egg yolk, apples |
| Astaxanthin | Seafood, tomato | |
| Fucoxanthin | Brown seaweeds | |
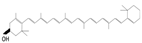 | Lutein | Broccoli, spinach, kale, kiwi, grapes, pumpkin |
| Zeaxanthin | Broccoli, spinach, kale, orange, peppers |
| Metabolic Pathway | Bioactive Compound | Mechanism | Function | Reference |
|---|---|---|---|---|
| ↓PI3K/AKT/mTOR | Curcumin | ↓miR 21/PTEN/Aktand IL-8 ↓AP-1 Activity | Inhibits growth, cell proliferation, and migration. Promotes apoptosis. | [63,64] |
| Quercetin | ↓p-AKT and Bcl-2 levels ↓pGSK3β | Decreases cell growth, proliferation, and migration. | [65,66] | |
| Resveratrol | ↓PI3K/PTEN ↓ AKT ↓ IL-8 | Decreases colon cell proliferation and formation. Regulates induced cell apoptosis (chemoprotection) and arrest in the G1 phase. | [67,68] | |
| Lycopene | ↓p-AKT ↓Cyclin D1expression | Inhibits cell proliferation. | [69] | |
| ↓EMT | Curcumin | ↑E-cadherin ↓Twist, vimentin | Reduces chemoresistance. Expression of BMI1, SUZ12, and EZH2 transcripts Suppresses migration and invasion. | [70] |
| Quercetin | [71] | |||
| Lycopene | ↑E-cadherin Inhibits PCN, and AP-1 | Reduces chemoresistance | [72] | |
| Cinnamic Acid | In combination with FOLFOX: ↓OCT4 ↓NANOG ↓ABCB1, ↓ALDH1A1 | Eliminate cancer stem cells. | [73] | |
| ↓Wnt/β-catenin | Curcumin | Acts as a PPARγ receptor agonist. | ↓ Inflammation TNF-α, growth, PGE2 levels | [71,74] |
| Quercetin | ↓MMP-2 ↓MMP-9 ↓TLR4 ↓NFKB ↓TNFα ↓COX-2 ↓IL-6 | |||
| Resveratrol | ↑IL-1β ↓Str1 ↓TGFb ↓NFKB ↓SUMO ↓WNT | Decreases invasion and inflammation. | [75] | |
| Reduces proliferation, formation of colonies, and invasion. Decreases spheroid formation and expression of CSC markers. | ||||
| EGCG | Promotes proteasome phosphorylation and degradation of β-catenin through a GSK-3B and PP2A-independent mechanism. | Decreases cell proliferation and induces apoptosis. Inhibits autophagy. Reduces inflammation. | [76,77,78] | |
| Lycopene | ↓CoX-2 ↓PGE2 ↓IL-1β ↓IL-6 ↓TNF-α ↓iNOS | Reduces invasion. | [79] | |
| ↓β-catenin ↓c-Myc ↓MMP-7 ↓MMP-9 | Regulate cell cycle, survival, autophagy, angiogenesis, and inflammation. | |||
| Silibinin | ↓COX-2 ↓NFKB ↓IL-6 ↓TNF-α ↓D1cyclin ↓Bcl-2 ↓VEGF ↓MMP2 ↓iNOS | Cell cycle regulation and apoptosis | [80] | |
| Quercetin | Inhibits D1 Cyclin, survuvin and GSK3 | [81,82] | ||
| Resveratrol | ↑Superoxide dismutase ↑glutathione peroxidase Sirt1/AMPK activation | [83] | ||
| Apoptosis | Curcumin | ↑ROS ↑Bax ↑caspases | Promote apoptosis | [84] |
| Lycopene | ↑DR5, Fas ↑p21 ↑Bax1, ↑caspase 3 | [85] | ||
| Cinnamic Acid | ↑Phase G0-G1 cells ↓PhaseS, G2/M cells ↑ROS ↑p21 ↑caspase 3 | Promote apoptosis | [86] | |
| JAK/STAT | Quercetin | Cell arrest in G0/G1 by p-STAT3 | Promote apoptosis and necrosis. | [87] |
| ↑Bax, ↑caspase3 ↑p53 | ||||
| EGCG | ↓Ki67 ↓promoter activity and transcription of STAT3 Inhibits AKT, ERK1/2 o P38 MAPK ↑Caspases-3, PARP, Bcl-2, Bim, Bak, MCL-1, E-cadherin, and Vimentin | Reduces proliferation and migration. Inhibits cell proliferation and promotes apoptosis. | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Gonzalez, P.; Garza-Treviño, E.N.; de la Garza Kalife, D.A.; Quiroz Reyes, A.; Hernández-Tobías, E.A. Bioactive Compounds of Dietary Origin and Their Influence on Colorectal Cancer as Chemoprevention. Life 2023, 13, 1977. https://doi.org/10.3390/life13101977
Delgado-Gonzalez P, Garza-Treviño EN, de la Garza Kalife DA, Quiroz Reyes A, Hernández-Tobías EA. Bioactive Compounds of Dietary Origin and Their Influence on Colorectal Cancer as Chemoprevention. Life. 2023; 13(10):1977. https://doi.org/10.3390/life13101977
Chicago/Turabian StyleDelgado-Gonzalez, Paulina, Elsa N. Garza-Treviño, David A. de la Garza Kalife, Adriana Quiroz Reyes, and Esther Alhelí Hernández-Tobías. 2023. "Bioactive Compounds of Dietary Origin and Their Influence on Colorectal Cancer as Chemoprevention" Life 13, no. 10: 1977. https://doi.org/10.3390/life13101977
APA StyleDelgado-Gonzalez, P., Garza-Treviño, E. N., de la Garza Kalife, D. A., Quiroz Reyes, A., & Hernández-Tobías, E. A. (2023). Bioactive Compounds of Dietary Origin and Their Influence on Colorectal Cancer as Chemoprevention. Life, 13(10), 1977. https://doi.org/10.3390/life13101977






