Impact of Particulate Matter Exposure and Aerobic Exercise on Circulating Biomarkers of Oxidative Stress, Antioxidant Status, and Inflammation in Young and Aged Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Maintenance
2.2. PM Intervention
2.3. Aerobic Exercise Intervention
2.4. Blood Sampling and Analysis
2.5. Statistical Analysis
3. Results
3.1. Changes in Biomarkers of Oxidative Stress
3.1.1. Changes in Serum 8-OHdG Levels
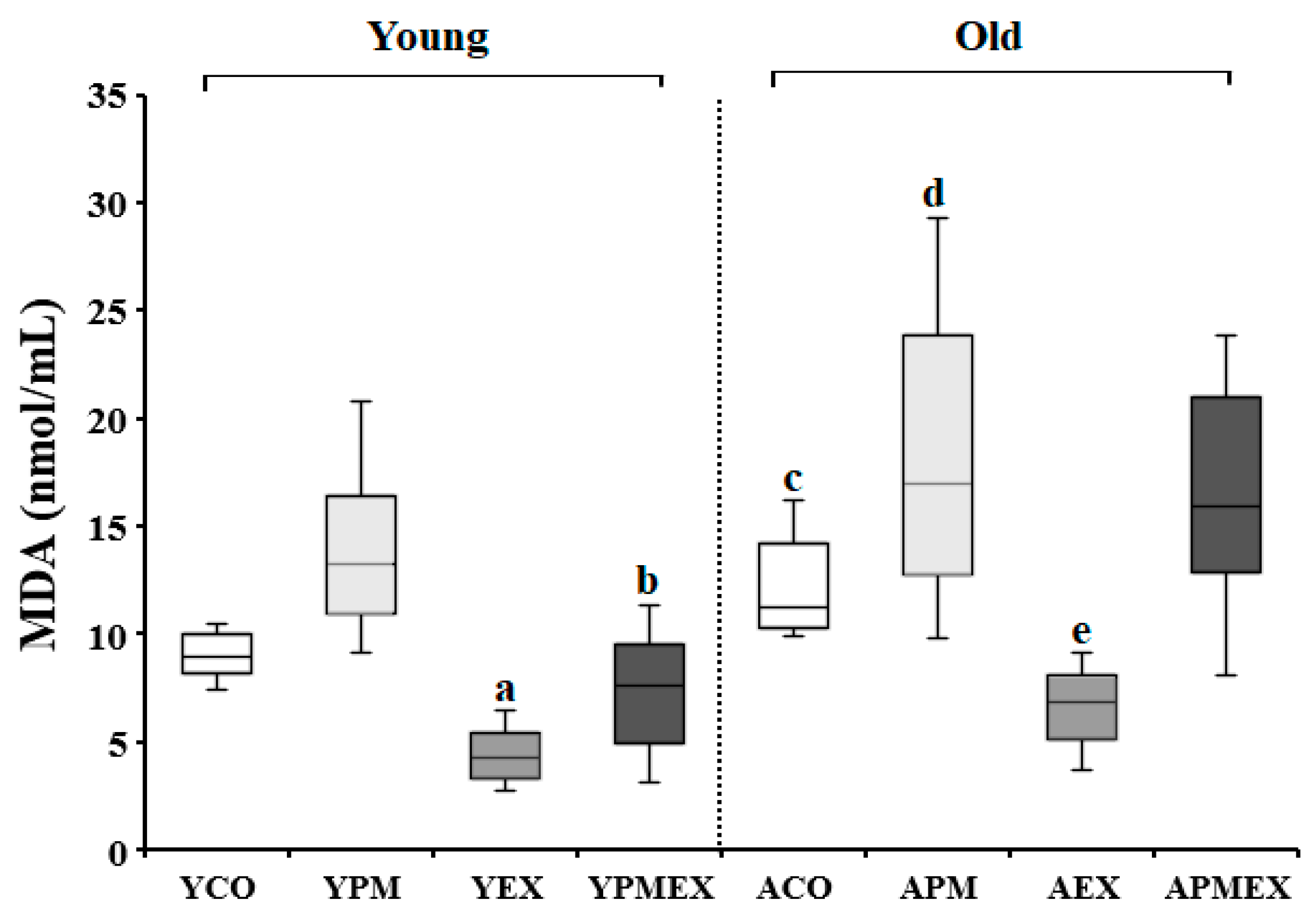
3.1.2. Changes in Serum MDA Levels
3.2. Changes in Biomarkers of Antioxidant Status
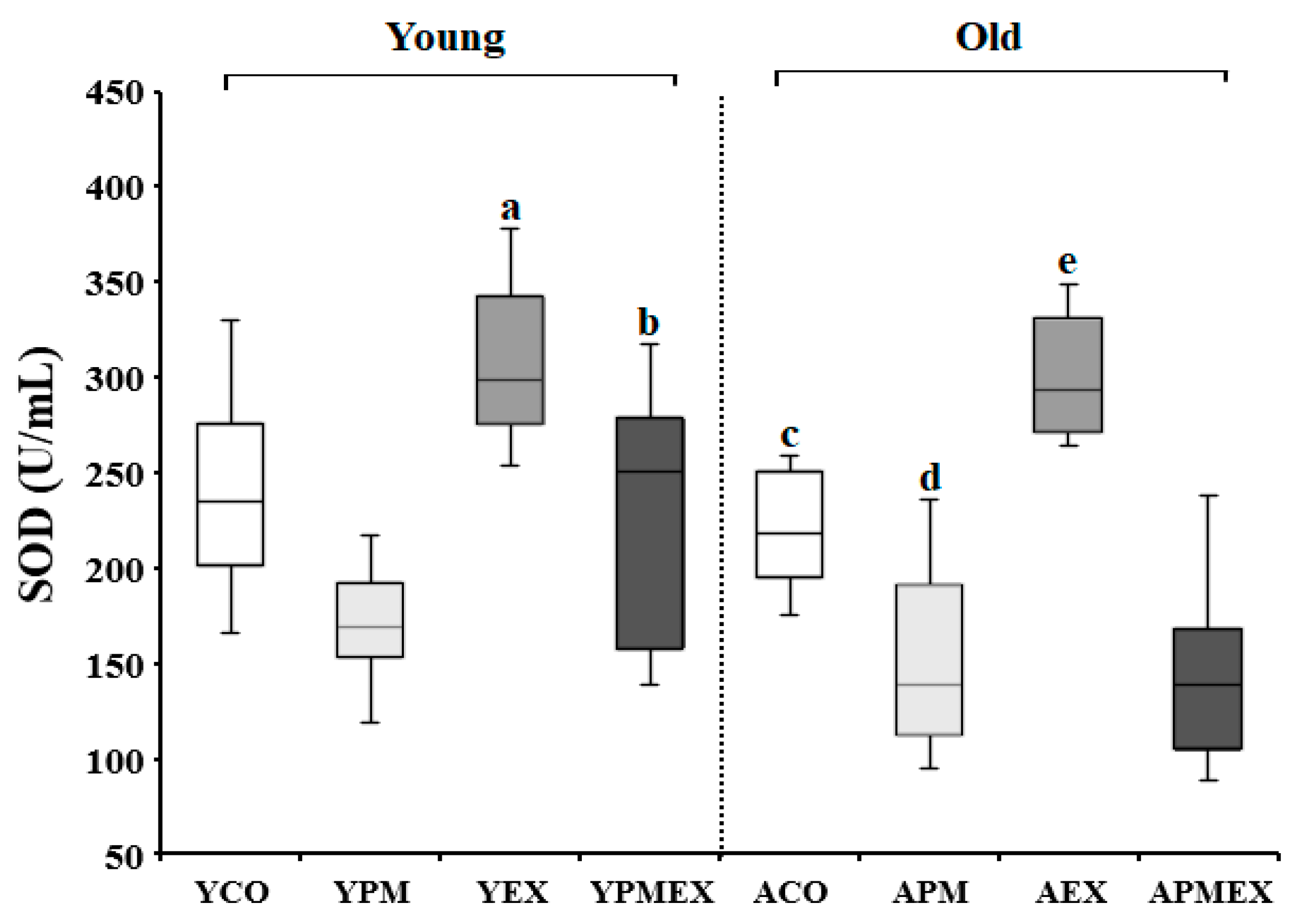
3.2.1. Changes in Serum SOD Activities
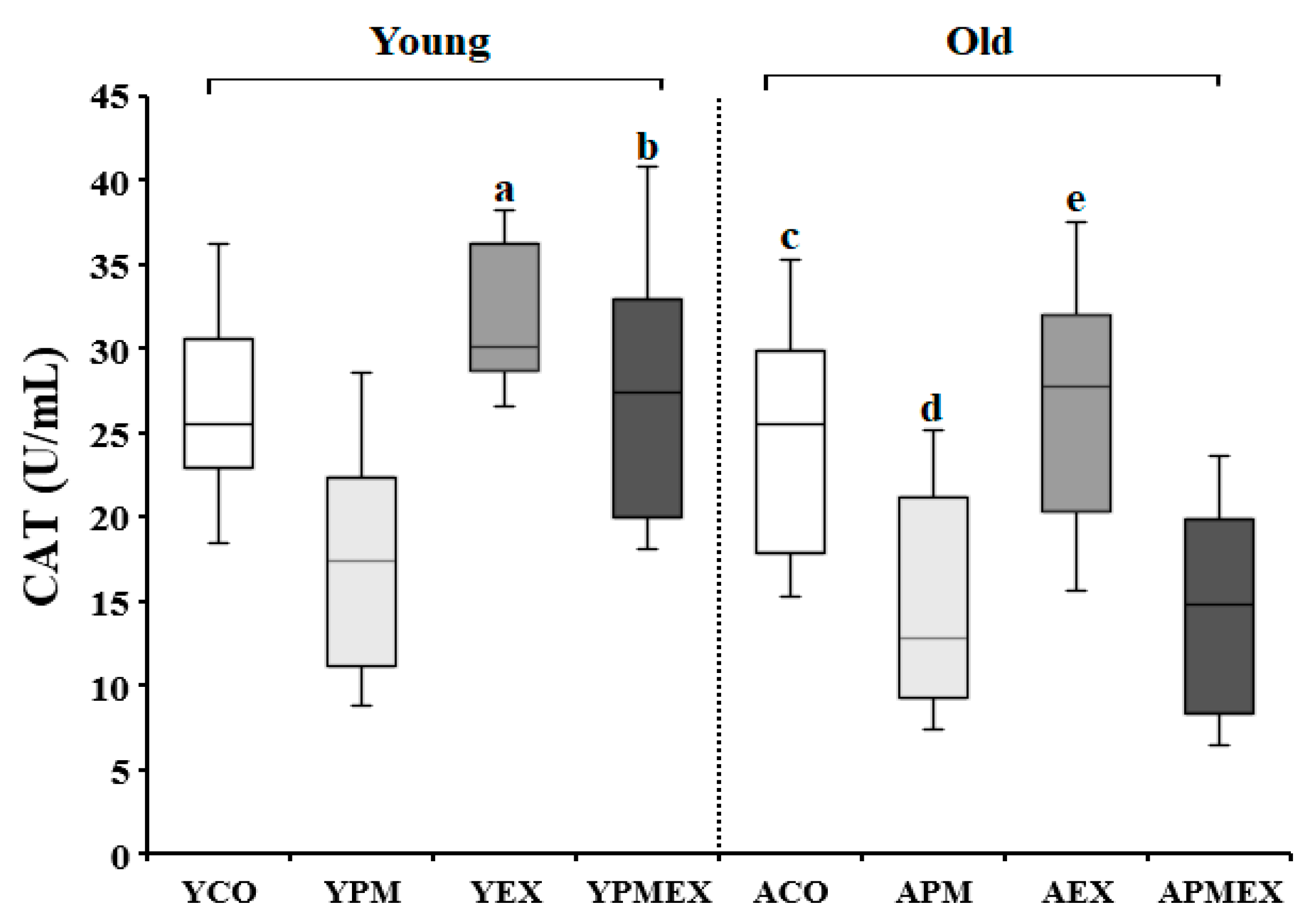
3.2.2. Changes in Serum CAT Activity
3.3. Changes in Biomarkers of Inflammation
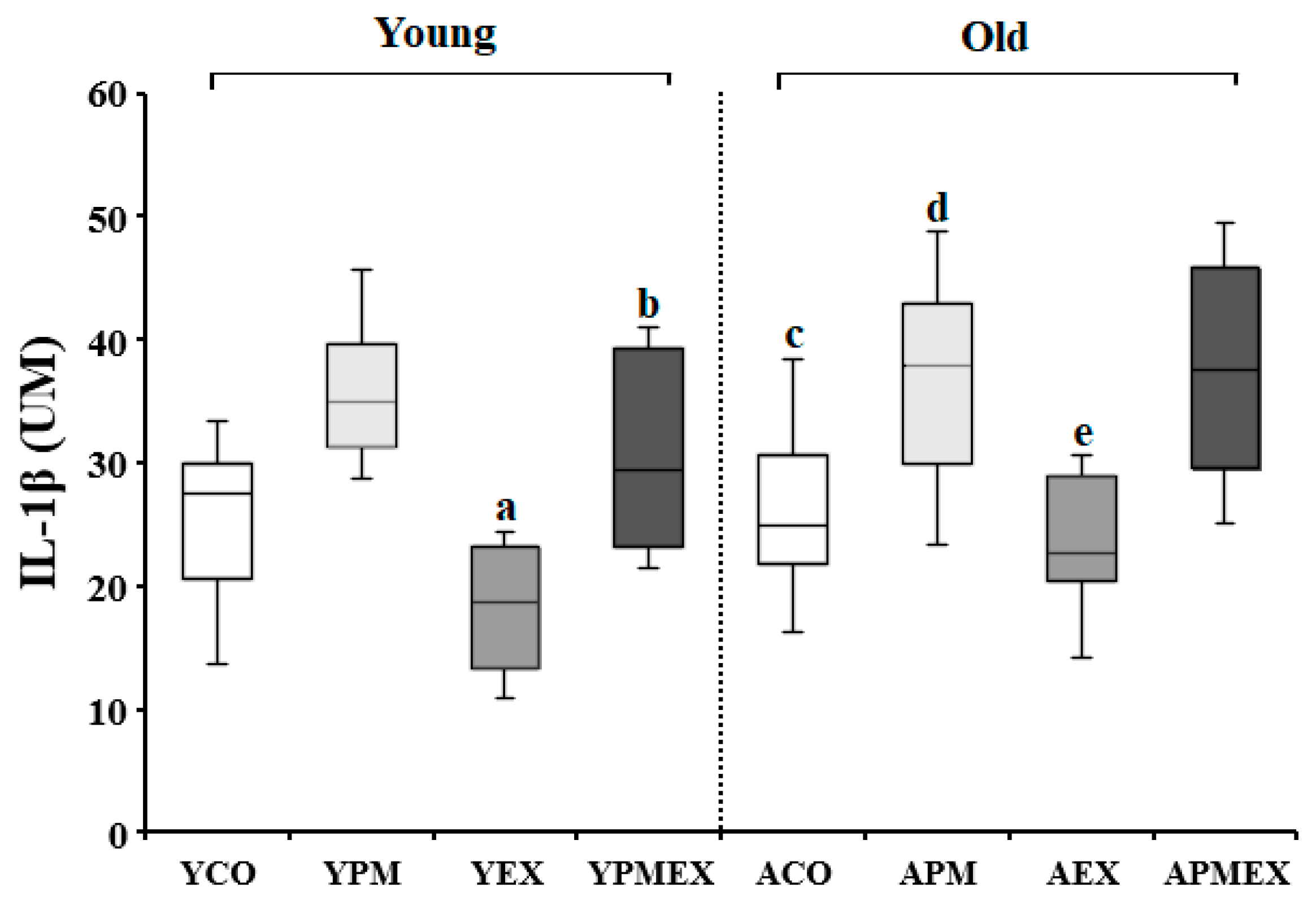
3.3.1. Changes in Serum IL-1β Levels
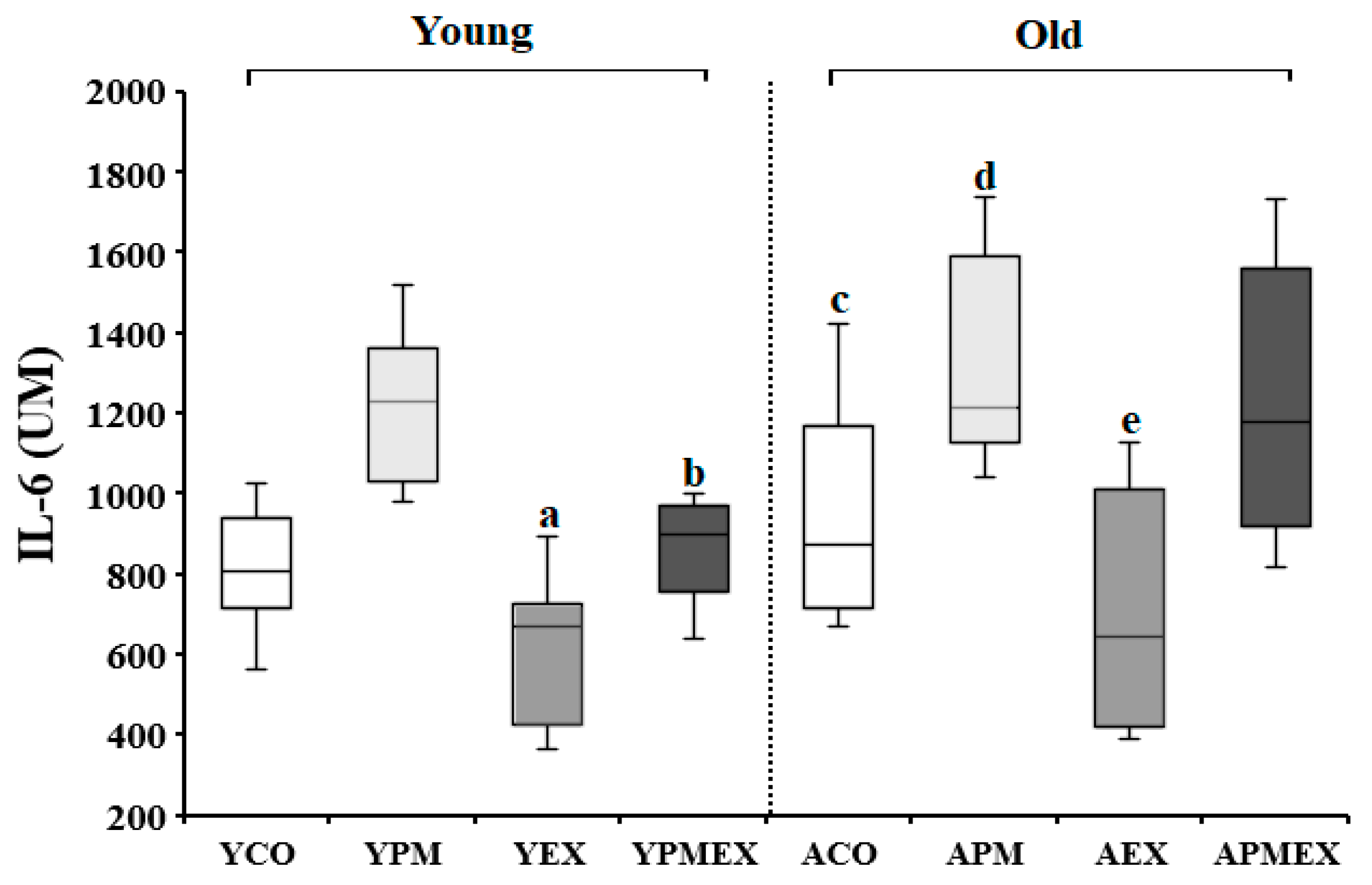
3.3.2. Changes in Serum IL-6 Levels
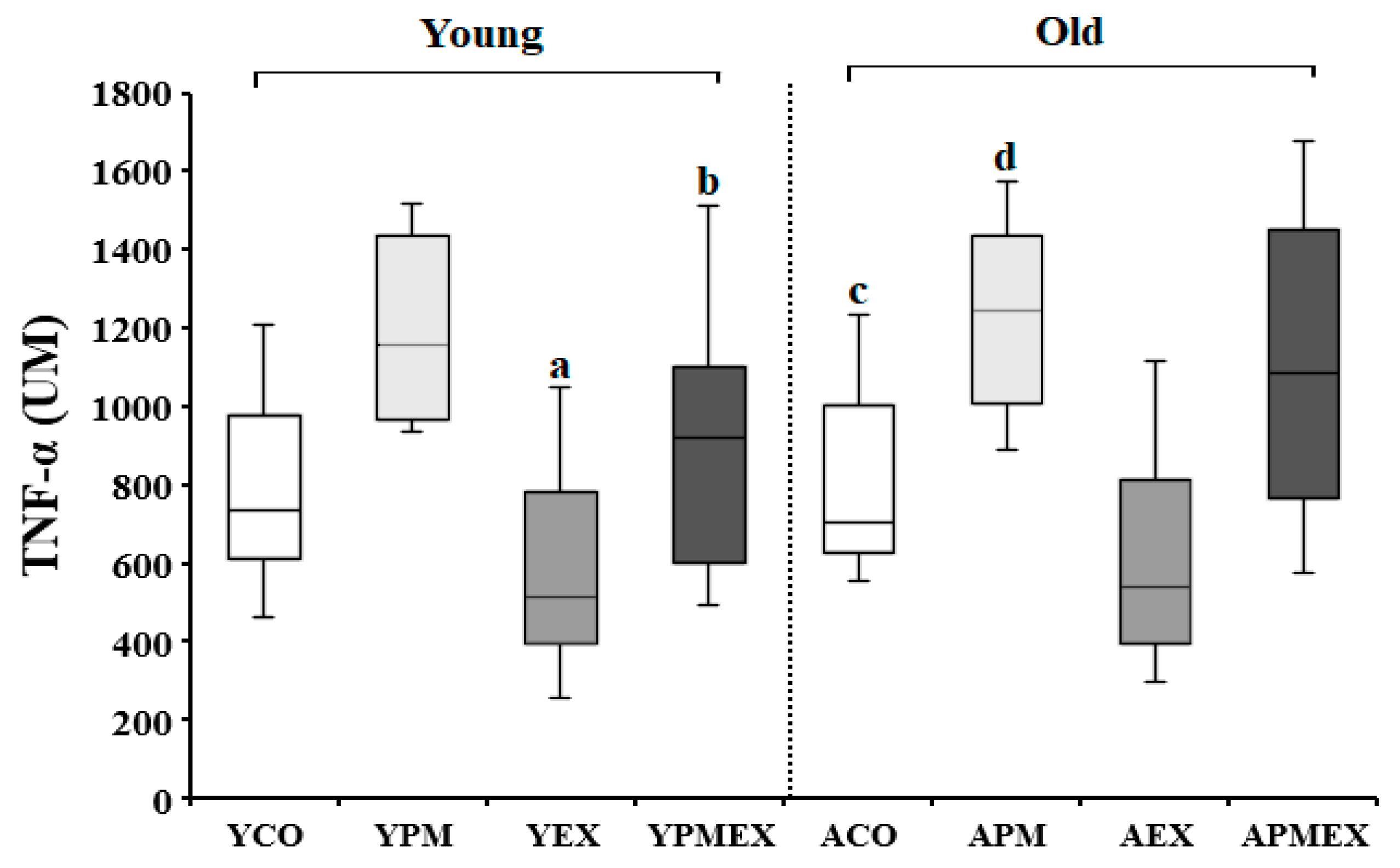
3.3.3. Changes in Serum TNF-α Levels
3.4. Correlation between OS, Antioxidant Status, and Inflammation Biomarkers
3.5. Multiple Linear Regression Analysis of Dependent Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. 2019. Chest 2019, 155, 409–416. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, H.; Meng, T.; Shen, M.; Ji, Q.; Xing, J.; Wang, Q.; Wang, T.; Niu, Y.; Yu, T.; et al. Reduced serum club cell protein as a pulmonary damage marker for chronic fine particulate matter exposure in Chinese population. Environ. Int. 2017, 112, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Hoek, G.; van den Brandt, P.A.; Goldbohm, R.A.; Fischer, P.; Schouten, L.J.; Jerrett, M.; Hughes, E.; Armstrong, B.; Brunekreef, B. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study). Environ. Health Perspect. 2008, 116, 196–202. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, Y.; Hu, H.; Hu, W.; Li, L.; Pang, Y.; Ma, S.; Niu, Y.; Zhang, R. Association between ambient particulate matter exposure and metabolic syndrome risk: A systematic review and meta-analysis. Sci. Total Environ. 2021, 782, 146855. [Google Scholar] [CrossRef]
- Fiordelisi, A.; Piscitelli, P.; Trimarco, B.; Coscioni, E.; Iaccarino, G.; Sorriento, D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail Rev. 2017, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Maldonado, E.M.; Sánchez-Pérez, Y.; Chirino, Y.I.; García-Cuellar, C.M. Airborne particulate matter induces oxidative damage, DNA adduct formation and alterations in DNA repair pathways. Environ. Pollut. 2021, 287, 117313. [Google Scholar] [CrossRef]
- Gong, S.Y.; Bae, H.J.; Hong, S.P.; Park, H.Y. A Study on the Health Impact and Management Policy of PM2.5 in Korea; Korea Environment Institute: Seoul, Korea, 2013; pp. 15–28. [Google Scholar]
- Wong, C.M.; Tsang, H.; Lai, H.K.; Thomas, G.N.; Lam, K.B.; Chan, K.P.; Zheng, Q.; Ayres, J.G.; Lee, S.Y.; Lam, T.H.; et al. Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiol. Biomark. Prev. 2016, 25, 839–845. [Google Scholar] [CrossRef]
- Zhou, H.; Geng, H.; Dong, C.; Bai, T. The short-term harvesting effects of ambient particulate matter on mortality in Taiyuan elderly residents: A time-series analysis with a generalized additive distributed lag model. Ecotoxicol. Environ. Saf. 2021, 207, 111235. [Google Scholar] [CrossRef]
- Zhang, X.; Staimer, N.; Gillen, D.L.; Tjoa, T.; Schauer, J.J.; Shafer, M.M.; Hasheminassab, S.; Pakbin, P.; Vaziri, N.D.; Sioutas, C.; et al. Associations of oxidative stress and inflammatory biomarkers with chemically-characterized air pollutant exposures in an elderly cohort. Environ. Res. 2016, 150, 306–319. [Google Scholar] [CrossRef]
- Lei, Y.C.; Hwang, J.S.; Chan, C.C.; Lee, C.T.; Cheng, T.J. Enhanced oxidative stress and endothelial dysfunction in streptozotocin-diabetic rats exposed to fine particles. Environ. Res. 2005, 99, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, A.; Egea-Guerrero, J.J.; Murillo-Cabezas, F.; Carrillo-Vico, A. Oxidative stress in traumatic brain injury. Curr. Med. Chem. 2014, 21, 1201–1211. [Google Scholar] [CrossRef]
- Jallali, N.; Ridha, H.; Thrasivoulou, C.; Underwood, C.; Butler, P.E.; Cowen, T. Vulnerability to ROS-induced cell death in ageing articular cartilage: The role of antioxidant enzyme activity. Osteoarthr. Cartil. 2005, 13, 614–622. [Google Scholar] [CrossRef]
- Martin, R.; Fitzl, G.; Mozet, C.; Martin, H.; Welt, K.; Wieland, E. Effect of age and hypoxia/reoxygenation on mRNA expression of antioxidative enzymes in rat liver and kidneys. Exp. Gerontol. 2002, 37, 1481–1487. [Google Scholar] [CrossRef]
- Fujiki, T.; Matsumoto, S.E.; Kishihara, K.; Katakura, Y. Age-related functional decline of human B cells. Cytotechnology 2022, 74, 319–327. [Google Scholar] [CrossRef]
- Aryal, A.; Harmon, A.C.; Dugas, T.R. Particulate matter air pollutants and cardiovascular disease: Strategies for intervention. Pharmacol. Ther. 2021, 223, 107890. [Google Scholar] [CrossRef]
- Benatti, F.B.; Pedersen, B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases—Myokine regulation. Nat. Rev. Rheumatol. 2015, 11, 86–97. [Google Scholar] [CrossRef]
- El Assar, M.; Álvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodríguez-Mañas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef]
- Qi, J.; Li, R.J.; Fu, L.Y.; Liu, K.L.; Qiao, J.A.; Yang, Y.; Yu, X.J.; Yu, J.Y.; Li, Y.; Tan, H.; et al. Exercise Training Attenuates Hypertension via Suppressing ROS/MAPK/NF-κB/AT-1R Pathway in the Hypothalamic Paraventricular Nucleus. Nutrients 2022, 14, 3968. [Google Scholar] [CrossRef] [PubMed]
- Chandwaney, R.; Leichtweis, S.; Leeuwenburgh, C.; Ji, L.L. Oxidative stress and mitochondrial function in skeletal muscle: Effects of aging and exercise training. Age 1998, 21, 109–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marmett, B.; Dorneles, G.P.; Nunes, R.B.; Peres, A.; Romão, P.R.T.; Rhoden, C.R. Exposure to fine particulate matter partially counteract adaptations on glucose metabolism, oxidative stress, and inflammation of endurance exercise in rats. Inhal. Toxicol. 2022, 34, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.C.; Yu, T.; Guo, C.; Lin, C.C.; Yang, H.T.; Chang, L.Y.; Thomas, G.N.; Tam, T.; Lau, A.K.H.; Lao, X.Q. Cardiovascular Mortality, Habitual Exercise, and Particulate Matter 2.5 Exposure: A Longitudinal Cohort Study. Am. J. Prev. Med. 2023, 64, 250–258. [Google Scholar] [CrossRef]
- So, B.; Park, J.; Jang, J.; Lim, W.; Imdad, S.; Kang, C. Effect of Aerobic Exercise on Oxidative Stress and Inflammatory Response During Particulate Matter Exposure in Mouse Lungs. Front. Physiol. 2022, 12, 773539. [Google Scholar] [CrossRef]
- Bouzid, M.A.; Filaire, E.; Matran, R.; Robin, S.; Fabre, C. Lifelong Voluntary Exercise Modulates Age-Related Changes in Oxidative Stress. Int. J. Sport. Med. 2018, 39, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Roh, H.T. Effects of Exercise Training on Neurotrophic Factors and Blood-Brain Barrier Permeability in Young-Old and Old-Old Women. Int. J. Environ. Res. Public Health 2022, 19, 16896. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; van Eeden, S.F. Systemic and vascular effects of circulating diesel exhaust particulate matter. Inhal. Toxicol. 2013, 25, 725–734. [Google Scholar] [CrossRef]
- Guo, J.; Xie, X.; Wu, J.; Yang, L.; Ruan, Q.; Xu, X.; Wei, D.; Wen, Y.; Wang, T.; Hu, Y.; et al. Association between fine particulate matter and coronary heart disease: A miRNA microarray analysis. Environ. Pollut. 2022, 313, 120163. [Google Scholar] [CrossRef]
- Wu, Y.; Song, P.; Lin, S.; Peng, L.; Li, Y.; Deng, Y.; Deng, X.; Lou, W.; Yang, S.; Zheng, Y.; et al. Global Burden of Respiratory Diseases Attributable to Ambient Particulate Matter Pollution: Findings From the Global Burden of Disease Study 2019. Front. Public Health. 2021, 9, 740800. [Google Scholar] [CrossRef]
- Donaldson, K.; MacNee, W. Potential mechanisms of adverse pulmonary and cardiovascular eCFects of particulate air pollution (PM10). Int. J. Hyg. Environ. Health 2001, 203, 411–415. [Google Scholar] [CrossRef]
- Kamal, A.; Qamar, K.; Gulfraz, M.; Anwar, M.A.; Malik, R.N. PAH exposure and oxidative stress indicators of human cohorts exposed to traffic pollution in Lahore city (Pakistan). Chemosphere 2015, 120, 59–67. [Google Scholar] [CrossRef]
- Lıu, X.; Deng, K.; Chen, S.; Zhang, Y.; Yao, J.; Weng, X.; Zhang, Y.; Gao, T.; Feng, G. 8-Hydroxy-2’-deoxyguanosine as a biomarker of oxidative stress in acute exacerbation of chronic obstructive pulmonary disease. Turk J. Med. Sci. 2019, 49, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Chen, J.; Zheng, J.; Zhang, M.; Zhang, H.; Wu, M.; Li, S.; Chen, B. The role of oxidative stress in cardiometabolic risk related to phthalate exposure in elderly diabetic patients from Shanghai. Environ. Int. 2018, 121, 340–348. [Google Scholar] [CrossRef]
- Rio, D.D.; Stewart, A.J.; Pellegrini, N. Areview of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar]
- Gao, Y.; Liu, W.; Wang, W.; Zhao, X.; Wang, F. Polyguluronate sulfate (PGS) attenuates immunological liver injury in vitro and in vivo. Int. J. Biol. Macromol. 2018, 114, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Gai, H.F.; An, J.X.; Qian, X.Y.; Wei, Y.J.; Williams, J.P.; Gao, G.L. Ovarian Damages Produced by Aerosolized Fine Particulate Matter (PM2.5) Pollution in Mice: Possible Protective Medications and Mechanisms. Chin. Med. J. 2017, 130, 1400–1410. [Google Scholar] [CrossRef]
- Billet, S.; Landkocz, Y.; Martin, P.J.; Verdin, A.; Ledoux, F.; Lepers, C.; André, V.; Cazie, R.F.; Sichel, F.; Shirali, P.; et al. Chemical characterization of fine and ultrafine PM, direct and indirect genotoxicity of PM and their organic extracts on pulmonary cells. J. Environ. Sci. 2018, 71, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Olszewski, M.B.; Groot, A.J.; Dastych, J.; Knol, E.F. TNF trafficking to human mast cell granules: Mature chain-dependent endocytosis. J. Immunol. 2007, 178, 5701–5709. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Mackos, A.R.; Floden, A.M.; Wold, L.E.; Combs, C.K. Particulate Matter Exposure Exacerbates Amyloid-β Plaque Deposition and Gliosis in APP/PS1 Mice. J. Alzheimers Dis. 2021, 80, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhu, G.; Zhu, M.; Song, J.; Cai, H.; Song, Y.; Wang, J.; Jin, M. Edaravone Attenuated Particulate Matter-Induced Lung Inflammation by Inhibiting ROS-NF-κB Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 6908884. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Geng, X.; Stone, C.; Cosky, E.E.P.; Ji, Y.; Du, H.; Zhang, K.; Sun, Q.; Ding, Y. PM2.5 exposure induces systemic inflammation and oxidative stress in an intracranial atherosclerosis rat model. Environ. Toxicol. 2019, 34, 530–538. [Google Scholar] [CrossRef]
- Yao, H.; Zhao, X.; Wang, L.; Ren, Y. Atorvastatin ameliorated PM2.5-induced atherosclerosis in rats. Arch. Environ. Occup. Health 2023, 78, 267–272. [Google Scholar] [CrossRef]
- Wei, Q.; Ji, Y.; Gao, H.; Yi, W.; Pan, R.; Cheng, J.; He, Y.; Tang, C.; Liu, X.; Song, S.; et al. Oxidative stress-mediated particulate matter affects the risk of relapse in schizophrenia patients: Air purification intervention-based panel study. Environ. Pollut. 2022, 292, 118348. [Google Scholar] [CrossRef]
- Lawler, J.M.; Kwak, H.B.; Song, W.; Parker, J.L. Exercise training reverses downregulation of HSP70 and antioxidant enzymes in porcine skeletal muscle after chronic coronary artery occlusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1756–R1763. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, A.H.; McKenzie, M.J.; Bloomer, R.J. Gender comparisons of exercise-induced oxidative stress: Influence of antioxidant supplementation. Appl. Physiol. Nutr. Metab. 2007, 32, 1124–1131. [Google Scholar] [CrossRef]
- Farhat, F.; Amérand, A.; Simon, B.; Guegueniat, N.; Moisan, C. Gender-dependent differences of mitochondrial function and oxidative stress in rat skeletal muscle at rest and after exercise training. Redox Rep. 2017, 22, 508–514. [Google Scholar] [CrossRef]

| 8-OHdG | MDA | SOD | CAT | IL-1β | IL-6 | TNF-α | |
|---|---|---|---|---|---|---|---|
| 8-OHdG | 1 | ||||||
| MDA | 0.565 * | 1 | |||||
| SOD | −0.611 * | −0.631 * | 1 | ||||
| CAT | −0.567 * | −0.590 * | 0.607 * | 1 | |||
| IL-1β | 0.599 * | 0.519 * | −0.561 * | −0.541 * | 1 | ||
| IL-6 | 0.602 * | 0.657 * | −0.571 * | −0.473 * | 0.539 * | 1 | |
| TNF-α | 0.490 * | 0.507 * | −0.491 * | −0.471 * | 0.510 * | 0.505 * | 1 |
| Dependent Variables | Explanatory Variables | B | Beta | t | p | F |
|---|---|---|---|---|---|---|
| 8-OHdG | Age | 8.763 | 0.458 | 6.902 | <0.001 | 50.334 * |
| PM | 9.858 | 0.515 | 7.765 | <0.001 | ||
| Exercise | −8.333 | −0.436 | −6.563 | <0.001 | ||
| R = 0.816, R2 = 0.665, Adjusted R2 = 0.652, Durbin-Watson = 1.784 | ||||||
| MDA | Age | 4.657 | 0.412 | 5.513 | <0.001 | 34.291 * |
| PM | 5.516 | 0.488 | 6.530 | <0.001 | ||
| Exercise | −4.615 | −0.408 | −5.463 | <0.001 | ||
| R = 0.758, R2 = 0.575, Adjusted R2 = 0.558, Durbin-Watson = 1.567 | ||||||
| SOD | Age | −34.620 | −0.243 | −3.292 | 0.002 | 35.757 * |
| PM | −91.670 | −0.644 | −8.716 | <0.001 | ||
| Exercise | 47.580 | 0.334 | 4.524 | <0.001 | ||
| R = 0.765, R2 = 0.585, Adjusted R2 = 0.569, Durbin-Watson = 1.688 | ||||||
| CAT | Age | −5.513 | −0.321 | −3.761 | <0.001 | 20.435 * |
| PM | −9.072 | −0.528 | −6.189 | <0.001 | ||
| Exercise | 4.363 | 0.254 | 2.97 | 0.004 | ||
| R = 0.668, R2 = 0.446, Adjusted R2 = 0.425, Durbin-Watson = 1.673 | ||||||
| IL-1β | Age | 3.603 | 0.197 | 2.409 | 0.018 | 24.744 * |
| PM | 11.838 | 0.646 | 7.915 | <0.001 | ||
| Exercise | −3.597 | −0.196 | −2.405 | 0.019 | ||
| R = 0.703, R2 = 0.494, Adjusted R2 = 0.474, Durbin-Watson = 1.721 | ||||||
| IL-6 | Age | 155.992 | 0.233 | 2.893 | 0.005 | 26.153 * |
| PM | 394.223 | 0.588 | 7.310 | <0.001 | ||
| Exercise | −220.052 | −0.328 | −4.081 | <0.001 | ||
| R = 0.713, R2 = 0.508, Adjusted R2 = 0.489, Durbin-Watson = 2.039 | ||||||
| TNF-α | Age | 66.327 | 0.095 | 1.114 | 0.269 | 20.636 * |
| PM | 418.062 | 0.598 | 7.022 | <0.001 | ||
| Exercise | −200.568 | −0.287 | −3.369 | 0.001 | ||
| R = 0.670, R2 = 0.449, Adjusted R2 = 0.427, Durbin-Watson = 1.611 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-Y.; Roh, H.-T. Impact of Particulate Matter Exposure and Aerobic Exercise on Circulating Biomarkers of Oxidative Stress, Antioxidant Status, and Inflammation in Young and Aged Mice. Life 2023, 13, 1952. https://doi.org/10.3390/life13101952
Cho S-Y, Roh H-T. Impact of Particulate Matter Exposure and Aerobic Exercise on Circulating Biomarkers of Oxidative Stress, Antioxidant Status, and Inflammation in Young and Aged Mice. Life. 2023; 13(10):1952. https://doi.org/10.3390/life13101952
Chicago/Turabian StyleCho, Su-Youn, and Hee-Tae Roh. 2023. "Impact of Particulate Matter Exposure and Aerobic Exercise on Circulating Biomarkers of Oxidative Stress, Antioxidant Status, and Inflammation in Young and Aged Mice" Life 13, no. 10: 1952. https://doi.org/10.3390/life13101952
APA StyleCho, S.-Y., & Roh, H.-T. (2023). Impact of Particulate Matter Exposure and Aerobic Exercise on Circulating Biomarkers of Oxidative Stress, Antioxidant Status, and Inflammation in Young and Aged Mice. Life, 13(10), 1952. https://doi.org/10.3390/life13101952






