Expression Analysis of Genes Involved in Transport Processes in Mice with MPTP-Induced Model of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Parkinson’s Disease Models
2.2. RNA Isolation and Expression Analysis of Individual Candidate Genes
2.3. Statistical Processing and Bioinformatic Analysis
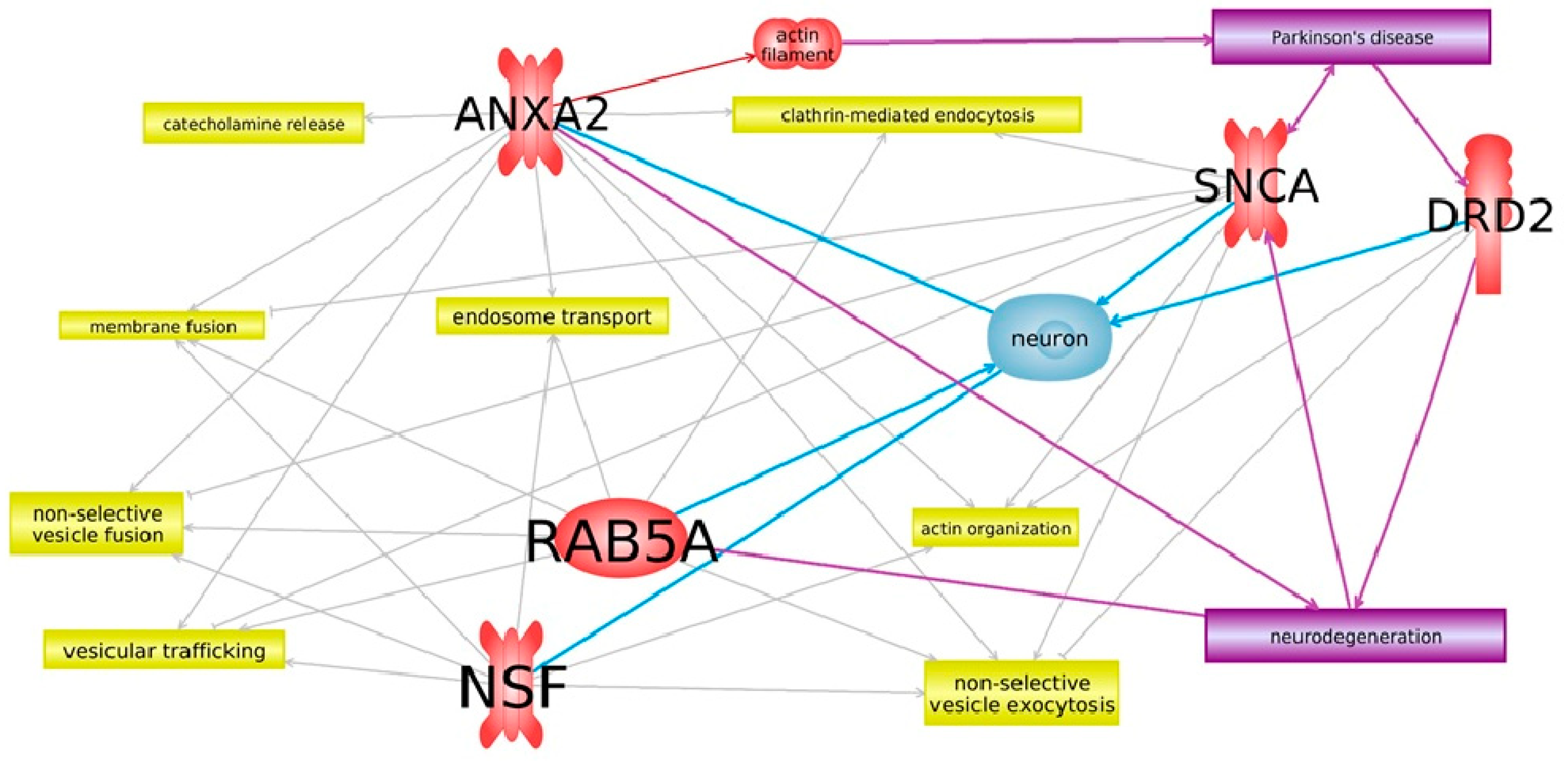
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.B. Cellular transport mechanisms. Annu. Rev. Biochem. 1978, 47, 933–965. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Keller, C.; Muller, M.J.; Klumpp, S.; Lipowsky, R. Co-operative transport by molecular motors. Biochem. Soc. Trans. 2011, 39, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Membrane trafficking and transport: Overview and neurologic implications. Neurology 2012, 79, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Abeliovich, A.; Gitler, A.D. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 2016, 539, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.S.; Yang, Z. Microtubule-based transport systems in neurons: The roles of kinesins and dyneins. Annu. Rev. Neurosci. 2000, 23, 39–71. [Google Scholar] [CrossRef]
- Maeder, C.I.; Shen, K.; Hoogenraad, C.C. Axon and dendritic trafficking. Curr. Opin. Neurobiol. 2014, 27, 165–170. [Google Scholar] [CrossRef]
- Bridgman, P.C. Myosin-dependent transport in neurons. J. Neurobiol. 2004, 58, 164–174. [Google Scholar] [CrossRef]
- Isola, A.L.; Chen, S. Exosomes: The Messengers of Health and Disease. Curr. Neuropharmacol. 2017, 15, 157–165. [Google Scholar] [CrossRef]
- Rizo, J.; Sudhof, T.C. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices—Guilty as charged? Annu. Rev. Cell Dev. Biol. 2012, 28, 279–308. [Google Scholar] [CrossRef]
- Kononenko, N.L.; Haucke, V. Molecular mechanisms of presynaptic membrane retrieval and synaptic vesicle reformation. Neuron 2015, 85, 484–496. [Google Scholar] [CrossRef]
- Gondre-Lewis, M.C.; Park, J.J.; Loh, Y.P. Cellular mechanisms for the biogenesis and transport of synaptic and dense-core vesicles. Int. Rev. Cell Mol. Biol. 2012, 299, 27–115. [Google Scholar] [CrossRef] [PubMed]
- Grafstein, B.; Forman, D.S. Intracellular transport in neurons. Physiol. Rev. 1980, 60, 1167–1283. [Google Scholar] [CrossRef] [PubMed]
- Hannah, M.J.; Schmidt, A.A.; Huttner, W.B. Synaptic vesicle biogenesis. Annu. Rev. Cell Dev. Biol. 1999, 15, 733–798. [Google Scholar] [CrossRef] [PubMed]
- Eccles, J.C. The Mechanism of Synaptic Transmission. In Ergebnisse der Physiologie Biologischen Chemie und Experimentellen Pharmakologie; Kramer, K., Krayer, O., Lehnartz, E., Muralt, A.V., Weber, H.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1961; pp. 299–430. [Google Scholar]
- Huettner, J.E. Kainate receptors and synaptic transmission. Prog. Neurobiol. 2003, 70, 387–407. [Google Scholar] [CrossRef]
- Katz, B.; Miledi, R. A study of synaptic transmission in the absence of nerve impulses. J. Physiol. 1967, 192, 407–436. [Google Scholar] [CrossRef]
- Bronfman, F.C.; Escudero, C.A.; Weis, J.; Kruttgen, A. Endosomal transport of neurotrophins: Roles in signaling and neurodegenerative diseases. Dev. Neurobiol. 2007, 67, 1183–1203. [Google Scholar] [CrossRef]
- Halim, N.D.; Weickert, C.S.; McClintock, B.W.; Hyde, T.M.; Weinberger, D.R.; Kleinman, J.E.; Lipska, B.K. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol. Psychiatry 2003, 8, 797–810. [Google Scholar] [CrossRef]
- Gabriel, S.M.; Davidson, M.; Haroutunian, V.; Powchik, P.; Bierer, L.M.; Purohit, D.P.; Perl, D.P.; Davis, K.L. Neuropeptide deficits in schizophrenia vs. Alzheimer’s disease cerebral cortex. Biol. Psychiatry 1996, 39, 82–91. [Google Scholar] [CrossRef]
- Shankar, G.M.; Walsh, D.M. Alzheimer′s disease: Synaptic dysfunction and Abeta. Mol. Neurodegener. 2009, 4, 48. [Google Scholar] [CrossRef]
- Waites, C.L.; Garner, C.C. Presynaptic function in health and disease. Trends Neurosci. 2011, 34, 326–337. [Google Scholar] [CrossRef]
- Willis, M.; Leitner, I.; Jellinger, K.A.; Marksteiner, J. Chromogranin peptides in brain diseases. J. Neural Transm. 2011, 118, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, J.C.; Rex, C.S.; Kramar, E.; Chen, L.Y.; Pandyarajan, V.; Lynch, G.; Gall, C.M. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 10685–10694. [Google Scholar] [CrossRef] [PubMed]
- Annangudi, S.P.; Luszpak, A.E.; Kim, S.H.; Ren, S.; Hatcher, N.G.; Weiler, I.J.; Thornley, K.T.; Kile, B.M.; Wightman, R.M.; Greenough, W.T.; et al. Neuropeptide Release is Impaired in a Mouse Model of Fragile X Mental Retardation Syndrome. ACS Chem. Neurosci. 2010, 1, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, K.; De Jonghe, P.; Coen, K.; Verpoorten, N.; Auer-Grumbach, M.; Kwon, J.M.; FitzPatrick, D.; Schmedding, E.; De Vriendt, E.; Jacobs, A.; et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am. J. Hum. Genet. 2003, 72, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.P.; Lin, L.; Prasad, A.; Paul, C.A.; Chang, T.Y.; Maue, R.A. Embryonic striatal neurons from niemann-pick type C mice exhibit defects in cholesterol metabolism and neurotrophin responsiveness. J. Biol. Chem. 2000, 275, 20179–20187. [Google Scholar] [CrossRef] [PubMed]
- Chevalier-Larsen, E.; Holzbaur, E.L. Axonal transport and neurodegenerative disease. Biochim. Biophys. Acta 2006, 1762, 1094–1108. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A. Effects of alpha-synuclein on axonal transport. Neurobiol. Dis. 2017, 105, 321–327. [Google Scholar] [CrossRef]
- Bossers, K.; Meerhoff, G.; Balesar, R.; Van Dongen, J.W.; Kruse, C.G.; Swaab, D.F.; Verhaagen, J. Analysis of Gene Expression in Parkinson’s Disease: Possible Involvement of Neurotrophic Support and Axon Guidance in Dopaminergic Cell Death. Brain Pathol. 2009, 19, 91–107. [Google Scholar] [CrossRef]
- Hauser, M.A.; Li, Y.-J.; Xu, H.; Noureddine, M.A.; Shao, Y.S.; Gullans, S.R.; Scherzer, C.R.; Jensen, R.V.; McLaurin, A.C.; Gibson, J.R.; et al. Expression Profiling of Substantia Nigra in Parkinson Disease, Progressive Supranuclear Palsy, and Frontotemporal Dementia With Parkinsonism. Arch Neurol. 2005, 62, 917–921. [Google Scholar] [CrossRef]
- Miller, R.M.; Kiser, G.L.; Kaysser-Kranich, T.M.; Lockner, R.J.; Palaniappan, C.; Federoff, H.J. Robust dysregulation of gene expression in substantia nigra and striatum in Parkinson’s disease. Neurobiol. Dis. 2006, 21, 305–313. [Google Scholar] [CrossRef]
- Bieri, G.; Gitler, A.D.; Brahic, M. Internalization, axonal transport and release of fibrillar forms of alpha-synuclein. Neurobiol. Dis. 2018, 109, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fukae, J.; Mori, H.; Mizuno, Y.; Hattori, N. Positive immunoreactivity for vesicular monoamine transporter 2 in Lewy bodies and Lewy neurites in substantia nigra. Neurosci. Lett. 2006, 396, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Power, J.H.; Barnes, O.L.; Chegini, F. Lewy Bodies and the Mechanisms of Neuronal Cell Death in Parkinson’s Disease and Dementia with Lewy Bodies. Brain Pathol. 2017, 27, 3–12. [Google Scholar] [CrossRef]
- Lin, M.K.; Farrer, M.J. Genetics and genomics of Parkinson’s disease. Genome Med. 2014, 6, 48. [Google Scholar] [CrossRef]
- Tsika, E.; Glauser, L.; Moser, R.; Fiser, A.; Daniel, G.; Sheerin, U.M.; Lees, A.; Troncoso, J.C.; Lewis, P.A.; Bandopadhyay, R.; et al. Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum. Mol. Genet. 2014, 23, 4621–4638. [Google Scholar] [CrossRef]
- Alter, S.P.; Lenzi, G.M.; Bernstein, A.I.; Miller, G.W. Vesicular integrity in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2013, 13, 362. [Google Scholar] [CrossRef]
- Mukherjee, A.; Biswas, A.; Das, S.K. Gut dysfunction in Parkinson’s disease. World J. Gastroenterol. 2016, 22, 5742–5752. [Google Scholar] [CrossRef]
- Alieva, A.K.; Filatova, E.V.; Kolacheva, A.A.; Rudenok, M.M.; Slominsky, P.A.; Ugrumov, M.V.; Shadrina, M.I. Transcriptome Profile Changes in Mice with MPTP-Induced Early Stages of Parkinson’s Disease. Mol. Neurobiol. 2017, 54, 6775–6784. [Google Scholar] [CrossRef]
- Alieva, A.K.; Zyrin, V.S.; Rudenok, M.M.; Kolacheva, A.A.; Shulskaya, M.V.; Ugryumov, M.V.; Slominsky, P.A.; Shadrina, M.I. Whole-Transcriptome Analysis of Mouse Models with MPTP-Induced Early Stages of Parkinson’s Disease Reveals Stage-Specific Response of Transcriptome and a Possible Role of Myelin-Linked Genes in Neurodegeneration. Mol. Neurobiol. 2018, 55, 7229–7241. [Google Scholar] [CrossRef]
- Ugrumov, M.V.; Khaindrava, V.G.; Kozina, E.A.; Kucheryanu, V.G.; Bocharov, E.V.; Kryzhanovsky, G.N.; Kudrin, V.S.; Narkevich, V.B.; Klodt, P.M.; Rayevsky, K.S.; et al. Modeling of presymptomatic and symptomatic stages of parkinsonism in mice. Neuroscience 2011, 181, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Kolacheva, A.A.; Kozina, E.A.; Volina, E.V.; Ugryumov, M.V. Time course of degeneration of dopaminergic neurons and respective compensatory processes in the nigrostriatal system in mice. Dokl. Biol.Sci. Proc. Acad. Sci. USSR Biol. Sci. Sect. Transl. Russ. 2014, 456, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Rudenok, M.M.; Alieva, A.K.; Starovatykh, J.S.; Nesterov, M.S.; Stanishevskaya, V.A.; Kolacheva, A.A.; Ugryumov, M.V.; Slominsky, P.A.; Shadrina, M.I. Expression analysis of genes involved in mitochondrial biogenesis in mice with MPTP-induced model of Parkinson’s disease. Mol. Genet. Metab. Rep. 2020, 23, 100584. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Ding, Y.; Dong, L.; Zhu, L.J.; Jensen, R.V.; Hsiao, L.L. Differential expression patterns of housekeeping genes increase diagnostic and prognostic value in lung cancer. PeerJ 2018, 6, e4719. [Google Scholar] [CrossRef]
- Haslinger, D.; Waltes, R.; Yousaf, A.; Lindlar, S.; Schneider, I.; Lim, C.K.; Tsai, M.M.; Garvalov, B.K.; Acker-Palmer, A.; Krezdorn, N.; et al. Loss of the Chr16p11.2 ASD candidate gene QPRT leads to aberrant neuronal differentiation in the SH-SY5Y neuronal cell model. Mol. Autism 2018, 9, 56. [Google Scholar] [CrossRef]
- Hoerndli, F.J.; Toigo, M.; Schild, A.; Gotz, J.; Day, P.J. Reference genes identified in SH-SY5Y cells using custom-made gene arrays with validation by quantitative polymerase chain reaction. Anal. Biochem. 2004, 335, 30–41. [Google Scholar] [CrossRef]
- Warrington, J.A.; Nair, A.; Mahadevappa, M.; Tsyganskaya, M. Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol. Genom. 2000, 2, 143–147. [Google Scholar] [CrossRef]
- Hettne, K.M.; Thompson, M.; van Haagen, H.H.; van der Horst, E.; Kaliyaperumal, R.; Mina, E.; Tatum, Z.; Laros, J.F.; van Mulligen, E.M.; Schuemie, M.; et al. The Implicitome: A Resource for Rationalizing Gene-Disease Associations. PLoS ONE 2016, 11, e0149621. [Google Scholar] [CrossRef]
- Zoetmulder, M.; Biernat, H.B.; Nikolic, M.; Korbo, L.; Friberg, L.; Jennum, P.J. Prepulse inhibition is associated with attention, processing speed, and 123I-FP-CIT SPECT in Parkinson’s disease. J. Parkinson’s Dis. 2014, 4, 77–87. [Google Scholar] [CrossRef]
- Brunger, A.T. Structural insights into the molecular mechanism of Ca(2+)-dependent exocytosis. Curr. Opin. Neurobiol. 2000, 10, 293–302. [Google Scholar] [CrossRef]
- Steger, M.; Tonelli, F.; Ito, G.; Davies, P.; Trost, M.; Vetter, M.; Wachter, S.; Lorentzen, E.; Duddy, G.; Wilson, S.; et al. Phosphoproteomics reveals that Parkinson′s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife 2016, 5, e12813. [Google Scholar] [CrossRef] [PubMed]
- Inoshita, T.; Arano, T.; Hosaka, Y.; Meng, H.; Umezaki, Y.; Kosugi, S.; Morimoto, T.; Koike, M.; Chang, H.Y.; Imai, Y.; et al. Vps35 in cooperation with LRRK2 regulates synaptic vesicle endocytosis through the endosomal pathway in Drosophila. Hum. Mol. Genet. 2017, 26, 2933–2948. [Google Scholar] [CrossRef]
- Esteves, A.R.; Swerdlow, R.H.; Cardoso, S.M. LRRK2, a puzzling protein: Insights into Parkinson′s disease pathogenesis. Exp. Neurol. 2014, 261, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Demirsoy, S.; Martin, S.; Motamedi, S.; van Veen, S.; Holemans, T.; Van den Haute, C.; Jordanova, A.; Baekelandt, V.; Vangheluwe, P.; Agostinis, P. ATP13A2/PARK9 regulates endo-/lysosomal cargo sorting and proteostasis through a novel PI(3, 5)P2-mediated scaffolding function. Hum. Mol. Genet. 2017, 26, 1656–1669. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Blair, N.F.; Sue, C.M. The role of ATP13A2 in Parkinson’s disease: Clinical phenotypes and molecular mechanisms. Mov. Disord. 2015, 30, 770–779. [Google Scholar] [CrossRef] [PubMed]
- German, D.C.; Nelson, E.L.; Liang, C.L.; Speciale, S.G.; Sinton, C.M.; Sonsalla, P.K. The neurotoxin MPTP causes degeneration of specific nucleus A8, A9 and A10 dopaminergic neurons in the mouse. Neurodegeneration 1996, 5, 299–312. [Google Scholar] [CrossRef]
- Meredith, G.E.; Totterdell, S.; Potashkin, J.A.; Surmeier, D.J. Modeling PD pathogenesis in mice: Advantages of a chronic MPTP protocol. Parkinsonism. Relat. Disord. 2008, 14 (Suppl. 2), S112–S115. [Google Scholar] [CrossRef]
- Gerlach, M.; Riederer, P. Animal models of Parkinson′s disease: An empirical comparison with the phenomenology of the disease in man. J. Neural. Transm. 1996, 103, 987–1041. [Google Scholar] [CrossRef]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef]
- Przedborski, S.; Jackson-Lewis, V.; Djaldetti, R.; Liberatore, G.; Vila, M.; Vukosavic, S.; Almer, G. The parkinsonian toxin MPTP: Action and mechanism. Restor. Neurol. Neurosci. 2000, 16, 135–142. [Google Scholar]
- Salari, S.; Bagheri, M. In vivo, in vitro and pharmacologic models of Parkinson’s disease. Physiol. Res. 2019, 68, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Heikkila, R.E.; Allis, B.; Cabbat, F.; Dembiec, D.; MacNamee, D.; Mytilineou, C.; Winston, B. Destruction of sympathetic nerve terminals by 6-hydroxydopamine: Protection by 1-phenyl-3-(2-thiazolyl)-2-thiourea, diethyldithiocarbamate, methimazole, cysteamine, ethanol and n-butanol. J. Pharmacol. Exp. Ther. 1976, 199, 336–352. [Google Scholar]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Benabid, A.L.; Sadoul, R.; Verna, J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson′s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s Disease in Rats: An Evaluation of 6-OHDA Lesions of the Nigrostriatal Pathway. Exp. Neurol. 2002, 175, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Lorigados, P.L.; Fuentes, N.P.; Alvarez, G.L.; McRae, A.; Serrano, S.T.; Blanco, L.L.; Macías, G.R. Nerve growth factor levels in Parkinson disease and experimental parkinsonian rats. Brain Res. 2002, 952, 122–127. [Google Scholar] [CrossRef]
- Dimatelis, J.J.; Hendricks, S.; Hsieh, J.; Vlok, N.M.; Bugarith, K.; Daniels, W.M.; Russell, V.A. Exercise partly reverses the effect of maternal separation on hippocampal proteins in 6-hydroxydopamine-lesioned rat brain. Exp. Physiol. 2013, 98, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sugiyama, K.; Asakawa, T.; Yamaguchi, H.; Akamine, S.; Ouchi, Y.; Magata, Y.; Namba, H. Dynamic changes of striatal dopamine D2 receptor binding at later stages after unilateral lesions of the medial forebrain bundle in Parkinsonian rat models. Neurosci. Lett. 2011, 496, 157–162. [Google Scholar] [CrossRef]
- Carman, L.S.; Gage, F.H.; Shults, C.W. Partial lesion of the substantia nigra relation between extent of lesion. Brain Res. 1991, 553, 275–283. [Google Scholar] [CrossRef]
- Gonzalez, S.; Mena, M.A.; Lastres-Becker, I.; Serrano, A.; de Yebenes, J.G.; Ramos, J.A.; Fernandez-Ruiz, J. Cannabinoid CB(1) receptors in the basal ganglia and motor response to activation or blockade of these receptors in parkin-null mice. Brain Res. 2005, 1046, 195–206. [Google Scholar] [CrossRef]
- Rial, D.; Castro, A.A.; Machado, N.; Garcao, P.; Goncalves, F.Q.; Silva, H.B.; Tome, A.R.; Kofalvi, A.; Corti, O.; Raisman-Vozari, R.; et al. Behavioral phenotyping of Parkin-deficient mice: Looking for early preclinical features of Parkinson’s disease. PLoS ONE 2014, 9, e114216. [Google Scholar] [CrossRef]
- Kelm-Nelson, C.A.; Yang, K.M.; Ciucci, M.R. Exercise Effects on Early Vocal Ultrasonic Communication Dysfunction in a PINK1 Knockout Model of Parkinson’s Disease. J. Parkinson’s Dis. 2015, 5, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.W.; Chakraborty, S.; Matthews, G.A.; Dougalis, A.; Wood, N.W.; Festenstein, R.; Ungless, M.A. Hyperexcitable substantia nigra dopamine neurons in PINK1- and HtrA2/Omi-deficient mice. J. Neurophysiol. 2010, 104, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, M.W.; Qu, D.; Hewitt, S.J.; Seang, S.; Kim, R.H.; Slack, R.S.; Schlossmacher, M.G.; Lagace, D.C.; Mak, T.W.; Park, D.S. Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease. Proc. Natl. Acad. Sci. USA 2012, 25, 15918–15923. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Lachenmayer, M.L. Genetic LRRK2 models of Parkinson’s disease: Dissecting the pathogenic pathway and exploring clinical applications. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Bernheimer, H.; Birkmayer, W.; Hornykiewicz, O.; Jellinger, K.; Seitelberger, F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 1973, 20, 415–455. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; Greenamyre, J.T. Animal models of Parkinson’s disease. BioEssays News Rev.Mol. Cell. Dev. Biol. 2002, 24, 308–318. [Google Scholar] [CrossRef]
- Fibiger, H.C.; Mogeer, E.G. Effect of acute and chronic methamphetamine treatment on tyrosine hydroxylase activity in brain and adrenal medulla. Eur. J. Pharmacol. 1971, 16, 176–180. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Nicklas, W.J.; Heikkila, R.E. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science 1989, 243, 398–400. [Google Scholar] [CrossRef]
- Noble, E.P. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am. J. Med. Genet. Part B Neuropsychiatr.Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2003, 116b, 103–125. [Google Scholar] [CrossRef]
- Burre, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Sudhof, T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef]
- Ozansoy, M.; Basak, A.N. The central theme of Parkinson’s disease: Alpha-synuclein. Mol. Neurobiol. 2013, 47, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Whiteheart, S.W.; Schraw, T.; Matveeva, E.A. N-ethylmaleimide sensitive factor (NSF) structure and function. Int. Rev. Cytol. 2001, 207, 71–112. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Taniguchi, S.; Ishizuka, Y.; Kim, H.S.; Wataya, Y.; Yamamoto, A.; Moriyama, Y. A homologue of N-ethylmaleimide-sensitive factor in the malaria parasite Plasmodium falciparum is exported and localized in vesicular structures in the cytoplasm of infected erythrocytes in the brefeldin A-sensitive pathway. J. Biol. Chem. 2001, 276, 15249–15255. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.; Haines, N.; Kuppuswamy, V.; Fleet, D.J.; Stewart, B.A. Synaptic vesicle mobility and presynaptic F-actin are disrupted in a N-ethylmaleimide-sensitive factor allele of Drosophila. Mol. Biol. Cell 2006, 17, 4709–4719. [Google Scholar] [CrossRef][Green Version]
- Faugaret, D.; Chouinard, F.C.; Harbour, D.; El Azreq, M.-A.; Bourgoin, S.G. An essential role for phospholipase D in the recruitment of vesicle amine transport protein-1 to membranes in human neutrophils. Biochem. Pharm. 2011, 81, 144–156. [Google Scholar] [CrossRef]
- Woodman, P. Vesicle transport: More work for the Rabs? Curr. Biol. 1998, 8, R199–R201. [Google Scholar] [CrossRef][Green Version]
- Tanmay, B.; Kumar, R.J. Rab proteins: The key regulators of intracellular vesicle transport. Exp. Cell Res. 2014, 328, 1–19. [Google Scholar] [CrossRef]
- Roth, B.L.; Willins, D.L.; Kroeze, W.K. G protein-coupled receptor (GPCR) trafficking in the central nervous system: Relevance for drugs of abuse. Drug Alcohol. Depend. 1998, 51, 73–85. [Google Scholar] [CrossRef]
- Delprato, A.; Merithew, E.; Lambright, D.G. Structure, exchange determinants, and family-wide rab specificity of the tandem helical bundle and Vps9 domains of Rabex-5. Cell 2004, 118, 607–617. [Google Scholar] [CrossRef]
- Luo, M.; Hajjar, K.A. Annexin A2 system in human biology: Cell surface and beyond. Semin. Thromb. Hemost. 2013, 39, 338–346. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Bydoun, M.; Holloway, R.; Waisman, D. Annexin A2 heterotetramer: Structure and function. Int. J. Mol. Sci. 2013, 14, 6259–6305. [Google Scholar] [CrossRef] [PubMed]
- Filipenko, N.R.; Waisman, D.M. The C Terminus of Annexin II Mediates Binding to F-actin. J. Biol. Chem. 2001, 276, 5310–5315. [Google Scholar] [CrossRef] [PubMed]
- Babbin, B.A.; Parkos, C.A.; Mandell, K.J.; Winfree, L.M.; Laur, O.; Ivanov, A.I.; Nusrat, A. Annexin 2 regulates intestinal epithelial cell spreading and wound closure through Rho-related signaling. Am. J. Pathol. 2007, 170, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Joanna, B.-P. Annexins in the Central Nervous System: Are they Neuroprotective or Proapoptotic Agents? Med. Chem. Rev. Online 2004, 1, 233–252. [Google Scholar] [CrossRef]
- Brandt, R.; Bakota, L. Microtubule dynamics and the neurodegenerative triad of Alzheimer′s disease: The hidden connection. J. Neurochem. 2017, 143, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Domon, M.M.; Besson, F.; Bandorowicz-Pikula, J.; Pikula, S. Annexin A6 is recruited into lipid rafts of Niemann-Pick type C disease fibroblasts in a Ca2+-dependent manner. Biochem. Biophys. Res. Commun. 2011, 405, 192–196. [Google Scholar] [CrossRef]
- Streubel-Gallasch, L.; Giusti, V.; Sandre, M.; Tessari, I.; Plotegher, N.; Giusto, E.; Masato, A.; Iovino, L.; Battisti, I.; Arrigoni, G.; et al. Parkinson’s Disease-Associated LRRK2 Interferes with Astrocyte-Mediated Alpha-Synuclein Clearance. Mol. Neurobiol. 2021, 58, 3119–3140. [Google Scholar] [CrossRef]
- Barbanti, P.; Fabbrini, G.; Ricci, A.; Cerbo, R.; Bronzetti, E.; Caronti, B.; Calderaro, C.; Felici, L.; Stocchi, F.; Meco, G.; et al. Increased expression of dopamine receptors on lymphocytes in Parkinson’s disease. Mov. Disord. 1999, 14, 764–771. [Google Scholar] [CrossRef]
- Buttarelli, F.R.; Capriotti, G.; Pellicano, C.; Prosperi, D.; Circella, A.; Festa, A.; Giovannelli, M.; Tofani, A.; Pontieri, F.E.; Scopinaro, F. Central and peripheral dopamine transporter reduction in Parkinson′s disease. Neurol. Res. 2009, 31, 687–691. [Google Scholar] [CrossRef]
- Nagai, Y.; Ueno, S.; Saeki, Y.; Soga, F.; Hirano, M.; Yanagihara, T. Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson’s disease. Neurology 1996, 46, 791–795. [Google Scholar] [CrossRef]
 p-Value ≤ 0.05.
p-Value ≤ 0.05.
 p-Value ≤ 0.05.
p-Value ≤ 0.05.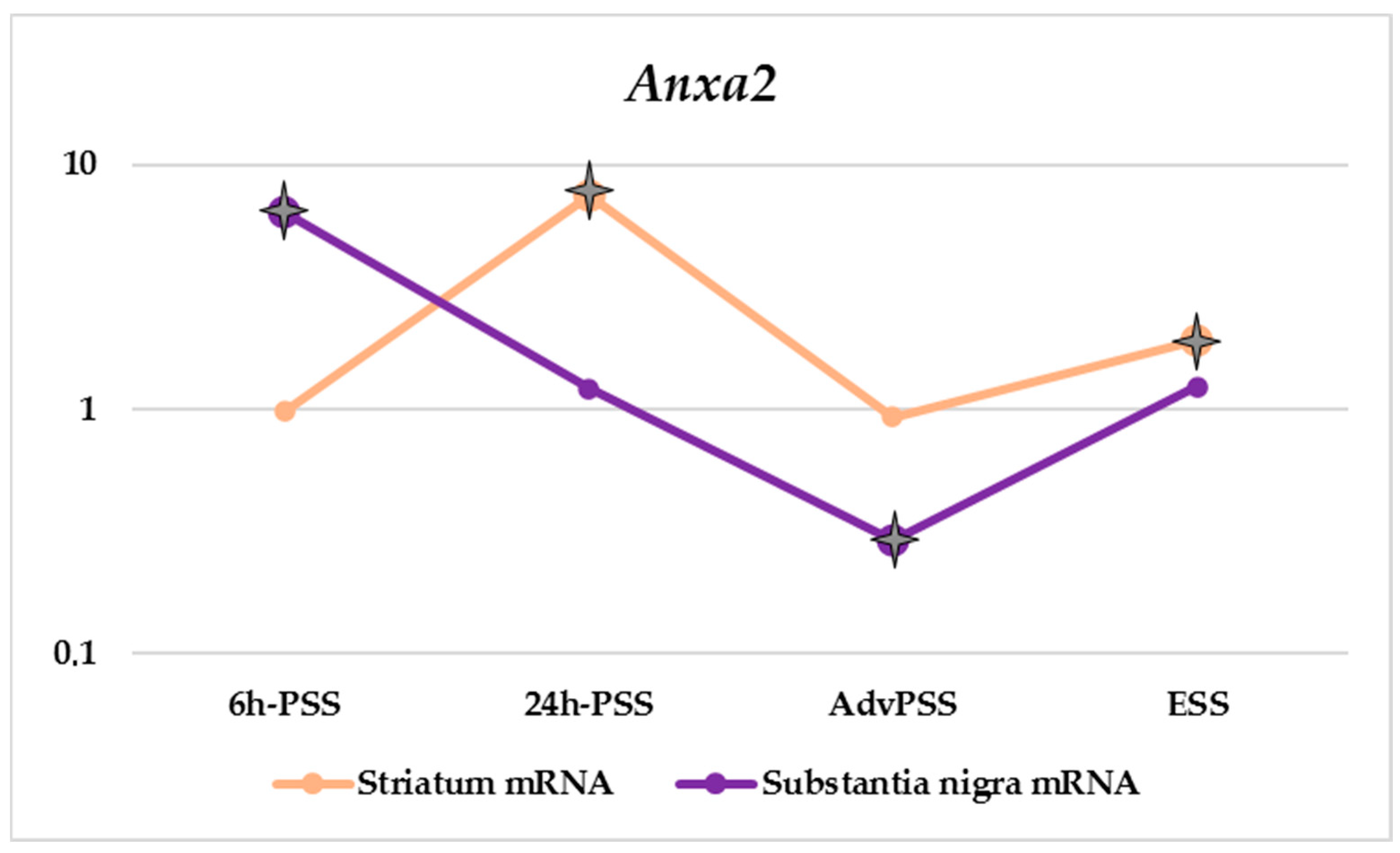
| 6 h-PSS | 24 h-PSS | AdvPSS | ESS | |
|---|---|---|---|---|
| Dose of MPTP | 12 mg/kg | 12 mg/kg | 12 mg/kg | 12 mg/kg |
| Number and frequency of injections | 4 injections at 2-h intervals | 4 injections at 2-h intervals | 2 injections at 2-h intervals | 4 injections at 2-h intervals |
| Withdrawal time after the last injection of MPTP | 6 h | 24 h | 2 weeks | 2 weeks |
| Change in DA levels in the striatum | 89% reduction | 72% increase | 56% reduction | 75% reduction |
| Death of DAergic neurons in substantia nigra | 33% reduction | unchanged | 26% reduction | 43% reduction |
| Changes in animal behavior | unchanged | unchanged | unchanged | a decrease in motor activity was detected |
| Gene | Nucleotide Sequence |
|---|---|
| Snca (Synuclein Alpha) NM_001042451.2 * | Probe: 5′-VIC-CCTCCTCACCCTTGCCCATCTGGTCC-BHQ2-3′ Forward primer: 5′-AGTGGAGGGAGCTGGGAATATAG-3′ Reverse primer: 5′-GCATGTCTTCCAGGATTCCTTCC-3′ |
| Drd2 (Dopamine Receptor D2) NM_010077.2 * | Probe: 5′-ROX-CAGCCAGCAGATGATGAACACACCAAGA-BHQ2-3′ Forward primer: 5′-TCCCAGCAGAAGGAGAAGAAAG-3′ Reverse primer: 5′-TGTATATTCAGGATGTGCGTGATG-3′ |
| Rab5a (RAB5A, Member RAS Oncogene Family) NM_025887.4 * | Probe: 5′-VIC-CATCTGCATAGGACTGTGCTTCC-BHQ2-3′ Forward primer: 5′-GCAAGTCCTAATATTGTGATA-3′ Reverse primer: 5′-CTCCATAAATAATAAGCTGTTG-3′ |
| Anxa2 (Annexin A2) NM_007585.3 * | Probe: 5′-VIC-AGAAGGACATCATCTCTGACACATCTG-BHQ2-3′ Forward primer: 5′-GAGTGTACAAGGAAATGTAC-3′ Reverse primer: 5′-CGTAGTCAATAACTGAGC-3′ |
| Nsf (N-ethylmaleimide sensitive fusion protein) NM_008740.4 * | Probe: 5′-VIC-CGTCCTCACCATCACATGCTG-BHQ2-3′ Forward primer: 5′-CCCTACTGATGAATTATCTTTA-3′ Reverse primer: 5′-TCCTCAGCGTAAATATGTA-3′ |
| Bcat2 (Branched chain aminotransferase 2) NM_001243053.1 * | Probe: 5′-FAM-CGGATACACTCCAACAGCTCCTGCTTGT-BHQ1-3′ Forward primer: 5′-TCAACATGGACAGGATGCTACG-3′ Reverse primer: 5′-CCAGTCTTTGTCTACTTCAATGAGC-3′ |
| Psmd7 (Proteasome 26S Subunit, Non-ATPase 7) NM_010817.2 * | Probe: 5′-FAM-AGTCCTAGGTCCTTTGGCTTCACGTCGA-BHQ1-3′ Forward primer: 5′-CTGCACAAGAATGATATCGCCATC-3′ Reverse primer: 5′-CTCCACTGAGATGTAGGCTTCG-3′ |
| Genes | Striatum | Substantia Nigra | Peripheral Blood | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 h-PSS (n = 10) | 24 h-PSS (n = 10) | Adv-PSS (n = 10) | ESS (n = 10) | 6 h-PSS (n = 10) | 24 h-PSS (n = 10) | Adv-PSS (n = 10) | ESS (n = 10) | 6 h-PSS (n = 10) | 24 h-PSS (n = 10) | Adv-PSS (n = 10) | ESS (n = 10) | |
| Drd2 | 0.94 1 | 1.76 | 0.90 * | 0.42 * | 1.34 | 0.57 | 3.55 * | 0.27 * | — | — | 0.90 * | 0.38 * |
| 0.77–1.18 2 | 1.25–2.39 | 0.17–1.09 | 0.33–0.86 | 1.24–1.46 | 0.46–0.81 | 3.14–5.80 | 0.13–0.59 | 0.75–1.52 | 0.27–0.44 | |||
| Snca | 1.10 | 0.87 | 0.93 * | 0.73 * | 0.87 | 0.49 | 7.52 * | 0.17 * | 3.37 | 0.74 | 0.69 * | 0.20 * |
| 1.01–1.33 | 0.78–1.41 | 0.12–1.00 | 0.61–1.30 | 0.81–1.13 | 0.33–1.06 | 6.42–8.36 | 0.16–0.36 | 2.12–4.29 | 0.60–0.93 | 0.21–1.82 | 0.14–0.36 | |
| Rab5a | 1.03 | 1.01 | 0.57 | 1.12 | 0.93 | 1.01 | 2.68 | 2.43 | 1.81 | 0.61 | 0.36 | 0.96 |
| 0.83–1.52 | 0.82–1.11 | 0.47–0.85 | 0.39–1.59 | 0.72–1.08 | 0.88–1.44 | 1.79–4.89 | 2.01–3.38 | 1.56–2.72 | 0.39–1.30 | 0.22–0.47 | 0.73–1.82 | |
| Anxa2 | 0.98 | 7.41 | 0.93 | 1.92 | 6.38 | 1.21 | 0.29 | 1.24 | 2.88 | 0.71 | 1.30 | 1.05 |
| 0.84–1.22 | 5.80–9.60 | 0.68–2.20 | 1.37–2.75 | 5.38–6.47 | 0.72–3.83 | 0.19–1.01 | 0.99–1.56 | 1.12–5.11 | 0.36–0.87 | 0.83–1.96 | 0.73–1.70 | |
| Nsf | 0.98 | 1.29 | 2.69 | 0.11 | 1.13 | 1.03 | 1.32 | 3.50 | 1.14 | 0.58 | 0.72 | 1.07 |
| 0.88–1.09 | 1.00–1.59 | 1.67–3.27 | 0.07–0.33 | 1.12–1.25 | 0.82–1.31 | 0.71–3.13 | 2.47–5.21 | 1.06–3.18 | 0.39–1.67 | 0.46–0.84 | 0.79–1.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudenok, M.M.; Shadrina, M.I.; Filatova, E.V.; Rybolovlev, I.N.; Nesterov, M.S.; Abaimov, D.A.; Ageldinov, R.A.; Kolacheva, A.A.; Ugrumov, M.V.; Slominsky, P.A.; et al. Expression Analysis of Genes Involved in Transport Processes in Mice with MPTP-Induced Model of Parkinson’s Disease. Life 2022, 12, 751. https://doi.org/10.3390/life12050751
Rudenok MM, Shadrina MI, Filatova EV, Rybolovlev IN, Nesterov MS, Abaimov DA, Ageldinov RA, Kolacheva AA, Ugrumov MV, Slominsky PA, et al. Expression Analysis of Genes Involved in Transport Processes in Mice with MPTP-Induced Model of Parkinson’s Disease. Life. 2022; 12(5):751. https://doi.org/10.3390/life12050751
Chicago/Turabian StyleRudenok, Margarita M., Maria I. Shadrina, Elena V. Filatova, Ivan N. Rybolovlev, Maxim S. Nesterov, Denis A. Abaimov, Ruslan A. Ageldinov, Anna A. Kolacheva, Michael V. Ugrumov, Petr A. Slominsky, and et al. 2022. "Expression Analysis of Genes Involved in Transport Processes in Mice with MPTP-Induced Model of Parkinson’s Disease" Life 12, no. 5: 751. https://doi.org/10.3390/life12050751
APA StyleRudenok, M. M., Shadrina, M. I., Filatova, E. V., Rybolovlev, I. N., Nesterov, M. S., Abaimov, D. A., Ageldinov, R. A., Kolacheva, A. A., Ugrumov, M. V., Slominsky, P. A., & Alieva, A. K. (2022). Expression Analysis of Genes Involved in Transport Processes in Mice with MPTP-Induced Model of Parkinson’s Disease. Life, 12(5), 751. https://doi.org/10.3390/life12050751









