The Impact of Weight Loss during Chemoradiotherapy for Unresectable Esophageal Cancer: Real-World Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Pretreatment Workup
2.3. Body Weight Measurement and Nutritional Counseling
2.4. Treatment
2.5. Follow-Up Schedule
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. BMI, Body Weight Change, Optimal Cut Point for Weight Change, and Feeding Condition
| Characteristic | Value |
|---|---|
| Age | |
| Mean | 58.5 ± 10.0 (38.8–78.7) |
| ≤65 | 50 (72.5%) |
| >65 | 19 (27.5%) |
| Gender | |
| Male | 68 (98.6%) |
| Female | 1 (1.4%) |
| BMI | |
| Mean | 22.8 ± 3.4 (18.5–32.3) |
| Comorbidities | |
| Hypertension | 17 (24.6%) |
| Diabetes mellitus | 5 (7.2%) |
| COPD | 4 (5.8%) |
| Liver disease | |
| Cirrhosis | 6 (8.7%) |
| Chronic HBV | 5 (7.2%) |
| Chronic HCV | 2 (2.9%) |
| Chronic renal disease | 1 (1.4%) |
| Personal history | |
| Smoking | 63 (91.3%) |
| Betel nut use | 42 (60.9%) |
| Alcohol use | 63 (91.3%) |
| Feeding route | |
| Oral | 29 (42.0%) |
| Nasogastric tube | 34 (49.3%) |
| Jejunostomy tube | 6 (8.7%) |
| Histology type | |
| SCC | 68 (98.6%) |
| Adenosquamous | 1 (1.4%) |
| Adenocarcinoma | 0 (0.0%) |
| Clinical lymph node category | |
| 1 | 24 (34.8%) |
| 2 | 30 (43.5%) |
| 3 | 15 (21.7%) |
| Tumor location | |
| Upper thoracic esophagus | 21 (30.4%) |
| Middle thoracic esophagus | 31 (44.9%) |
| Lower thoracic esophagus | 17 (24.7%) |
| Tumor invasion site | |
| Great vessels | 28 (40.6%) |
| Airway | 23 (33.3%) |
| Both | 18 (26.1%) |
| Tumor length | |
| ≤6 cm | 33 (47.8%) |
| >6 cm | 36 (52.2%) |
3.3. Treatment Response, Toxicities, and Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anandavadivelan, P.; Lagergren, P. Cachexia in patients with oesophageal cancer. Nat. Rev. Clin. Oncol. 2015, 13, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; De Montreuil, C.B.; Schneider, S.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients with Cancer. J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Allen, K. Nutritional Management of Patients with Esophageal and Esophagogastric Junction Cancer. Cancer Control 1999, 6, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Yano, M.; Yasuda, T.; Hamano, R.; Yamasaki, M.; Hou, E.; Motoori, M.; Shiraishi, O.; Tanaka, K.; Mori, M.; et al. Randomized study of clinical effect of enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Clin. Nutr. 2011, 31, 330–336. [Google Scholar] [CrossRef]
- Bansal, A.; Kapoor, R.; Kumar, S.; Miriyala, R.T. Factors influencing compliance to radical treatment of middle thoracic esophageal cancer: An audit from a regional cancer centre. Indian J. Palliat. Care 2016, 22, 288–294. [Google Scholar] [CrossRef]
- Yu, X.-L.; Yang, J.; Chen, T.; Liu, Y.-M.; Xue, W.-P.; Wang, M.-H.; Bai, S.-M. Excessive Pretreatment Weight Loss Is a Risk Factor for the Survival Outcome of Esophageal Carcinoma Patients Undergoing Radical Surgery and Postoperative Adjuvant Chemotherapy. Can. J. Gastroenterol. Hepatol. 2018, 2018, 6075207. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Ji, W.; Zheng, W.; Li, B.; Cao, C.; Mao, W. Influence of body mass index on the long-term outcomes of patients with esophageal squamous cell carcinoma who underwent esophagectomy as a primary treatment. Medicine 2016, 95, e4204. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yang, H.; Luo, K.J.; Huang, Q.Y.; Chen, J.Y.; Yang, F.; Cai, X.L.; Xie, X.; Liu, Q.W.; E Bella, A.; et al. The impact of body mass index on complication and survival in resected oesophageal cancer: A clinical-based cohort and meta-analysis. Br. J. Cancer 2013, 109, 2894–2903. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Powell, C.; Carter, B.; Hurt, C.; Mukherjee, S.; Crosby, T.D.L. Role of nutritional status and intervention in oesophageal cancer treated with definitive chemoradiotherapy: Outcomes from SCOPE1. Br. J. Cancer 2016, 115, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Netwrok, N.C.C. Esophageal and Esophagogastric Junction Cancers. 2022; Version 2. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf (accessed on 18 February 2022).
- Pan, P.; Tao, G.; Sun, X. Subjective global assessment and prealbumin levels of esophageal cancer patients undergoing concurrent chemoradiotherapy. Nutr. Hosp. 2015, 31, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Deans, D.A.C.; Tan, B.H.; Wigmore, S.J.; A Ross, J.; De Beaux, A.C.; Paterson-Brown, S.; Fearon, K.C.H. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br. J. Cancer 2009, 100, 63–69. [Google Scholar] [CrossRef]
- Bower, M.R.; Martin, R.C. Nutritional management during neoadjuvant therapy for esophageal cancer. J. Surg. Oncol. 2009, 100, 82–87. [Google Scholar] [CrossRef]
- Sanders, K.J.; Hendriks, L.E.; Troost, E.G.; Bootsma, G.P.; Houben, R.M.; Schols, A.M.; Dingemans, A.-M.C. Early Weight Loss during Chemoradiotherapy Has a Detrimental Impact on Outcome in NSCLC. J. Thorac. Oncol. 2016, 11, 873–879. [Google Scholar] [CrossRef]
- Capuano, G.; Grosso, A.; Gentile, P.C.; Battista, M.; Bianciardi, F.; Di Palma, A.; Pavese, I.; Satta, F.; Tosti, M.; Rn, A.P.; et al. Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head Neck 2007, 30, 503–508. [Google Scholar] [CrossRef]
- Lin, J.; Peng, J.; Qdaisat, A.; Li, L.; Chen, G.; Lu, Z.; Wu, X.; Gao, Y.; Zeng, Z.; Ding, P.; et al. Severe weight loss during preoperative chemoradiotherapy compromises survival outcome for patients with locally advanced rectal cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 2551–2560. [Google Scholar] [CrossRef]
- Clavier, J.-B.; Antoni, D.; Atlani, D.; Ben Abdelghani, M.; Schumacher, C.; Dufour, P.; Kurtz, J.-E.; Noel, G. Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis. Esophagus 2012, 27, 560–567. [Google Scholar] [CrossRef]
- Di Fiore, F.; Lecleire, S.; Pop, D.; Rigal, O.; Hamidou, H.; Paillot, B.; Ducrotté, P.; Lerebours, E.; Michel, P. Baseline Nutritional Status Is Predictive of Response to Treatment and Survival in Patients Treated by Definitive Chemoradiotherapy for a Locally Advanced Esophageal Cancer. Am. J. Gastroenterol. 2007, 102, 2557–2563. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Trotti, A.L.; Greene, F.L. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Huang, T.-T.; Li, S.-H.; Chen, Y.-H.; Lu, H.-I.; Lo, C.-M.; Fang, F.-M.; Chou, S.-Y.; Chiu, Y.-C.; Chou, Y.-P.; Wang, Y.-M. Definitive chemoradiotherapy for clinical T4b esophageal cancer—Treatment outcomes, failure patterns, and prognostic factors. Radiother. Oncol. 2021, 157, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009. Available online: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed on 7 December 2021).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Ogłuszka, M.; Orzechowska, M.; Jędroszka, D.; Witas, P.; Bednarek, A.K. Evaluate Cutpoints: Adaptable continuous data distribution system for determining survival in Kaplan-Meier estimator. Comput. Methods Programs Biomed. 2019, 177, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.P.; Mandrekar, J. Cutpoint Determination Methods in Survival Analysis using SAS®: Updated %FINDCUT macro. In Proceedings of the SAS Global Forum 2015, Dallas, TX, USA, 26–29 April 2015. [Google Scholar]
- Smith, M.; Zhou, M.; Whitlock, G.; Yang, G.; Offer, A.; Hui, G.; Peto, R.; Huang, Z.; Chen, Z. Esophageal cancer and body mass index: Results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int. J. Cancer 2007, 122, 1604–1610. [Google Scholar] [CrossRef]
- Qiu, Y.; You, J.; Wang, K.; Cao, Y.; Hu, Y.; Zhang, H.; Fu, R.; Sun, Y.; Chen, H.; Yuan, L.; et al. Effect of whole-course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: A randomized control trial. Nutrition 2019, 69, 110558. [Google Scholar] [CrossRef]
- Chen, M.-J.; Wu, I.-C.; Chen, Y.-J.; Wang, T.-E.; Chang, Y.-F.; Yang, C.-L.; Huang, W.-C.; Chang, W.-K.; Sheu, B.-S.; Wu, M.-S.; et al. Nutrition therapy in esophageal cancer—Consensus statement of the Gastroenterological Society of Taiwan. Dis. Esophagus 2018, 31, doy016. [Google Scholar] [CrossRef]
- Odelli, C.; Burgess, D.; Bateman, L.; Hughes, A.; Ackland, S.; Gillies, J.; Collins, C.E. Nutrition Support Improves Patient Outcomes, Treatment Tolerance and Admission Characteristics in Oesophageal Cancer. Clin. Oncol. 2005, 17, 639–645. [Google Scholar] [CrossRef]
- Lyu, J.; Li, T.; Xie, C.; Li, J.; Xing, L.; Zhang, X.; Shen, L.; Zhao, K.; Zhao, R.; Yang, D.; et al. Enteral nutrition in esophageal cancer patients treated with radiotherapy: A Chinese expert consensus 2018. Futur. Oncol. 2019, 15, 517–531. [Google Scholar] [CrossRef]
- Vasson, M.-P.; Talvas, J.; Perche, O.; Dillies, A.-F.; Bachmann, P.; Pezet, D.; Achim, A.-C.; Pommier, P.; Racadot, S.; Weber, A.; et al. Immunonutrition improves functional capacities in head and neck and esophageal cancer patients undergoing radiochemotherapy: A randomized clinical trial. Clin. Nutr. 2013, 33, 204–210. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Sikalidis, A.K. Amino Acids and Immune Response: A Role for Cysteine, Glutamine, Phenylalanine, Tryptophan and Arginine in T-cell Function and Cancer? Pathol. Oncol. Res. 2015, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of Selected Vitamins and Trace Elements to Immune Function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Yoon, H.G.; Oh, D.; Ahn, Y.C.; Noh, J.M.; Pyo, H.; Cho, W.K.; Song, Y.M.; Park, M.; Hwang, N.Y.; Sun, J.-M.; et al. Prognostic Impact of Sarcopenia and Skeletal Muscle Loss During Neoadjuvant Chemoradiotherapy in Esophageal Cancer. Cancers 2020, 12, 925. [Google Scholar] [CrossRef]
- Impact of Sarcopenia in Patients with Unresectable Locally Advanced Esophageal Cancer Receiving Chemoradiotherapy. In Vivo 2018, 32, 603–610. [CrossRef]
- Järvinen, T.; Ilonen, I.; Kauppi, J.; Salo, J.; Räsänen, J. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: A retrospective cohort study. World J. Surg. Oncol. 2018, 16, 27. [Google Scholar] [CrossRef]
- Reisinger, K.W.; Bosmans, J.W.A.M.; Uittenbogaart, M.; Gs, A.A.; Poeze, M.; Sosef, M.N.; Derikx, J.P.M. Loss of Skeletal Muscle Mass During Neoadjuvant Chemoradiotherapy Predicts Postoperative Mortality in Esophageal Cancer Surgery. Ann. Surg. Oncol. 2015, 22, 4445–4452. [Google Scholar] [CrossRef]
- Radaideh, K.M. Dosimetric impact of weight loss and anatomical changes at organs at risk during intensity-modulated radiotherapy for head-and-neck cancer. J. Radiat. Res. Appl. Sci. 2020, 13, 301–308. [Google Scholar] [CrossRef]
- Hou, W.-H.; Wang, C.-W.; Tsai, C.-L.; Hsu, F.-M.; Cheng, J.C.-H. The ratio of weight loss to planning target volume significantly impacts setup errors in nasopharyngeal cancer patients undergoing helical tomotherapy with daily megavoltage computed tomography. Radiol. Oncol. 2016, 50, 427–432. [Google Scholar] [CrossRef][Green Version]
- Sakanaka, K.; Fujii, K.; Ishida, Y.; Miyamoto, S.; Horimatsu, T.; Muto, M.; Mizowaki, T. Nutritional and clinical outcomes of chemoradiotherapy for clinical T1N0M0 esophageal carcinoma. Cancer Manag. Res. 2019, 11, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.; Iyengar, P.; Pandita, T.K. The Role of Inflammatory Pathways in Cancer-Associated Cachexia and Radiation Resistance. Mol. Cancer Res. 2013, 11, 967–972. [Google Scholar] [CrossRef]
- Hinz, M.; Krappmann, D.; Eichten, A.; Heder, A.; Scheidereit, C.; Strauss, M. NF-κB Function in Growth Control: Regulation of Cyclin D1 Expression and G0/G1-to-S-Phase Transition. Mol. Cell. Biol. 1999, 19, 2690–2698. [Google Scholar] [CrossRef] [PubMed]
- Harant, H.; de Martin, R.; Andrew, P.J.; Foglar, E.; Dittrich, C.; Lindley, I.J.D. Synergistic Activation of Interleukin-8 Gene Transcription by All-trans-retinoic Acid and Tumor Necrosis Factor-α Involves the Transcription Factor NF-κB. J. Biol. Chem. 1996, 271, 26954–26961. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.C.; Cheshire, J.K.; Akira, T.; Kishimoto, T.; Woo, P. The role of NF-kappa B and NF-IL6 transactivating factors in the synergistic activation of human serum amyloid A gene expression by interleukin-1 and interleukin-6. J. Biol. Chem. 1993, 268, 25624–25631. [Google Scholar] [CrossRef]
| Variable | No. (%) |
|---|---|
| Weight change during | |
| ≤4% | 25 (36.2%) |
| >4% | 44 (63.8%) |
| RT modality | |
| 3D-CRT | 1 (1.4%) |
| 2D + IMRT | 12 (17.4%) |
| IMRT | 56 (81.2%) |
| Post-dCRT complication | |
| ≥Grade 3 esophagitis | 4 (5.8%) |
| ≥Grade 3 radiation pneumonitis | 0 (0%) |
| Tracheo-esophageal fistula | 5 (7.2%) |
| Tracheo-aortic fistula | 1 (1.4%) |
| Treatment response | |
| Complete response | 21 (30.4%) |
| Partial response | 35 (50.8%) |
| Stable/progression disease | 9 (13.0%) |
| Non-accessible | 4 (5.8%) |
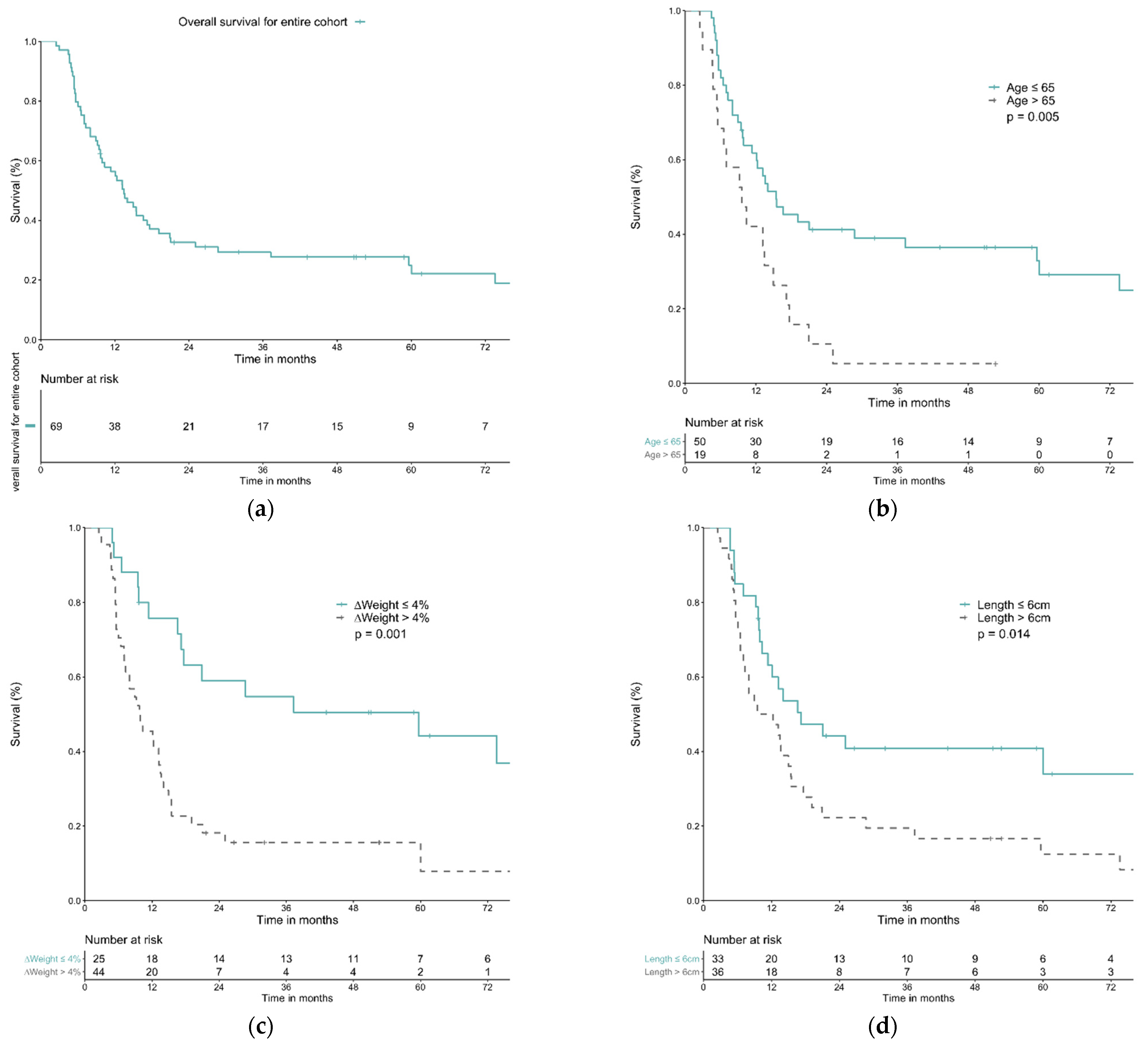
| Variable | N | OS (%) | MS (Months) | p Value | Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-Year | 3-Year | 5-Year | HR | 95% CI | p Value | HR | 95% CI | p Value | ||||
| All | 69 | 56.4% | 29.5% | 24.9% | 13.5 | |||||||
| Age | ||||||||||||
| ≤65 | 50 | 61.8% | 38.9% | 32.8% | 15.4 | 0.005 * | 2.28 | 1.26–4.10 | 0.006 * | 1.91 | 1.06–3.44 | 0.032 * |
| >65 | 19 | 42.1% | 5.3% | 5.3% | 9.6 | |||||||
| Weight change | ||||||||||||
| ≤4% | 25 | 75.8% | 54.7% | 44.2% | 59.6 | 0.001 * | 2.83 | 1.53–5.26 | 0.001 * | 2.61 | 1.40–4.85 | 0.002 * |
| >4% | 44 | 45.5% | 15.6% | 15.6% | 9.7 | |||||||
| Clinical N stage | ||||||||||||
| cN1 | 24 | 70.6% | 52.9% | 45.4% | 59.6 | 0.010 * | 2.22 | 1.19–4.13 | 0.012 * | N.S. | ||
| cN2-3 | 45 | 48.9% | 16.9% | 14.1% | 11.4 | |||||||
| Tumor location | ||||||||||||
| U/3 EC | 21 | 57.1% | 23.8% | 23.8% | 13.2 | 0.377 | N.S. | N.S. | ||||
| M/3 EC | 31 | 58.1% | 41.4% | 31.0% | 16.6 | |||||||
| L/3 EC | 17 | 51.8% | 12.9% | 12.9% | 12.1 | |||||||
| Tumor invasion | ||||||||||||
| Great vessels | 28 | 60.3% | 22.6% | 18.1% | 13.5 | 0.457 | N.S. | N.S. | ||||
| Airway | 23 | 65.2% | 38.6% | 30.9% | 17.6 | |||||||
| Both | 18 | 38.9% | 27.8% | 27.8% | 8.0 | |||||||
| Tumor length | ||||||||||||
| ≤6 cm | 33 | 63.1% | 40.8% | 40.8% | 17.2 | 0.014 * | 1.98 | 1.13–3.46 | 0.016 * | 1.83 | 1.05–3.22 | 0.035 * |
| >6 cm | 36 | 50.0% | 19.4% | 12.5% | 9.5 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.-T.; Chou, S.-Y.; Lin, Y.-H.; Li, S.-H.; Chen, Y.-H.; Lu, H.-I.; Lo, C.-M.; Fang, F.-M.; Chiu, Y.-C.; Chou, Y.-P.; et al. The Impact of Weight Loss during Chemoradiotherapy for Unresectable Esophageal Cancer: Real-World Results. Life 2022, 12, 706. https://doi.org/10.3390/life12050706
Huang T-T, Chou S-Y, Lin Y-H, Li S-H, Chen Y-H, Lu H-I, Lo C-M, Fang F-M, Chiu Y-C, Chou Y-P, et al. The Impact of Weight Loss during Chemoradiotherapy for Unresectable Esophageal Cancer: Real-World Results. Life. 2022; 12(5):706. https://doi.org/10.3390/life12050706
Chicago/Turabian StyleHuang, Tzu-Ting, Shang-Yu Chou, Yun-Hsuan Lin, Shau-Hsuan Li, Yen-Hao Chen, Hung-I Lu, Chien-Ming Lo, Fu-Min Fang, Yi-Chun Chiu, Yeh-Pin Chou, and et al. 2022. "The Impact of Weight Loss during Chemoradiotherapy for Unresectable Esophageal Cancer: Real-World Results" Life 12, no. 5: 706. https://doi.org/10.3390/life12050706
APA StyleHuang, T.-T., Chou, S.-Y., Lin, Y.-H., Li, S.-H., Chen, Y.-H., Lu, H.-I., Lo, C.-M., Fang, F.-M., Chiu, Y.-C., Chou, Y.-P., & Wang, Y.-M. (2022). The Impact of Weight Loss during Chemoradiotherapy for Unresectable Esophageal Cancer: Real-World Results. Life, 12(5), 706. https://doi.org/10.3390/life12050706






