Apocynin Attenuates Diabetes-Induced Skeletal Muscle Dysfunction by Mitigating ROS Generation and Boosting Antioxidant Defenses in Fast-Twitch and Slow-Twitch Muscles
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Insulin Tolerance Test
2.4. Isometric Tension Measurements
2.5. Measurement of Reactive Oxygen Species Levels
2.6. Determination of Glutathione Status
2.7. Total RNA Extraction and Real-Time RT-qPCR for mRNA Expression Analyzes
2.8. Statistical Analysis
3. Results
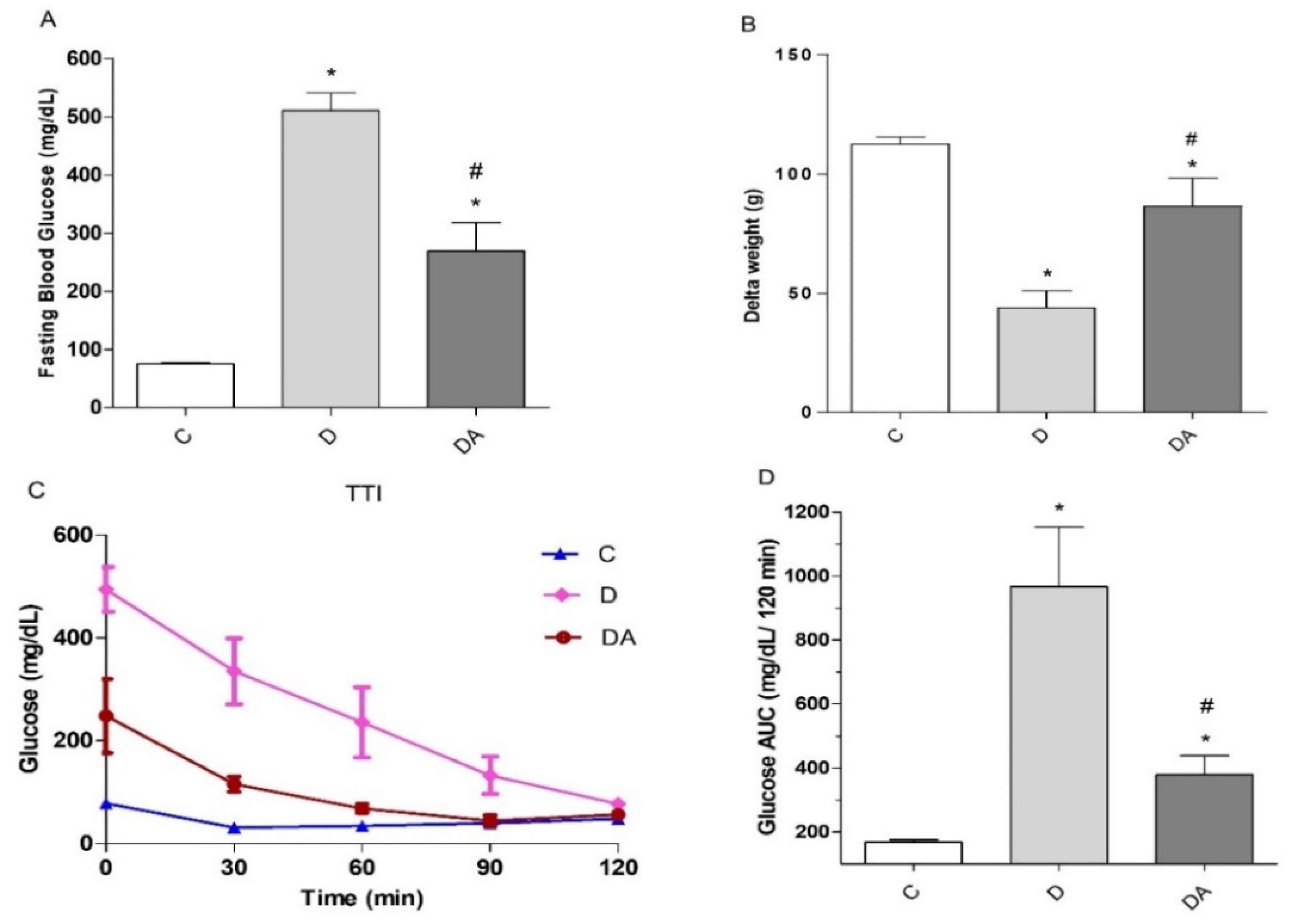
3.1. Effect of Apocynin Treatment on Fasting Blood Glucose, Body Weight, and Insulin Tolerance in Diabetes
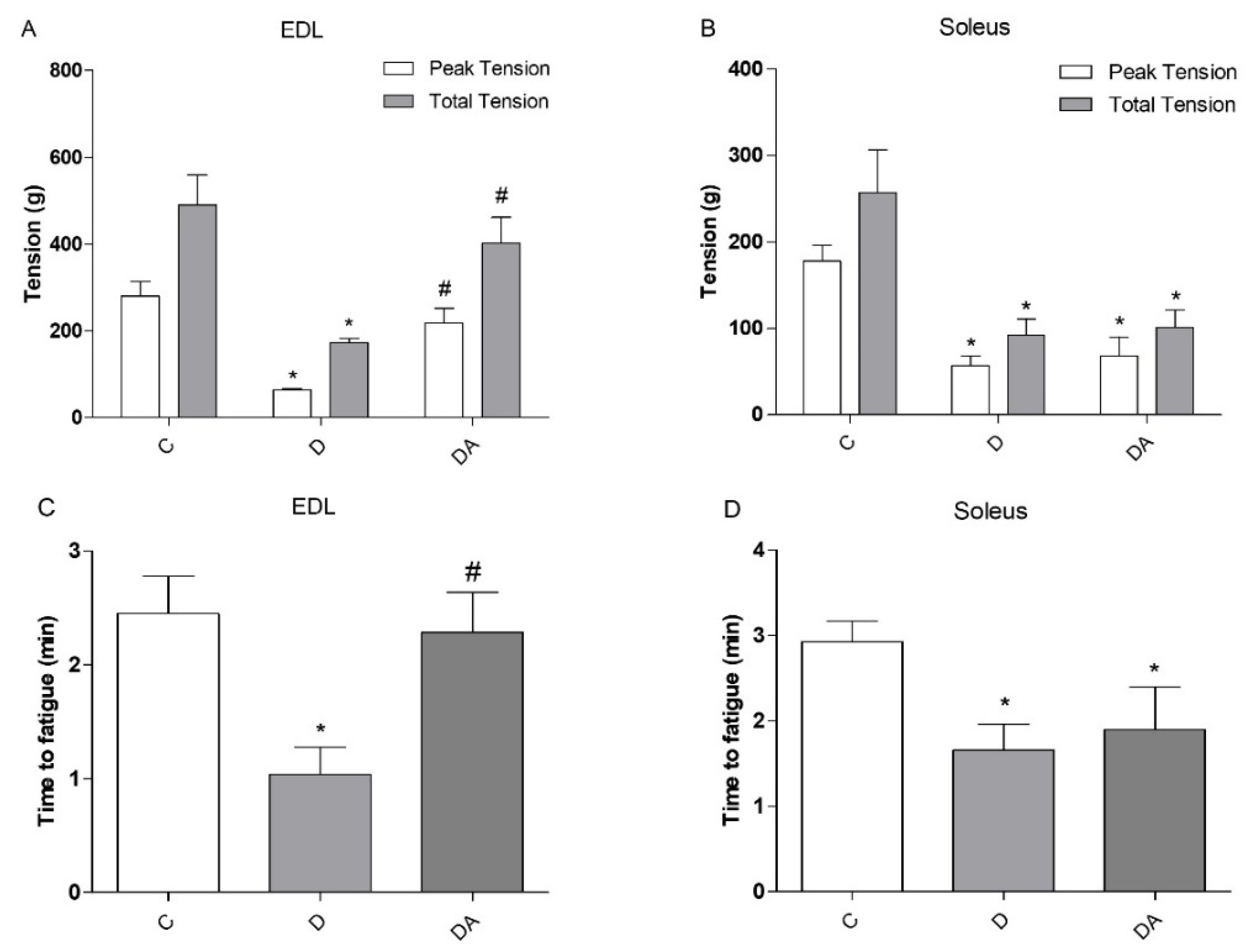
3.2. Effects of Apocynin on Contractile Properties and Fatigue of Slow-Twitch and Fast-Twitch Muscles
3.3. Apocynin Reduces the Levels of Reactive Oxygen Species in Fast and Slow Diabetic Skeletal Muscles
3.4. Apocynin Improved Glutathione Redox Status in Fast and Slow Skeletal Muscle in Diabetic Rats
3.5. Effects of Apocynin on NOX2 and NOX4 Expression in Fast- and Slow-Twitch Skeletal Muscle in Diabetic Rats
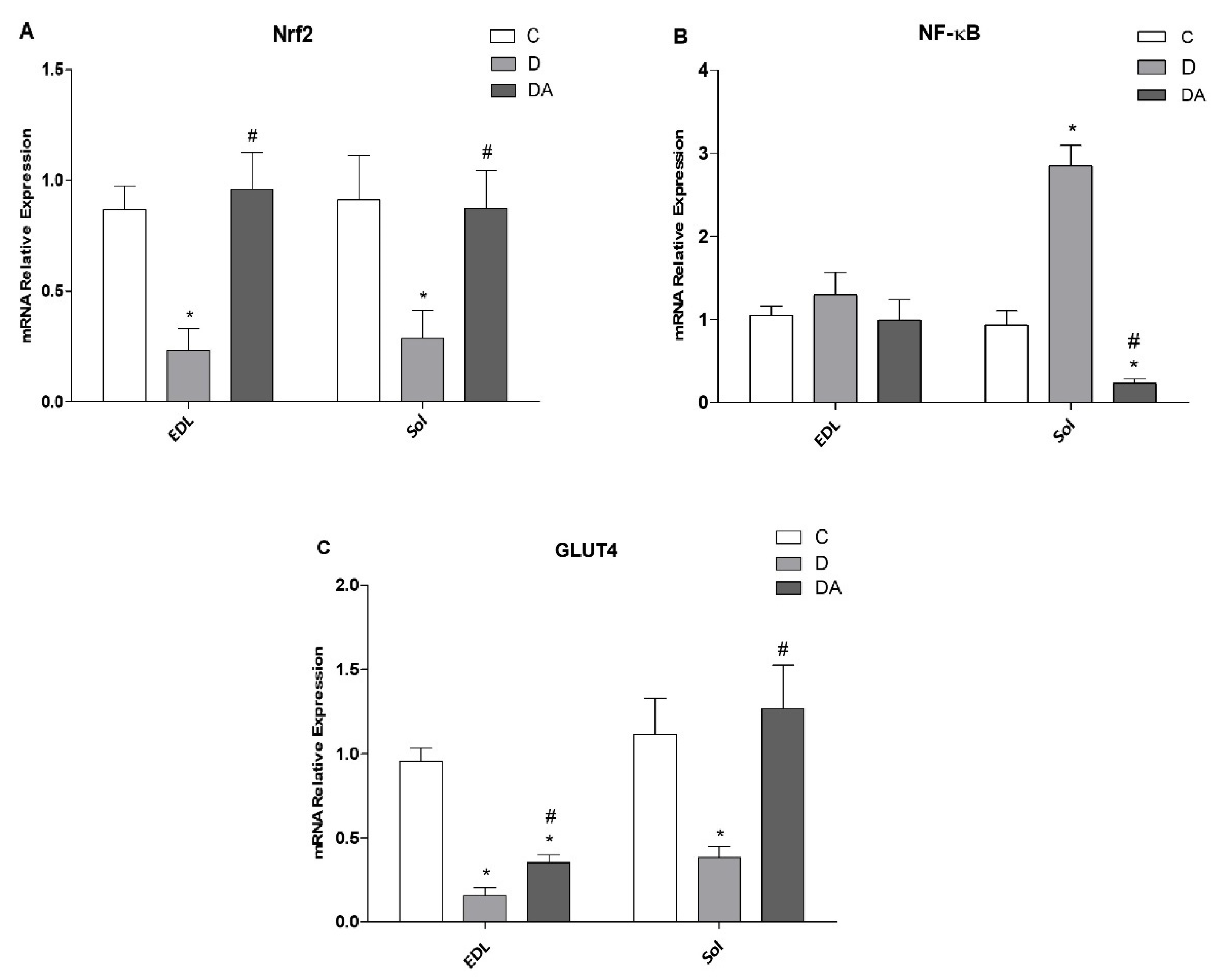
3.6. Effect of Apocynin on Gene Expression of Nrf2, NF-ҡβ, and GLUT4 in the Fast and Slow Muscles of Rats with Diabetes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.M.; Al-Sajee, D.; Hawke, T.J. Diabetic myopathy: Impact of diabetes mellitus on skeletal muscle progenitor cells. Front. Physiol. 2013, 4, 379. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.K.; Rebalka, I.A.; D’Souza, D.M.; Hawke, T.J. Skeletal muscle as a therapeutic target for delaying type 1 diabetic complications. World J. Diabetes 2015, 6, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.C. Diabetes and Muscle Dysfunction in Older Adults. Ann. Geriatr. Med. Res. 2019, 23, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Meex, R.; Blaak, E.E.; van Loon, L. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 2019, 2, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef]
- Sánchez-Duarte, S.; Márquez-Gamiño, S.; Montoya-Pérez, R.; Villicaña-Gómez, E.A.; Vera-Delgado, K.S.; Caudillo-Cisneros, C.; Sotelo-Barroso, F.; Melchor-Moreno, M.T.; Sánchez-Duarte, E. Nicorandil decreases oxidative stress in slow- and fast-twitch muscle fibers of diabetic rats by improving the glutathione system functioning. J. Diabetes Investig. 2021, 12, 1152–1161. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Yokota, T.; Kinugawa, S.; Hirabayashi, K.; Matsushima, S.; Inoue, N.; Ohta, Y.; Hamaguchi, S.; Sobirin, M.A.; Ono, T.; Suga, T.; et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1069–H1077. [Google Scholar] [CrossRef]
- Souto Padron de Figueiredo, A.; Salmon, A.B.; Bruno, F.; Jimenez, F.; Martinez, H.G.; Halade, G.V.; Ahuja, S.S.; Clark, R.A.; DeFronzo, R.A.; Abboud, H.E.; et al. Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. J. Biol. Chem. 2015, 290, 13427–13439. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH oxidases in skeletal muscle. Free. Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Urner, S.; Ho, F.; Jha, J.C.; Ziegler, D.; Jandeleit-Dahm, K. NADPH Oxidase Inhibition: Preclinical and Clinical Studies in Diabetic Complications. Antioxid. Redox Signal. 2020, 33, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, S.; Karunakaran, U.; Moon, J.S.; Won, K.C. NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction. Cells 2021, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.S.; Aasum, E.; Hafstad, A.D. The role of NADPH oxidases in diabetic cardiomyopathy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 1908–1913. [Google Scholar] [CrossRef] [PubMed]
- Bechara, L.R.; Moreira, J.B.; Jannig, P.R.; Voltarelli, V.A.; Dourado, P.M.; Vasconcelos, A.R.; Scavone, C.; Ramires, P.R.; Brum, P.C. NADPH oxidase hyperactivity induces plantaris atrophy in heart failure rats. Int. J. Cardiol. 2014, 175, 499–507. [Google Scholar] [CrossRef]
- Savla, S.R.; Laddha, A.P.; Kulkarni, Y.A. Pharmacology of apocynin: A natural acetophenone. Drug Metab. Rev. 2021, 53, 542–562. [Google Scholar] [CrossRef]
- Min, S.; Kim, O.J.; Bae, J.; Chung, T.N. Effect of Pretreatment with the NADPH Oxidase Inhibitor Apocynin on the Therapeutic Efficacy of Human Placenta-Derived Mesenchymal Stem Cells in Intracerebral Hemorrhage. Int. J. Mol. Sci. 2018, 19, 3679. [Google Scholar] [CrossRef]
- Du, Z.D.; Yu, S.; Qi, Y.; Qu, T.F.; He, L.; Wei, W.; Liu, K.; Gong, S.S. NADPH oxidase inhibitor apocynin decreases mitochondrial dysfunction and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging model in rats. Neurochem. Int. 2019, 124, 31–40. [Google Scholar] [CrossRef]
- Bravo-Sánchez, E.; Peña-Montes, D.; Sánchez-Duarte, S.; Saavedra-Molina, A.; Sánchez-Duarte, E.; Montoya-Pérez, R. Effects of Apocynin on Heart Muscle Oxidative Stress of Rats with Experimental Diabetes: Implications for Mitochondria. Antioxidants 2021, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, R.; Gimenes, C.; Rosa, C.M.; Xavier, N.P.; Campos, D.; Fernandes, A.; Cezar, M.; Guirado, G.N.; Pagan, L.U.; Chaer, I.D.; et al. Influence of apocynin on cardiac remodeling in rats with streptozotocin-induced diabetes mellitus. Cardiovasc. Diabetol. 2018, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.M. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care 1994, 17, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.; Lee, J.E.; Yenari, M.A. NOX Inhibitors—A Promising Avenue for Ischemic Stroke. Exp. Neurobiol. 2017, 26, 195–205. [Google Scholar] [CrossRef]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes—A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem. Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef]
- Winiarska, K.; Grabowski, M.; Rogacki, M.K. Inhibition of renal gluconeogenesis contributes to hypoglycaemic action of NADPH oxidase inhibitor, apocynin. Chem. Biol. Interact. 2011, 189, 119–126. [Google Scholar] [CrossRef]
- Meng, R.; Zhu, D.L.; Bi, Y.; Yang, D.H.; Wang, Y.P. Anti-oxidative effect of apocynin on insulin resistance in high-fat diet mice. Ann. Clin. Lab. Sci. 2011, 41, 236–243. [Google Scholar] [PubMed]
- Liang, M.; Li, A.; Lou, A.; Zhang, X.; Chen, Y.; Yang, L.; Li, Y.; Yang, S.; Hou, F.F. Advanced oxidation protein products promote NADPH oxidase-dependent β-cell destruction and dysfunction through the Bcl-2/Bax apoptotic pathway. Lab. Investig. 2017, 97, 792–805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barclay, C.J. Mechanical efficiency and fatigue of fast and slow muscles of the mouse. J. Physiol. 1996, 497, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Pouvreau, C.; Dayre, A.; Butkowski, E.G.; de Jong, B.; Jelinek, H.F. Inflammation and oxidative stress markers in diabetes and hypertension. J. Inflamm. Res. 2018, 11, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, F.J.; Espinosa-Díez, C.; Dubey, M.; Dikshit, M.; Lamas, S. S-glutathionylation: Relevance in diabetes and potential role as a biomarker. Biol. Chem. 2013, 394, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, R.; Nair, K.S. Skeletal muscle mitochondrial dysfunction & diabetes. Indian J. Med. Res. 2007, 125, 399–410. [Google Scholar] [PubMed]
- Loureiro, A.C.; do Rêgo-Monteiro, I.C.; Louzada, R.A.; Ortenzi, V.H.; de Aguiar, A.P.; de Abreu, E.S.; Cavalcanti-de-Albuquerque, J.P.; Hecht, F.; de Oliveira, A.C.; Ceccatto, V.M.; et al. Differential Expression of NADPH Oxidases Depends on Skeletal Muscle Fiber Type in Rats. Oxidative Med. Cell. Longev. 2016, 2016, 6738701. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Goldblatt, C.S.; Meyer, C.J.; Li, X.; Cai, L.; et al. Diabetic downreg-ulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef]
- Chowdhry, S.; Nazmy, M.H.; Meakin, P.J.; Dinkova-Kostova, A.T.; Walsh, S.V.; Tsujita, T.; Dillon, J.F.; Ashford, M.L.; Hayes, J.D. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free. Radic. Biol. Med. 2010, 48, 357–371. [Google Scholar] [CrossRef]
- Meng, R.; Zhu, D.L.; Bi, Y.; Yang, D.H.; Wang, Y.P. Apocynin improves insulin resistance through suppressing inflammation in high-fat diet-induced obese mice. Mediat. Inflamm. 2010, 2010, 858735. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dou, W.; Ni, Z.; Wen, Q.; Zhang, R.; Qin, M.; Wang, X.; Tang, H.; Cao, Y.; Wang, J.; et al. Deletion of Nrf2 leads to hepatic insulin resistance via the activation of NF-κB in mice fed a high-fat diet. Mol. Med. Rep. 2016, 14, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Whitman, S.A.; Long, M.; Wondrak, G.T.; Zheng, H.; Zhang, D.D. Nrf2 modulates contractile and metabolic properties of skeletal muscle in streptozotocin-induced diabetic atrophy. Exp. Cell Res. 2013, 319, 2673–2683. [Google Scholar] [CrossRef]
- Landis, R.C.; Quimby, K.R.; Greenidge, A.R. M1/M2 Macrophages in Diabetic Nephropathy: Nrf2/HO-1 as Therapeutic Targets. Curr. Pharm. Des. 2018, 24, 2241–2249. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Nam, S.J.; Chun, W.; Kim, S.I.; Park, S.C.; Kang, C.D.; Lee, S.J. Anti-inflammatory effects of apocynin on dextran sulfate sodium-induced mouse colitis model. PLoS ONE 2019, 14, e0217642. [Google Scholar] [CrossRef]
- Pan, L.; Qian, S. Apocynin promotes neural function recovery and suppresses neuronal apoptosis by inhibiting Tlr4/NF-κB signaling pathway in a rat model of cerebral infarction. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418817700. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, W.; Li, D.; Guo, Y.; Ding, H. Overactivation of NF-κB impairs insulin sensitivity and mediates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Endocrine 2010, 37, 157–166. [Google Scholar] [CrossRef]
- Garvey, W.T.; Huecksteadt, T.P.; Birnbaum, M.J. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science 1989, 245, 60–63. [Google Scholar] [CrossRef]



| Gene | Forward | Reverse |
|---|---|---|
| NOX2 | 5′-CAATTCACACCATTGCACATC-3′ | 5′-CGAGTCACAGCCACATACAG-3′ |
| NOX4 | 5′-TCCATCAAGCCAAGATTCTGAG-3′ | 5′-GGTTTCCAGTCATCCA-TAGAG-3′ |
| Nrf2 | 5′-CACATCCAGACAGACACCAGT-3′ | 5′-CTACAAATG-GAATGTCTCTGC-3′ |
| NF-κB | 5′-ATGGCAGACGACGATCCTTTC-3′ | 5′-TGTTGACAGTG-TATATCTGTTG-3′ |
| GLUT4 | 5′-TCCATCAAGCCAAGATTCTGAG-3′ | 5′-GGTTTCCAGTCATCCA-TAGAG-3′ |
| 18s | 5′-GCAAATTACCCACTCCCGAC-3′ | 5′-CCGCTCCCAAGA TCCAACTA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Duarte, S.; Montoya-Pérez, R.; Márquez-Gamiño, S.; Vera-Delgado, K.S.; Caudillo-Cisneros, C.; Sotelo-Barroso, F.; Sánchez-Briones, L.A.; Sánchez-Duarte, E. Apocynin Attenuates Diabetes-Induced Skeletal Muscle Dysfunction by Mitigating ROS Generation and Boosting Antioxidant Defenses in Fast-Twitch and Slow-Twitch Muscles. Life 2022, 12, 674. https://doi.org/10.3390/life12050674
Sánchez-Duarte S, Montoya-Pérez R, Márquez-Gamiño S, Vera-Delgado KS, Caudillo-Cisneros C, Sotelo-Barroso F, Sánchez-Briones LA, Sánchez-Duarte E. Apocynin Attenuates Diabetes-Induced Skeletal Muscle Dysfunction by Mitigating ROS Generation and Boosting Antioxidant Defenses in Fast-Twitch and Slow-Twitch Muscles. Life. 2022; 12(5):674. https://doi.org/10.3390/life12050674
Chicago/Turabian StyleSánchez-Duarte, Sarai, Rocío Montoya-Pérez, Sergio Márquez-Gamiño, Karla S. Vera-Delgado, Cipriana Caudillo-Cisneros, Fernando Sotelo-Barroso, Luis A. Sánchez-Briones, and Elizabeth Sánchez-Duarte. 2022. "Apocynin Attenuates Diabetes-Induced Skeletal Muscle Dysfunction by Mitigating ROS Generation and Boosting Antioxidant Defenses in Fast-Twitch and Slow-Twitch Muscles" Life 12, no. 5: 674. https://doi.org/10.3390/life12050674
APA StyleSánchez-Duarte, S., Montoya-Pérez, R., Márquez-Gamiño, S., Vera-Delgado, K. S., Caudillo-Cisneros, C., Sotelo-Barroso, F., Sánchez-Briones, L. A., & Sánchez-Duarte, E. (2022). Apocynin Attenuates Diabetes-Induced Skeletal Muscle Dysfunction by Mitigating ROS Generation and Boosting Antioxidant Defenses in Fast-Twitch and Slow-Twitch Muscles. Life, 12(5), 674. https://doi.org/10.3390/life12050674






